Abstract
Secondary metabolites have important defense and signaling roles, and they contribute to the overall quality of developing and ripening fruits. Blueberries, bilberries, cranberries, and other Vaccinium berries are fleshy berry fruits recognized for the high levels of bioactive compounds, especially anthocyanin pigments. Besides anthocyanins and other products of the phenylpropanoid and flavonoid pathways, these berries also contain other metabolites of interest, such as carotenoid derivatives, vitamins and flavor compounds. Recently, new information has been achieved on the mechanisms related with developmental, environmental, and genetic factors involved in the regulation of secondary metabolism in Vaccinium fruits. Especially light conditions and temperature are demonstrated to have a prominent role on the composition of phenolic compounds. The present review focuses on the studies on mechanisms associated with the regulation of key secondary metabolites, mainly phenolic compounds, in Vaccinium berries. The advances in the research concerning biosynthesis of phenolic compounds in Vaccinium species, including specific studies with mutant genotypes in addition to controlled and field experiments on the genotype × environment (G×E) interaction, are discussed. The recently published Vaccinium transcriptome and genome databases provide new tools for the studies on the metabolic routes.
Keywords: anthocyanins, bilberry, blueberry, carotenoids, flavonoids, fruits, light, temperature
Introduction
Genus Vaccinium includes over 450 deciduous or evergreen species distributed in cool temperate regions and mountains of the northern and southern hemispheres. The genus contains economically important cultivated and wild berry species, such as blueberries (e.g., Vaccinium corymbosum, V. angustifolium), bilberry (V. myrtillus), cranberries (V. macrocarpon, V. oxycoccos), and lingonberry (V. vitis-idaea; Figure 1A). Numerous studies have given evidence on the beneficial health effects of these berries, for instance in reducing risk of metabolic syndrome and various microbial and degenerative diseases (Kolehmainen et al., 2012; Blumberg et al., 2013; Norberto et al., 2013; Patel, 2014). These health-benefits are mostly attributed to the various phenolic compounds. Vaccinium berries are rich with flavonoids, including anthocyanins, flavonols, and proanthocyanidins (Määttä-Riihinen et al., 2004; Rodrigues-Mateos et al., 2012; Ancillotti et al., 2016), which are linked to many biological activities such as anti-inflammatory, antimutagenic, antimicrobial, anticancer, antiobesity, and antioxidant properties (Szajdek and Borowska, 2008; He and Giusti, 2010; Nile and Park, 2014). However, these berries also contain other valuable compounds, such as carotenoids and their derivatives, other flavor compounds and vitamins. This review covers the current knowledge on the developmental and environmental regulation of the biosynthesis of key metabolites in Vaccinium berries. Most studies in this topic have been performed on flavonoids but other compounds, such as other phenylpropanoids, carotenoid derivatives, and vitamin C are also covered.
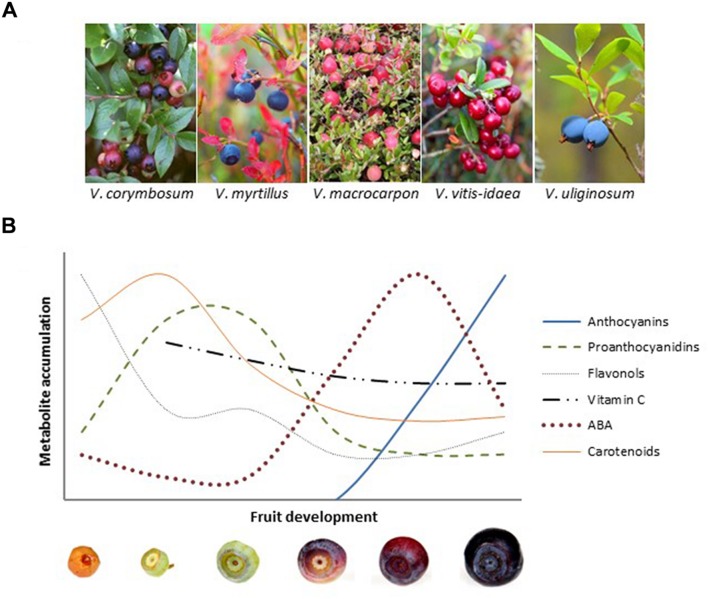
FIGURE 1.
(A) Vaccinium berries: highbush blueberry (V. corymbosum), bilberry (V. myrtillus), cranberry (V. macrocarpon), lingonberry (V. vitis-idaea), and bog bilberry (V. uliginosum). (B) Schematic representation of the accumulation of key metabolites during bilberry fruit development and ripening. The highest mean values of different compounds are 3960 μg g-1 FW for anthocyanins, 216 μg g-1 FW for proanthocyanidins, 130 μg g-1 FW for flavonols, 82.5 μg g-1 FW for vitamin C, 6.2 μg g-1 FW for ABA and 81.8 μg g-1 DW (14.4 μg g-1 FW) for carotenoids, according to Jaakola et al. (2002), Cocetta et al. (2012), and Karppinen et al. (2013, 2016).
