Abstract
Chronic hemolytic anemia has increasingly been identified as an important risk factor for the development of pulmonary hypertension. Within the thalassemia syndromes, there are multiple mechanisms, both distinct and overlapping, by which pulmonary hypertension develops and that differ among β-thalassemia major or intermedia patients. Pulmonary hypertension in β-thalassemia major correlates with the severity of hemolysis, yet in patients whose disease is well treated with chronic transfusion therapy, the development of pulmonary hypertension can be related to cardiac dysfunction and the subsequent toxic effects of iron overload rather than hemolysis. β-thalassemia intermedia, on the other hand, has a higher incidence of pulmonary hypertension owing to the low level of hemolysis that exists over years without the requirement for frequent transfusions, while splenectomy is shown to play an important role in both types. Standard therapies such as chronic transfusion have been shown to mitigate pulmonary hypertension, and appropriate chelation therapy can avoid the toxic effects of iron overload, yet is not indicated in many patients. Limited evidence exists for the use of pulmonary vasodilators or other therapies, such as l-carnitine, to treat pulmonary hypertension associated with thalassemia. Here we review the most recent findings regarding the pathogenic mechanisms, epidemiology, presentation, diagnosis, and treatment of pulmonary hypertension in thalassemia syndromes.
Keywords: pulmonary hypertension, thalassemia, chronic hemolytic anemia
Introduction
As a common confounder of many chronic diseases, pulmonary hypertension (PH) universally portends a poor prognosis. The recognition of elevated pressure in the pulmonary circulation as a complication of the thalassemia syndromes α- and β-thalassemia is now widely accepted. Here we review the presence of pulmonary hypertension in chronic hemolytic anemia, which is followed by a focused examination of pulmonary hypertension in β-thalassemia. We will discuss the mechanisms by which pulmonary hypertension develops owing to chronic hemolysis and organ system dysfunction, followed by a discussion of therapeutic measures to both prevent and subsequently treat pulmonary hypertension in this population.
Pulmonary hypertension in hemoglobinopathies
Pulmonary hypertension represents a clinical spectrum of disease that has increasingly been identified as a significant source of morbidity and mortality in many common disease entities. Alterations in the pulmonary vasculature, which include sustained vasoconstriction, vascular remodeling, and changes in the extracellular matrix, lead to increased pulmonary vascular resistance (PVR) and subsequently to elevated pulmonary arterial pressures (PAP). Elevated PAP produces right ventricular strain, which can eventually progress to right ventricular failure and death.1 Increases in PVR can result from many different mechanisms and at different anatomical locations within the pulmonary circulation; therefore, a universal classification system has been used to characterize the various types of pulmonary hypertension.2 Under this system, PH is arranged into five separate groups based on common manifestations, clinical presentation, and therapeutic strategies, which include (1) pulmonary arterial hypertension (PAH), (2) pulmonary hypertension owing to left heart disease, (3) pulmonary hypertension owing to lung diseases and/or hypoxia, (4) chronic thromboembolic pulmonary hypertension, and (5) pulmonary hypertension with unclear multifactorial mechanisms. While PH due to chronic hemolytic anemia shares many of the canonical features of group 1 PAH, in the most recent classification it is included in group 5 owing to its multifactorial mechanisms, as the response to therapies used for PAH has not been well elucidated and the pathogenic mechanisms appear to be unique with regard to chronic hemolysis.
Echocardiography is often employed as a screening tool to identify subjects at high risk for pulmonary hypertension. The flow of retrograde blood across the tricuspid valve during systole, termed TR jet velocity (TRV), estimates right ventricular systolic pressure and correlates with mean PAP.3 Limitations to this technique, particularly in situations of high cardiac output, such as the chronic hemolytic anemias, necessitate the direct measurement of pulmonary hemodynamics by right heart catheterization (RHC) to confirm PH. RHC with mean PAP ≥ 25 mmHg at rest defines PH, while the presence of pulmonary artery occlusion pressure (PAOP) ≤ 15 mmHg and an elevated PVR > 3 Wood units confirm the presence of precapillary pulmonary hypertension that is not due to passive back pressure from derangements in the left heart and pulmonary venous congestion.3
The consumption, destruction, or dysfunction of erythrocytes leads to numerous changes in the cardiovascular system, both as compensatory mechanisms and as pathologic consequences of the disease. Anemia, a hallmark of these disorders, results in reduced blood viscosity, elevated cardiac output, decreased cardiac filling pressures, and reduced systemic and pulmonary vascular resistance, particularly when hemoglobin values are less than 7 g/dL.4 In addition to changes resulting directly from anemia, organ dysfunction may contribute to pathologic changes in the cardiovascular system. Liver disease, cardiac dysfunction, renal impairment, splenic changes, and thrombosis may all be confounding factors when examining the mechanisms by which PH develops in chronic hemolytic anemia.
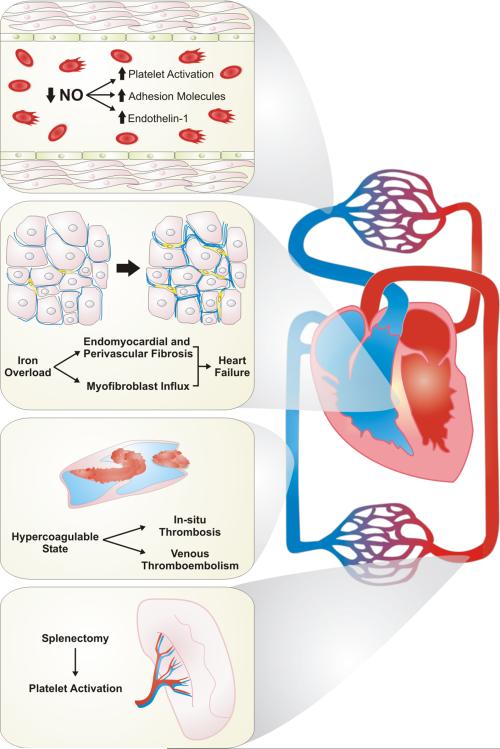
The development of PH in thalassemia syndromes is thought to be the result of numerous, multifactorial pathogenic mechanisms, which include chronic hemolysis, iron overload due to transfusion therapy, hypercoagulability, and changes to circulating cells as a consequence of splenectomy (Fig. 1).5 Erythrocyte dysfunction and by-products of chronic hemolysis contribute to impaired NO bioavailability. Free hemoglobin inactivates NO and its vasodilatory properties within the pulmonary circulation,6,7, while NO synthesis is inhibited by release of arginase during hemolysis and subsequent depletion of the essential NO substrate l-arginine.8 l-Arginine provides the substrate for both NO synthases and arginases; therefore, excess arginase activity will predominately drive l -arginine breakdown to ornithine rather than NO production through NO synthases, which produces citrulline as a breakdown product. Significantly higher arginase activity and lower arginine bioavailability has been shown in thalassemia subjects at high risk of having pulmonary hypertension—those with an elevated TRV by echocardiography—compared to those with normal TRV.9 In the subjects at highest risk for PH, plasma arginine levels were half as much as the subjects with normal TRV, and the arginase concentration was more than 10-fold higher. Hemolysis and impaired NO bioavailability also contribute to platelet activation, endothelial dysfunction, and increased oxidative stress, which result in tissue damage throughout the cardiovascular system.10,11 Anemia and associated hypoxemia heightens cardiovascular stress, increasing the cardiac output and amplifying hypoxic pulmonary vasoconstriction, which leads to untoward effects on PVR.12,13
Figure 1.
