Abstract
Vibrio cholerae O1 exists as two major serotypes, Inaba and Ogawa, which are associated with the O antigen of the lipopolysaccharide and are capable of unequal reciprocal interconversion. The 20-kilobase rfb regions encoding O-antigen biosynthesis in strains 569B (Inaba) and O17 (Ogawa) have been cloned in Escherichia coli K-12 and the nucleotide sequences have been determined. Besides several base substitutions and a small deletion in the 569B sequence relative to O17, there is a single nucleotide change resulting in a TGA stop codon within the gene for the 32-kDa RfbT protein. We have demonstrated that rfbT is responsible for serotype conversion (Inaba to Ogawa). The construction of a specific rfbT mutation in the Ogawa strain O17, and the ability of the gene from O17 to complement Inaba strains to Ogawa, confirmed rfbT as the gene required for the Ogawa serotype. By Southern hybridization and sequencing of PCR products of a number of strains, we have shown that the changes observed in one Inaba strain (569B) are not conserved in other Inaba strains. This may explain why some Inaba strains are able to convert to Ogawa whereas others are not. The protein encoded by rfbT has been identified and expressed in E. coli K-12 using a phage T7 expression system. Amino-terminal analysis of partially purified protein has identified the translational start of the protein. Primer extension studies have enabled the 5' end of the mRNA to be defined. It exists as a separate transcript from the rest of the rfb region, and the distinctive G + C content of rfbT suggests that it has been acquired from a non-Vibrio source.
Full text
PDF



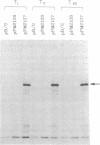
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- BHASKARAN K., GORRILL R. H. A study of antigenic variation in Vibrio cholerae. J Gen Microbiol. 1957 Jun;16(3):721–729. doi: 10.1099/00221287-16-3-721. [DOI] [PubMed] [Google Scholar]
- Gangarosa E. J., Sanati A., Saghari H., Feeley J. C. Multiple serotypes of vibrio cholerae isolated from a case of cholera. Evidence suggesting in-vivo mutation. Lancet. 1967 Mar 25;1(7491):646–648. doi: 10.1016/s0140-6736(67)92542-1. [DOI] [PubMed] [Google Scholar]
- Imbesi F., Manning P. A. Biotype-specific restriction and modification of DNA in Vibrio cholerae. J Clin Microbiol. 1982 Sep;16(3):552–554. doi: 10.1128/jcm.16.3.552-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Unger P., Gustafsson B., Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr Res. 1982 Mar 1;100:341–349. doi: 10.1016/s0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond J. W., Korsch M. J., Jackson G. D. Immunochemical studies to the O-antigens of vibrio cholerae. Partial characterization of an acid-labile antigenic determinant. Aust J Exp Biol Med Sci. 1973 Apr;51(2):229–235. doi: 10.1038/icb.1973.20. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1979 May 1;584(2):346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K. Somatic antigen variation in Vibrio cholerae. Jpn J Med Sci Biol. 1971 Apr;24(2):93–100. doi: 10.7883/yoken1952.24.93. [DOI] [PubMed] [Google Scholar]
- Sharma D. P., Stroeher U. H., Thomas C. J., Manning P. A., Attridge S. R. The toxin-coregulated pilus (TCP) of Vibrio cholerae: molecular cloning of genes involved in pilus biosynthesis and evaluation of TCP as a protective antigen in the infant mouse model. Microb Pathog. 1989 Dec;7(6):437–448. doi: 10.1016/0882-4010(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Sheehy T. W., Sprinz H., Augerson W. S., Formal S. B. Laboratory Vibrio cholerae infection in the United States. JAMA. 1966 Aug 1;197(5):321–326. [PubMed] [Google Scholar]
- Shimada T., Sakazaki R. Additional serovars and inter-O antigenic relationships of Vibrio cholerae. Jpn J Med Sci Biol. 1977 Oct;30(5):275–277. doi: 10.7883/yoken1952.30.275. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. M., Morelli G., Kamke M., Morona R., Yeadon J., Hackett J. A., Manning P. A. A physical map of the chromosomal region determining O-antigen biosynthesis in Vibrio cholerae O1. Gene. 1987;55(2-3):197–204. doi: 10.1016/0378-1119(87)90280-0. [DOI] [PubMed] [Google Scholar]