Pathogenic mechanisms associated with the development of pulmonary hypertension in thalassemia. The development of pulmonary hypertension in thalassemia is associated with multiple mechanisms that are both distinct and overlapping. Chronic hemolysis is associated with decreased nitric oxide bioavailability, resulting in platelet activation, increased expression of adhesion molecules, and increase of the vasoactive peptide endothelin-1. Iron overload as a consequence of chronic transfusion therapy leads to fibrosis of both the endomyocardial and perivascular space within the heart, leading to heart dysfunction and failure. Hypercoagulability can lead to both macrovascular and microvascular thrombosis. Therapeutic splenectomy is associated with platelet activation, combining with many of the above mechanisms. NO, nitric oxide.
The consequences of chronic transfusion therapy appear to play a distinct role in the development of PH in thalassemia. Iron overload increases oxidative stress and endothelial dysfunction,14–16 with evidence of these changes occurring very early in life.17 Iron overload is also associated with both right and left cardiac dysfunction through secondary hemochromatosis.18–20 Evaluation of iron accumulation in cardiac tissue by magnetic resonance imaging shows a significant iron burden in transfusion-dependent β-thalassemia patients with left heart failure and is thought to be the major cause of left ventricular dysfunction in this population.21 Iron overload also has effects on the lungs, with excess hemosiderin deposits in the pulmonary parenchyma resulting in fibrosis, as well as arterial wall stiffening, which can lead to increases in PVR.22–25 Indeed, excess sideroblasts identified in a pediatric population of transfusion-dependent β-thalassemia major is shown to mimic the findings of idiopathic pulmonary hemosiderosis.26
Splenectomy has been shown to be an important risk factor in the development of PH.27,28 The spleen functions as a filter for damaged erythrocytes and other circulating blood cells. Loss of this function is thought to result in platelet activation, abnormal erythrocyte aggregation, and release of procoagulant factors, likely as a response to having a greater number of abnormal erythrocytes or erythrocyte breakdown products in the circulation.28–31 Splenectomy also appears to be associated with increased release of early, nucleated erythrocytes that exhibit enhanced expression of adhesion molecules.28,32 Each of these factors may affect the propensity for intravascular thrombosis and cause changes within the pulmonary vasculature that lead to PH. Indeed, the prevalence of splenectomy shares a significant correlation with the prevalence of PH in β-thalassemia patients from multiple previous studies (Fig. 2).
Figure 2.

The prevalence of pulmonary hypertension in β-thalassemia is correlated with splenectomy. Analysis of prior studies of pulmonary hypertension in β-thalassemia shows that the presence of splenectomy in each cohort of patients is directly correlated with the prevalence of pulmonary hypertension. Using Pearson product correlation, the r value of 0.6 shows a direct correlation between these variables with a P-value of 0.02.
Hypercoagulability and increased incidence of thromboembolic disease are well known in thalassemia.31,33,34 Factors that contribute to a procoagulant state are similar to those described above and include endothelial dysfunction, abnormal erythrocyte aggregation, platelet activation, abnormal circulating factors due to hemolysis, and circulating erythrocyte breakdown products. The incidence of clinically evident thromboembolism is 1–4%, with a majority of events occurring before 30 years of age.35,36 The presence of both coinherited and acquired thrombophilias has been proposed as another mechanism leading to a higher incidence of thrombosis in thalassemia patients.37–39 The multifactorial mechanisms described above can work both independently and in combination with other mechanisms or organ dysfunction to result in clinically apparent PH (Table 1).
Table 1.
Pathophysiologic factors contributing to pulmonary hypertension in β-thalassemia
| Chronic hemolysis |
| Splenectomy |
| Oxidative stress |
| Iron overload |
| Inflammation |
| Hypercoagulability |
| Hypoxemia |
| RBC alterations |
| Impaired NO availability |
| Arginine dysregulation |
| Arginine excess |
Thalassemias
Thalassemia refers to disorders associated with defective synthesis of either the α-globulin or β-globulin subunit of hemoglobin A. These are inherited disorders, with the highest prevalence found in the Mediterranean and Middle East, as well as the tropical and subtropical regions of Africa and Southeast Asia.40 Genetic mutations on chromosomes 11 and 16 account for the cases of β-thalassemia and α-thalassemia, respectively, with more than 150 different mutations identified.40 The absence or severely impaired production of β-globulin in β-thalassemia results in an overproduction of α-globulin chains in erythrocyte precursors, leading to destruction in the bone marrow and peripheral blood.4 Conversely, the absence of α-globulin in α-thalassemia results in relative overproduction of β-globulin chains, which heterodimerize in erythrocytes, impairing oxygen delivery owing to impaired dissociation of oxygen from hemoglobin.41 Treatment relies on controlling the chronic hemolysis that is associated with both symptomatic anemia and organ dysfunction in these disorders.42 Chronic transfusion therapy, iron chelation, hydroxyurea therapy, and splenectomy are often employed. The increased recognition and successful treatment of children with thalassemia syndromes has resulted in significantly improved survival, with many patients now surviving into adulthood.43 As a consequence of increased survival, we are now recognizing the chronic complications of thalassemia, including severe cardiovascular complications.44,45
α–Thalassemia
More than 100 genetic mutations of α-thalassemia have been identified, and the severity of the disorder clearly correlates with the number of mutated copies of the α-globulin subunits.46 Asymptomatic carriers have a single genetic mutation, while those individuals with two genetic mutations also have a fairly benign course that rarely necessitates transfusion. Patients with three genetic mutations, termed HgH disease, are often symptomatic, but the clinical course can vary widely from those requiring transfusion only during episodes of severe infection or stressors to individuals requiring chronic transfusion therapy early in life. Finally, the presence of four genetic mutations often results in stillbirth, termed hydrops fetalis, though cases have survived to early childhood with regular transfusions started either intrauterine or immediately upon delivery, termed Bart's disease.47
Pulmonary hypertension in α-thalassemia appears to be an extremely rare complication of this disease. In one study examining pulmonary hemodynamic measurements by echocardiogram in α-thalassemia patients, only 3/80 (4%) patients with either HgH or Bart's disease had echocardiographic evidence of pulmonary hypertension as defined by a TRV greater than 2.9 m/s.48 In the largest study of HgH patients, out of 198 patients, 13 patients (7%) had TRVs greater than 2.5 m/s as measured by echocardiogram, and only three (2%) of those had TRVs greater than 3.0 m/s.49 Interestingly, splenectomy was negatively correlated with an elevated TRV, which conflicts with findings in splenectomized β-thalassemia subjects. Due to the rare presentation of pulmonary hypertension in α-thalassemia, to our knowledge there are no reports on the use of pulmonary vasodilators to treat PH in this population, and thus any potential therapies would be entirely experimental.
β-Thalassemia
β–thalassemia is characterized by a relative excess of α-globulin chains.43 Individuals with one defective gene are asymptomatic carriers of the β-thalassemia trait. β–thalassemia intermedia (TI) is characterized by mild-to-moderate decreases in β-globulin production in patients with two defective genes. ΤΙ generally presents with a mild course and clinically symptomatic anemia isolated to times of concurrent illness or infection. β-thalassemia major (TM) is the most severe form in patients with two defective β-globulin genes and severe decreases in β-globulin production.43 Patients at birth are asymptomatic owing to the presence of hemoglobin F (HbF) but subsequently develop transfusion-dependent anemia, hepatosplenomegaly, and skeletal deformities as early as 6 months of age. Patients are particularly susceptible to infection and commonly present with pathologic fractures. Pulmonary complications independent of pulmonary hypertension are rare, though both restrictive and obstructive defects have been reported, each of which are rarely associated with clinically significant lung disease.22,23,50
Both TI and TM are associated with pulmonary hypertension. Evidence suggestive of PH on echocardiography has been described in 40–50% and 10–75% of TI and TM patients, respectively (Table 2). In these series, the incidence of an elevated estimated right ventricular systolic pressure was associated with the severity of hemolysis.51,52 Other factors associated with the presence of PH include older age,53 splenectomy,27 prothrombotic state,31,54 and iron overload13 (Table 3). Biochemical studies looking at markers of arginine dysregulation and impaired NO bioavailability have shown that these to correlate with the presence of PH.55 Longitudinal data on the impact of pulmonary hypertension with respect to overall survival or morbidity in β-thalassemia is limited, though data from the Thalassemia Clinical Research Network has noted three deaths in 148 patients: two were considered likely to have PH on the basis of echocardiography, while the third died from cancer.53 Five deaths were also recorded in patients who had not undergone echocardiography; the cause of death in each of these subjects was cardiovascular causes or sudden death (in one subject), highlighting the possibility that PH may have contributed to the mortality. It is important to note that a majority of the studies identifying the incidence and prevalence of PH in thalassemia syndromes have focused on echocardiographic evidence for elevated pulmonary arterial pressure, which may overestimate the actual presence of PH in patients with chronic hemolytic anemia, compared to the gold standard of invasive hemodynamic measurements on RHC.
Table 2.
The prevalence of pulmonary hypertension in β-thalassemia.
| Study | β-thalassemia type | Assessment | n | Age (years) | Splenectomy (%) | PH prevalence (%) |
|---|---|---|---|---|---|---|
| Aessopos et al.51 | Intermedia | Echo | 110 | 32.5 | 61 (56) | 59 |
| Aessopos et al.44 | Intermedia | Echo | 74 | 23.2 | 42 (57) | 23 |
| Taher et al I.95 | Intermedia | Echo | 534 | 25.4 | 325 (56) | 11 |
| Derchi et al.59 | Intermedia | RHC | 332 | 42.8 | 194 (58) | 4.8 |
| Grisaru et al.96 | Major | Echo | 35 | 19.8 | 29 (33) | 75 |
| Du et al.97 | Major | Echo | 33 | 12.1 | 21 (64) | 79 |
| Derchi et al.98 | Major | Echo | 130 | 25 | N/A | 10 |
| Aessopos et al.12 | Major | Echo | 202 | 27.3 | 39 (19) | Absent |
| Wu et al.99 | Major | Echo | 39 | 12.9 | 15 (38) | 31 |
| Aessopos et al.44 | Major | Echo | 131 | 27.9 | 27 (21) | Absent |
| Hagar et al.57 | Major | Echo | 36 | 26 | 20 (56) | 57 |
| Vlahos et al.100 | Major | Echo | 27 | 38 | 17 (63) | 19 |
| Derchi et al.59 | Major | RHC | 977 | 34.3 | 439 (45) | 1.1 |
| Phrommintikul et al.28 | β-thal, E/β-thal, HbH | Echo | 68 | 29.3 | 32 (47) | 43 |
| Morris et al.53 | β-thal, E/β-thal, HbH | Echo | 148 | 25.9 | 100 (68) | 33 |
β-thal, β-thalassemia; E/β-thal, hemoglobin E/β-thalassemia; HbH, hemoglobin H
Table 3.
Risk factors for pulmonary hypertension in β-thalassemia
| Advanced age |
| Splenectomy |
| Severe hemolysis |
| Iron overload |
| Hepatitis C |
| Smoking |
| Previous venous thromboembolism |
Clinical presentation and diagnosis
Pulmonary hypertension is associated with numerous nonspecific symptoms, including dyspnea, weakness, fatigue, chest pain, and syncope.56 Initially, symptoms are more pronounced with exercise or exertion, but, as severity advances, the level of symptoms at rest intensifies. It can be difficult to distinguish symptoms directly related to pulmonary hypertension versus those of comorbid conditions, such as anemia, asthma, or parenchymal lung disease. The signs on physical exam of pulmonary hypertension can be broken down into two groups, those that represent increased PVR and signs of right heart dysfunction and failure. Signs of increased PVR include a parasternal lift; accentuated pulmonary component of the second heart sound; a soft, pansystolic murmur of tricuspid regurgitation; audible S4; and a diastolic murmur of pulmonary insufficiency. Signs of right heart dysfunction and failure are rare, as elevated cardiac output is most commonly encountered, but include a third heart sound arising from the right ventricle, best heard at the lower left sternal border, which increases with inspiration. Jugular venous distension, peripheral edema, hepatomegaly, hepatojugular reflux, and cool, mottled extremities in the setting of severe pulmonary hypertension may also indicate right heart failure.
Echocardiography can identify thalassemia patients at risk for PH in whom TRV is used to estimate right ventricular systolic pressures.12,57 It is not clear how well correlated TRV is in the thalassemia syndromes, yet TRV in thalassemia is thought to be similar to that of other chronic hemolytic anemias in which echocardiographic findings may overestimate the risk of PH.58 In β-thalassemia patients, a threshold of 3.2 m/s is shown to have a high positive predictive value for the diagnosis of PH on RHC, though the positive predictive value of lower thresholds, such as 2.5 m/s, remains unknown.59 Thus, most experts suggest that a TRV greater than 3.0 m/s in thalassemia patients identifies a population at increased risk of PH who should undergo RHC.60,61 Most data regarding RHC in both TM and TI patients have been either case reports or small case series describing predominately precapillary PH with elevated mPAP in the setting of normal PAOP,5,25,51,62,63 yet cases of postcapillary PH related to left heart disease have also been described.20,27
Given the presence of both pre- and postcapillary PH in this population and the presence of high cardiac output with only modestly elevated PVR, RHC remains a powerful tool in defining both the pathophysiology and severity of PH in thalassemia patients. The largest trial using RHC to identify PH was published by Derchi and colleagues, and it provides a better understanding of the precise prevalence in both TI and TM, as well as the limitations of echocardiography in estimating pulmonary hemodynamics in these patients.59 Of 1309 patients prospectively enrolled in this study, about 25% of the subjects had TI, while the remainder had TM. A great majority of patients (94%) were considered unlikely to have PH following echocardiography with findings of TRV less than 3.0 m/sec. Thirty-three subjects at the highest risk for having PH, with TRVs greater than 3.2 m/sec, underwent RHC. Of these 33 subjects, one TM patient and one TI patient had normal pulmonary artery pressures and therefore did not have PH. Of the remaining subjects, 27 had precapillary PH (11 TM subjects and 16 TI subjects) and four had postcapillary PH (one TM subject and three TI subjects). While the strict cutoff for TRV likely underestimates the true prevalence of PH in β-thalassemia, this study does suggest a higher prevalence in TI (4.8%) than TM (1.1%).
Supplementary data that are useful in the evaluation of PH in thalassemia patients include markers of right heart dysfunction, such as NT-proBNP and troponin T levels. Evaluation of liver and renal function, in addition to monitoring the degree of hemolysis with hematocrit, LDH, reticulocyte count, and bilirubin levels, can guide treatment. Assessing the severity of iron overload is also imperative to determine its effects on end-organ damage, including cardiomyopathy and the need for chelation therapy. Six-minute walk testing is the cornerstone of determining exertional capacity, and baseline walk distance has been shown to have significant prognostic value in PH.64 Pulmonary function testing can help identify both restrictive and obstructive defects that are common in thalassemia patients, as well as diffusing limitations that are often associated with PH. Cardiac MRI is shown to be comparable to echocardiography when determining the risk of PH in many disease states, and is also a reliable tool for determining the degree of iron burden and damage that may be present in chronically transfused individuals.65,66 Finally, the exclusion of other forms of pulmonary arterial hypertension should always be considered when evaluating a patient with newly diagnosed PH. History, physical exam findings, and laboratory assessment should ensure the absence of HIV, severe liver disease, collagen vascular disease, and/or the use of toxins associated with PAH. Additionally, a careful assessment for chronic thromboembolic disease is imperative, as thalassemia is a known hypercoagulable state.36,54,67 A systematic approach to evaluating PH associated with thalassemia syndromes will ensure a timely diagnosis while confirming the absence of any other potentially treatable comorbid conditions.
Therapeutic options
Transfusion therapy
Treatment of β-thalassemia relies on an appropriate transfusion strategy, particularly in more severe TM, in order to control chronic hemolysis. A chronic transfusion protocol, in addition to iron-chelating therapy to prevent iron overload, is thought to prevent or even ameliorate PH in these patients.63 In one large group of patients treated since early infancy with intensive transfusion and chelation therapy, echocardiography failed to show any evidence of PH.12
Hydroxyurea therapy, in order to increase HbF production, has also been shown to protect from the development of PH. In a study of 50 TI patients on chronic hydroxyurea therapy, echocardiographic evidence of PH was again absent, suggesting a potential protective effect.68 In each of these studies, invasive hemodynamics had not been measured, and thus the true incidence of PH was not directly assessed. These studies examined the cardiovascular effects of therapies that were already being used, and subsequent randomized, controlled trials have yet to be performed.
Stem cell transplant
The only known curative approach to TM remains stem cell transplant (SCT) early in life, which was first reported in 1982.69 Transplantation from unrelated donors carries high success rates exceeding 70%,70,71 and when transplants are performed between siblings, the rate of success has been reported to be as high as 94%.72–74 SCT appears to be a durable cure for these individuals, with markers of hemolysis, as well as the hypercoagulability associated with thalassemia, returning to normal following treatment.75,76
l-Carnitine
Mitochondrial dysfunction has more recently gained much attention in the pathophysiology of both pulmonary arterial hypertension and PH associated with many other diseases, including chronic hemolytic anemia. l-Carnitine is necessary for fatty acid oxidation and normal mitochondrial function. In a study of 12 children with TM and echocardiographic evidence of PH, administration of oral l-carnitine was associated with improvement in hemodynamics measurements, with mean pulmonary artery systolic pressure on echocardiography being reduced by nearly 10 mmHg after three months of therapy with l-carnitine.77 Invasive hemodynamics were again not measured; therefore, the true prevalence of PH could not be assessed, and patients receiving oral l-carnitine were not compared to a similar, placebo control group. Therefore, larger randomized controlled clinical trials will be necessary to confirm a potential benefit.
Supportive care
Similar to all patients with PH, supportive measures must be utilized promptly after identification of PH in thalassemia patients. Oxygen therapy should be administered in order to avoid hypoxia both at rest and during exertion, preventing the detrimental effects of hypoxic vasoconstriction in the pulmonary vasculature. Physical therapy and lifestyle modifications are often employed to improve symptoms and walk distance. Routine immunization with pneumococcal and annual influenza vaccination is recommended. Diuretics, digoxin, and/or dobutamine are used for cardiac dysfunction and arrhythmias. Prothombotic states may also be present, and careful evaluation for thromboembolic disease and appropriate therapy with anticoagulants and/or antiplatelet agents must be considered. Additionally, if the burden of thromboembolic disease is thought to be a significant contributor to pulmonary hypertension, then evaluation for pulmonary endarterectomy must be considered. Successful results have been reported in at least one case of β-thalassemia major.78
PH-specific therapies
There are limited data available regarding the use of pulmonary vasodilator therapies in β-thalassemia, and there are no prospective, randomized trials available in this patient population to support the routine use of such therapy (Table 4). Much of the data comes from treatment of PH associated with other chronic hemolytic anemias, in particular sickle cell disease. Initial studies using sildenafil, a phosphodiesterase type 5 inhibitor (PDE-5 inhibitor), in patients with PH as a result of sickle cell disease demonstrated improvements in 6-minute walk distance and hemodynamic measurements by echocardiography, but a subsequent randomized controlled trial was stopped early owing to increased risk of hospitalization for painful episodes related to sickle cell disease, and at the time of termination there was no significant clinical effect observed.79,80 Early studies of endothelin receptor antagonists (ERAs), particularly bosentan, on sickle cell– related PH have shown that treatment is effective at increasing 6-minute walk distance and pulmonary hemodynamic measurements by RHC in a small group of patients.81 Subsequent studies show that ERAs are well tolerated, but efficacy could not be assessed owing to poor patient recruitment.82
Table 4.
Treatment of pulmonary hypertension in β-thalassemia.
| Study | Diagnosis | n | Treatment | Duration | Assessment | Findings |
|---|---|---|---|---|---|---|
| Littera et al.85 | β-thalassemia intermedia | 1 | Sildenafil 50–100 mg/day | 15 months | Echo | Symptoms, echo improvement |
| Derchi et al.84 | β-thalassemia intermedia, major, and sickle/β-thalassemia | 7 | Sildenafil 100 mg/day | 4 months to 4 years | Echo | Symptoms, NYHA, 6-MWD, echo improvement |
| Correale et al.86 | β-thalassemia major | 1 | Sildenafil 120 mg/day | 2 years | Echo | NYHA, echo improvement |
| Morris et al.87 | β-thalassemia intermedia and major | 10 | Sildenafil 50–100 mg three times daily | 12 weeks | Echo | NYHA, echo improvement, 6-MWD unchanged |
| Anthi et al.62 | β-thalassemia intermedia | 1 | Bosentan 125 mg twice daily | 1 year | RHC | NYHA, hemodynamic improvement |
| Tam et al.93 | β-thalassemia major | 1 | Epoprostenol | 5 years | RHC | Symptoms, hemodynamic improvement |
| Ussavarungsi and Burger94 | β-thalassemia intermedia | 1 | Epoprostenol | 3 years | RHC | Symptoms, NYHA, 6-MWD, hemodynamic improvements |
| El-Beshlawy et al.77 | β-thalassemia major | 32 | L-carnitine 50 mg/kg daily | 3 months | Echo | Echo improvement |
NYHA, New York Heart Association functional class; 6-MWD, 6-minute walk distance
Phosphodiesterase type 5 inhibitors
The cyclic guanosine monophosphate (cGMP) pathway is an important target of endothelium-derived nitric oxide (NO), an essential vasodilatory agent.83 Phosphodiesterase type 5 (PDE5) rapidly hydrolyzes cGMP, inactivating its vasodilatory effects. PDE5 inhibitors have been developed that prolong the effect of cGMP by inhibiting its degradation.
The efficacy of sildenafil has been suggested in case reports and a small case series in which four patients with TI and two patients with TM showed improvements in 6-minute walk distance, symptoms, functional class, and hemodynamic measurements by echocardiography after long-term treatment.84–86 A pilot study of sildenafil in patients with β-thalassemia and echocardiographic findings suggestive of pulmonary hypertension over 12 weeks showed improvements in TRV and a trend toward improved functional classification without improvement of 6-minute walk distance.87
Endothelin receptor antagonists
Mainly synthesized in endothelial cells, but also described in vascular smooth muscle cells, endothelin (ET-1) is a potent vasoconstrictor that has also been shown to increase smooth muscle cell proliferation and decrease apoptosis.88 Increased levels of circulating ET-1, shown in PAH, lead to activation of endothelin receptors and contribute to pulmonary vasoconstriction and vascular remodeling.89
A case report of a TI patient with PH confirmed by RHC was treated with bosentan for over 1 year.62 Clinical improvements in functional classification and hemodynamic parameters were observed, while the therapy was well tolerated without any significant adverse effects or changes in liver function testing. Liver disease is the second most common cause of mortality in patients with TM, and bosentan is associated with increases in liver function markers, particularly transaminitis. Bosentan is contraindicated in patients with moderate to severe liver disease, and while newer agents may have less liver toxicity, the class should be used with caution in patients with liver disease.
Soluble guanylate cyclase stimulator
Soluble guanylate cyclase (sGC) converts guanosine triphosphate (GTP) to cGMP, leading to vasorelaxation. Randomized, placebo-controlled trials in both PAH and CTEPH patients have shown improvements in WHO functional classification, 6-minute walk distance, and invasive pulmonary hemodynamics with the use of the sGC stimulator riociguat.90,91 Neither trial included patients with PH related to chronic hemolytic anemia, nor are we aware of any reports on the use of sGC stimulators in this setting. Despite this, patients with thalassemia are at higher risk of thromboembolic disease, and if this is a major contributor to pulmonary pressure overload, there may be a consideration for treatment with riociguat if no surgical options are available.
Prostacyclin analogs
A product of the metabolism of arachidonic acid, prostacyclin binds to the prostacyclin receptor (IPR) on pulmonary artery smooth muscle cells, leading to increased levels of cyclic adenosine monophosphate (cAMP), which promotes vasodilation and inhibits vascular remodeling; as well as having effects on various other cell lines including platelet inhibition.92
A case study of TM complicated by PH reported improvements in both functional class and invasive hemodynamic measurements by RHC after treatment with epoprostenol, a synthetic prostacyclin.93 Another case report describes a patient with TI and severe PH diagnosed on RHC who was successfully treated with epoprostenol for over three years with improvements in symptoms, functional classification, 6-minute walk distance, and invasive hemodynamic measurements.94
Conclusions
In recent years, PH has become a commonly identified comorbid illness for many chronic diseases, including the hemolytic anemias. The pathophysiology of PH in thalassemia is both complex and unique among the different syndromes. Thus, a comprehensive evaluation for both the presence and cause of PH is imperative when determining appropriate therapies. Pulmonary vasodilator therapy, the basis of PAH treatment, has only limited evidence in the treatment of PH associated with chronic hemolytic anemias, and is even sparser for thalassemia patients. Until more comprehensive data regarding the diagnostic evaluation, natural history, and response to therapy of thalassemia patients with PH is available, there can be no standardization with regard to the management of these individuals, and we must continue to rely on anecdotal evidence and small case series. Sustained research efforts are imperative to developing best practices in this vulnerable patient population.
Acknowledgments
This work was supported in parts by NIH Grant HL127342. D.R.F was supported by a training grant from the NIH (HL082547).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Benza RL, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, et al. Definitions and Diagnosis of Pulmonary Hypertension. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Rund D, Rachmilewitz E. Beta-thalassemia. The New England journal of medicine. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 5.Aessopos A, et al. Pulmonary hypertension and right heart failure in patients with beta-thalassemia intermedia. Chest. 1995;107:50–53. doi: 10.1378/chest.107.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Reiter CD, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle- cell disease. Nature medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 7.Rother RP, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Jama. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 8.Morris CR, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris CR, et al. Dysregulated arginine metabolism and cardiopulmonary dysfunction in patients with thalassaemia. British journal of haematology. 2015;169:887–898. doi: 10.1111/bjh.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagger D, et al. Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 1995;6:93–99. doi: 10.1097/00001721-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Villagra J, et al. Platelet activation in patients with sickle disease, hemolysis- associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aessopos A, et al. Cardiac status in well-treated patients with thalassemia major. European journal of haematology. 2004;73:359–366. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 13.Wood JC, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Annals of the New York Academy of Sciences. 2005;1054:386–395. doi: 10.1196/annals.1345.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livrea MA, et al. Oxidative stress and antioxidant status in beta-thalassemia major: iron overload and depletion of lipid-soluble antioxidants. Blood. 1996;88:3608–3614. [PubMed] [Google Scholar]
- 15.Sengsuk C, et al. Association of Iron Overload with Oxidative Stress, Hepatic Damage and Dyslipidemia in Transfusion-Dependent beta-Thalassemia/HbE Patients. Indian journal of clinical biochemistry : IJCB. 2014;29:298–305. doi: 10.1007/s12291-013-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung YF, Chan GC, Ha SY. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation. 2002;106:2561–2566. doi: 10.1161/01.cir.0000037225.92759.a7. [DOI] [PubMed] [Google Scholar]
- 17.Detchaporn P, et al. Altered vascular function, arterial stiffness, and antioxidant gene responses in pediatric thalassemia patients. Pediatric cardiology. 2012;33:1054–1060. doi: 10.1007/s00246-012-0225-8. [DOI] [PubMed] [Google Scholar]
- 18.Kremastinos DT, et al. Left ventricular diastolic Doppler characteristics in beta- thalassemia major. Circulation. 1993;88:1127–1135. doi: 10.1161/01.cir.88.3.1127. [DOI] [PubMed] [Google Scholar]
- 19.Kremastinos DT, et al. Heart failure in beta thalassemia: a 5-year follow-up study. The American journal of medicine. 2001;111:349–354. doi: 10.1016/s0002-9343(01)00879-8. [DOI] [PubMed] [Google Scholar]
- 20.Hahalis G, et al. Right ventricular cardiomyopathy in beta-thalassaemia major. European heart journal. 2002;23:147–156. doi: 10.1053/euhj.2001.2709. [DOI] [PubMed] [Google Scholar]
- 21.Liguori C, et al. Magnetic resonance comparison of left-right heart volumetric and functional parameters in thalassemia major and thalassemia intermedia patients. BioMed research international. 2015;2015:857642. doi: 10.1155/2015/857642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnelli V, et al. Pulmonary dysfunction in transfusion-dependent patients with thalassemia major. American journal of respiratory and critical care medicine. 2003;168:180–184. doi: 10.1164/rccm.200211-1292OC. [DOI] [PubMed] [Google Scholar]
- 23.Factor JM, et al. Pulmonary function abnormalities in thalassemia major and the role of iron overload. American journal of respiratory and critical care medicine. 1994;149:1570–1574. doi: 10.1164/ajrccm.149.6.8004315. [DOI] [PubMed] [Google Scholar]
- 24.Piatti G, et al. Lung function in beta-thalassemia patients: a longitudinal study. Acta haematologica. 2006;116:25–29. doi: 10.1159/000092344. [DOI] [PubMed] [Google Scholar]
- 25.Zakynthinos E, et al. Pulmonary hypertension, interstitial lung fibrosis, and lung iron deposition in thalassaemia major. Thorax. 2001;56:737–739. doi: 10.1136/thorax.56.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priftis KN, et al. Quantification of siderophages in bronchoalveolar fluid in transfusional and primary pulmonary hemosiderosis. Pediatric pulmonology. 2006;41:972–977. doi: 10.1002/ppul.20479. [DOI] [PubMed] [Google Scholar]
- 27.Atichartakarn V, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. International journal of hematology. 2003;78:139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 28.Phrommintikul A, et al. Splenectomy: a strong risk factor for pulmonary hypertension in patients with thalassaemia. Heart. 2006;92:1467–1472. doi: 10.1136/hrt.2005.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruf A, et al. In-vivo platelet activation correlates with red cell anionic phospholipid exposure in patients with beta-thalassaemia major. British journal of haematology. 1997;98:51–56. doi: 10.1046/j.1365-2141.1997.1502965.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, et al. Enhanced aggregability of red blood cells of beta-thalassemia major patients. The American journal of physiology. 1996;270:H1951–1956. doi: 10.1152/ajpheart.1996.270.6.H1951. [DOI] [PubMed] [Google Scholar]
- 31.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- 32.Garozzo G, et al. Detection of ICAM-1, ICAM-2, ICAM-3, PECAM-1 and VCAM-1, evaluation of hypercoagulable state and platelet aggregation in hemoglobinopathy patients with erythroblasts. Haematologica. 2001;86:778–779. [PubMed] [Google Scholar]
- 33.Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. Haematologica. 2007;92:658–665. doi: 10.3324/haematol.10915. [DOI] [PubMed] [Google Scholar]
- 34.Musallam KM, Taher AT. Thrombosis in thalassemia: why are we so concerned? Hemoglobin. 2011;35:503–510. doi: 10.3109/03630269.2011.605499. [DOI] [PubMed] [Google Scholar]
- 35.Haghpanah S, Karimi M. Cerebral thrombosis in patients with beta-thalassemia: a systematic review. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2012;23:212–217. doi: 10.1097/MBC.0b013e3283502975. [DOI] [PubMed] [Google Scholar]
- 36.Taher A, et al. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thrombosis and haemostasis. 2006;96:488–491. [PubMed] [Google Scholar]
- 37.Cappellini MD, et al. Redefining thalassemia as a hypercoagulable state. Annals of the New York Academy of Sciences. 2010;1202:231–236. doi: 10.1111/j.1749-6632.2010.05548.x. [DOI] [PubMed] [Google Scholar]
- 38.Mustafa NY, et al. Hypercoagulable state and methylenetetrahydrofolate reductase (MTHFR) C677T mutation in patients with beta-thalassemia major in Kuwait. Acta haematologica. 2010;123:37–42. doi: 10.1159/000260069. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, et al. Lupus anticoagulant and anticardiolipin antibodies in polytransfused beta thalassemia major. Hematology. 2006;11:287–290. doi: 10.1080/10245330600954130. [DOI] [PubMed] [Google Scholar]
- 40.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piel FB, Weatherall DJ. The alpha-thalassemias. The New England journal of medicine. 2014;371:1908–1916. doi: 10.1056/NEJMra1404415. [DOI] [PubMed] [Google Scholar]
- 42.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118:3479–3488. doi: 10.1182/blood-2010-08-300335. [DOI] [PubMed] [Google Scholar]
- 43.Olivieri NF. The beta-thalassemias. The New England journal of medicine. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 44.Aessopos A, Farmakis D. Pulmonary hypertension in beta-thalassemia. Annals of the New York Academy of Sciences. 2005;1054:342–349. doi: 10.1196/annals.1345.041. [DOI] [PubMed] [Google Scholar]
- 45.Kremastinos DT, et al. Beta-thalassemia cardiomyopathy: history, present considerations, and future perspectives. Circulation. Heart failure. 2010;3:451–458. doi: 10.1161/CIRCHEARTFAILURE.109.913863. [DOI] [PubMed] [Google Scholar]
- 46.Giardine B, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic acids research. 2014;42:D1063–1069. doi: 10.1093/nar/gkt911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chui DH, Waye JS. Hydrops fetalis caused by alpha-thalassemia: an emerging health care problem. Blood. 1998;91:2213–2222. [PubMed] [Google Scholar]
- 48.Teawtrakul N, et al. Effect of genotype on pulmonary hypertension risk in patients with thalassemia. European journal of haematology. 2014;92:429–434. doi: 10.1111/ejh.12261. [DOI] [PubMed] [Google Scholar]
- 49.Yin XL, et al. Pulmonary hypertension risk in patients with hemoglobin h disease: low incidence and absence of correlation with splenectomy. Acta haematologica. 2013;130:153–159. doi: 10.1159/000347177. [DOI] [PubMed] [Google Scholar]
- 50.Abu-Ekteish FM, et al. Pulmonary function tests in children with beta-thalassemia major. Chronic respiratory disease. 2007;4:19–22. doi: 10.1177/1479972306070376. [DOI] [PubMed] [Google Scholar]
- 51.Aessopos A, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–3416. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 52.Atichartakarn V, et al. Prevalence and risk factors for pulmonary hypertension in patients with hemoglobin E/beta-thalassemia disease. European journal of haematology. 2014;92:346–353. doi: 10.1111/ejh.12242. [DOI] [PubMed] [Google Scholar]
- 53.Morris CR, et al. Risk factors and mortality associated with an elevated tricuspid regurgitant jet velocity measured by Doppler-echocardiography in thalassemia: a Thalassemia Clinical Research Network report. Blood. 2011;118:3794–3802. doi: 10.1182/blood-2010-11-319152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cappellini MD, et al. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. British journal of haematology. 2000;111:467–473. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- 55.Morris CR, et al. Hemolysis-associated pulmonary hypertension in thalassemia. Annals of the New York Academy of Sciences. 2005;1054:481–485. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jing ZC, et al. Pulmonary function testing in patients with pulmonary arterial hypertension. Respiratory medicine. 2009;103:1136–1142. doi: 10.1016/j.rmed.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Hagar RW, Morris CR, Vichinsky EP. Pulmonary hypertension in thalassaemia major patients with normal left ventricular systolic function. British journal of haematology. 2006;133:433–435. doi: 10.1111/j.1365-2141.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 58.Parent F, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. The New England journal of medicine. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 59.Derchi G, et al. Prevalence and risk factors for pulmonary arterial hypertension in a large group of beta-thalassemia patients using right heart catheterization: a Webthal study. Circulation. 2014;129:338–345. doi: 10.1161/CIRCULATIONAHA.113.002124. [DOI] [PubMed] [Google Scholar]
- 60.Galie N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). The European respiratory journal. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 61.Machado RF, Gladwin MT. Hemolytic anemia association pulmonary hypertension. In: J., M., T. D., editors. Pulmonary Vascular Disease. Elsevier; Philadelphia, PA.: 2006. pp. 170–187. [Google Scholar]
- 62.Anthi A, et al. Treatment with bosentan in a patient with thalassemia intermedia and pulmonary arterial hypertension. Blood. 2012;120:1531–1532. doi: 10.1182/blood-2012-04-422568. [DOI] [PubMed] [Google Scholar]
- 63.Atichartakarn V, et al. Correction of hypercoagulability and amelioration of pulmonary arterial hypertension by chronic blood transfusion in an asplenic hemoglobin E/beta-thalassemia patient. Blood. 2004;103:2844–2846. doi: 10.1182/blood-2003-09-3094. [DOI] [PubMed] [Google Scholar]
- 64.Miyamoto S, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 65.Nogami M, et al. Utility of phase contrast MR imaging for assessment of pulmonary flow and pressure estimation in patients with pulmonary hypertension: comparison with right heart catheterization and echocardiography. Journal of magnetic resonance imaging : JMRI. 2009;30:973–980. doi: 10.1002/jmri.21935. [DOI] [PubMed] [Google Scholar]
- 66.Wood JC, Ghugre N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin. 2008;32:85–96. doi: 10.1080/03630260701699912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taher A, et al. Pulmonary thromboembolism in beta-thalassemia intermedia: are we aware of this complication? Hemoglobin. 2002;26:107–112. doi: 10.1081/hem-120005447. [DOI] [PubMed] [Google Scholar]
- 68.Karimi M, et al. Echocardiographic finding in beta-thalassemia intermedia and major: absence of pulmonary hypertension following hydroxyurea treatment in beta-thalassemia intermedia. European journal of haematology. 2009;82:213–218. doi: 10.1111/j.1600-0609.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 69.Thomas ED, et al. Marrow transplantation for thalassaemia. Lancet. 1982;2:227–229. doi: 10.1016/s0140-6736(82)90319-1. [DOI] [PubMed] [Google Scholar]
- 70.Jaing TH, et al. Unrelated cord blood transplantation for thalassaemia: a single- institution experience of 35 patients. Bone marrow transplantation. 2012;47:33–39. doi: 10.1038/bmt.2011.39. [DOI] [PubMed] [Google Scholar]
- 71.La Nasa G, et al. Unrelated bone marrow transplantation for beta-thalassemia patients: The experience of the Italian Bone Marrow Transplant Group. Annals of the New York Academy of Sciences. 2005;1054:186–195. doi: 10.1196/annals.1345.023. [DOI] [PubMed] [Google Scholar]
- 72.Ali N, et al. Outcome of match related allogeneic stem cell transplantation procedures performed from 2004 till 2011. Experimental hematology & oncology. 2012;1:13. doi: 10.1186/2162-3619-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hongeng S, et al. Reduced intensity stem cell transplantation for treatment of class 3 Lucarelli severe thalassemia patients. American journal of hematology. 2007;82:1095–1098. doi: 10.1002/ajh.21002. [DOI] [PubMed] [Google Scholar]
- 74.Sabloff M, et al. HLA-matched sibling bone marrow transplantation for beta- thalassemia major. Blood. 2011;117:1745–1750. doi: 10.1182/blood-2010-09-306829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lucarelli G, Andreani M, Angelucci E. The cure of the thalassemia with bone marrow transplantation. Bone marrow transplantation. 2001;28(Suppl 1):S11–13. doi: 10.1038/sj.bmt.1703169. [DOI] [PubMed] [Google Scholar]
- 76.Sirachainan N, et al. Normalized coagulation markers and anticoagulation proteins in children with severe beta-thalassemia disease after stem cell transplantation. Thrombosis research. 2012;129:765–770. doi: 10.1016/j.thromres.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 77.El-Beshlawy A, et al. Pulmonary hypertension in beta-thalassemia major and the role of L-carnitine therapy. Pediatric hematology and oncology. 2008;25:734–743. doi: 10.1080/08880010802244035. [DOI] [PubMed] [Google Scholar]
- 78.Roggero S, et al. Management of pulmonary arterial hypertension associated to thalassemia: when pulmonary endarterectomy is the best therapeutical option? A case report. Journal of thrombosis and thrombolysis. 2015;39:139–143. doi: 10.1007/s11239-014-1073-6. [DOI] [PubMed] [Google Scholar]
- 79.Machado RF, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machado RF, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. British journal of haematology. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minniti CP, et al. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. British journal of haematology. 2009;147:737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barst RJ, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. British journal of haematology. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sausbier M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circulation research. 2000;87:825–830. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- 84.Derchi G, et al. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–458. [PubMed] [Google Scholar]
- 85.Littera R, et al. Long-term treatment with sildenafil in a thalassemic patient with pulmonary hypertension. Blood. 2002;100:1516–1517. doi: 10.1182/blood-2002-04-1171. [DOI] [PubMed] [Google Scholar]
- 86.Correale M, et al. Long-term treatment with high-dose of sildenafil in a thalassemic patient with pulmonary hypertension. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace / Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo. 2012;78:105–106. doi: 10.4081/monaldi.2012.131. [DOI] [PubMed] [Google Scholar]
- 87.Morris CR, et al. Sildenafil therapy in thalassemia patients with Doppler-defined risk of pulmonary hypertension. Haematologica. 2013;98:1359–1367. doi: 10.3324/haematol.2012.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levin ER. Endothelins. The New England journal of medicine. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 89.Giaid A, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. The New England journal of medicine. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 90.Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. The New England journal of medicine. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 91.Ghofrani HA, et al. Riociguat for the treatment of pulmonary arterial hypertension. The New England journal of medicine. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 92.Olschewski H, et al. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacology & therapeutics. 2004;102:139–153. doi: 10.1016/j.pharmthera.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 93.Tam DH, Farber HW. Pulmonary hypertension and beta-thalassemia major: report of a case, its treatment, and a review of the literature. American journal of hematology. 2006;81:443–447. doi: 10.1002/ajh.20603. [DOI] [PubMed] [Google Scholar]
- 94.Ussavarungsi K, Burger CD. Pulmonary arterial hypertension in a patient with beta-thalassemia intermedia and reversal with infusion epoprostenol then transition to oral calcium channel blocker therapy: review of literature. Pulmonary circulation. 2014;4:520–526. doi: 10.1086/677367. [DOI] [PMC free article] [PubMed] [Google Scholar]