Developmental Regulation
Development and ripening of fleshy fruits include major changes in fruit structure and in overall metabolism. At the metabolic level, development of Vaccinium berries is characterized by the production of high amounts of flavonoids, especially red/blue-pigmented anthocyanins coloring the ripe fruits (Figure 1A). At the early stages of berry development, proanthocyanidins, flavonols, and hydroxinnamic acids are the major phenolic compounds in these berries, and the accumulation of anthocyanins begins at the onset of ripening (Jaakola et al., 2002; Vvedenskaya and Vorsa, 2004; Castrejón et al., 2008; Zifkin et al., 2012; Gibson et al., 2013; Figure 1B). However, the flavonoid profiles vary between Vaccinium berries most of which accumulate anthocyanins only in the skin at ripening. Bilberry, which is recognized as one of the richest source of anthocyanins, accumulates these compounds also in flesh of ripe fruits with 15 different major anthocyanin glycosides identified (Jaakola et al., 2002; Zoratti et al., 2014b). The profile of anthocyanins in ripe bilberries and blueberries comprises glycosides of cyanidin, delphinidin, peonidin, petunidin, and malvidin anthocyanidins (Lohachoompol et al., 2008; Zoratti et al., 2014b). In red-colored Vaccinium berries, the profile of anthocyanins is less diverse, cyanidin glycosides being the major anthocyanins in ripe lingonberries, in addition to peonidins in ripe cranberries (Lee and Finn, 2012; Grace et al., 2014; Česonienė et al., 2015). However, proanthocyanidin content in ripe berries is typically higher in red-colored Vaccinium berries compared with blueberries. The proanthocyanidin profile of ripe Vaccinium berries includes procyanidins with rare A-type linkages (Määttä-Riihinen et al., 2005; Lätti et al., 2011; Grace et al., 2014). In addition to the role of anthocyanins in seed dispersal, the variation in flavonoid profile during berry development is considered to be related in defense responses. For instance, the astringent proanthocyanidins are suggested to provide protection against predation in unripe berries (Harborne, 1997).
Fleshy fruits are traditionally defined as either climacteric or non-climacteric according to the differences in respiration rate and production of ethylene at ripening (Gapper et al., 2013; McAtee et al., 2013; Osorio et al., 2013). In recent years, regulatory role of abscisic acid (ABA) has been established at molecular level in ripening initiation as well as in control of ripening-related anthocyanin biosynthesis of non-climacteric fruits (Jia et al., 2011; Li et al., 2011; Shen et al., 2014; Kadomura-Ishikawa et al., 2015), which includes Vaccinium berries. The increase in ABA levels at fruit ripening has been demonstrated in several non-climacteric fruits (Wheeler et al., 2009; Jia et al., 2011; Luo et al., 2014), also in bilberry (Karppinen et al., 2013; Figure 1B) and highbush blueberry (Zifkin et al., 2012), suggesting a role for ABA in ripening regulation in Vaccinium berries.
The flavonoid biosynthetic routes in plants are well understood and they are known to be regulated mainly through transcriptional control of structural genes (Hichri et al., 2011). The flavonoid pathway has been intensively studied also in Vaccinium berries, especially in bilberries and blueberries. The main structural genes have been isolated from bilberry (Jaakola et al., 2002), highbush blueberry (Zifkin et al., 2012), cranberry (Polashock et al., 2002; Sun et al., 2015), and bog bilberry (V. uliginosum; Primetta et al., 2015). The studies have indicated the increase in transcription levels of especially chalcone synthase (CHS), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), and UDP-glucose flavonoid 3-O-glucosyltransferase (UFGT) at the ripening stage leading to anthocyanin accumulation.
The key regulators of the flavonoid pathway have been characterized as R2R3 MYB transcription factors, MYC-like basic helix-loop-helix (bHLH) and WD40-repeat proteins, which comprise so called MBW-complex (Ferreyra et al., 2012; Xu et al., 2015). In Vaccinium species, potential R2R3 MYB genes involved in flavonoid biosynthesis have been identified in bilberry (Jaakola et al., 2010), highbush blueberry (Li X. et al., 2012; Zifkin et al., 2012; Gupta et al., 2015), and bog bilberry (Primetta et al., 2015). However, the upstream signaling network behind flavonoid biosynthesis is still unclear. At least part of the regulatory network controlling fleshy fruit ripening seems to be conserved during the evolution throughout climacteric and non-climacteric fruits (Seymour et al., 2013). In bilberry, a link between anthocyanin biosynthesis and one of the key regulators of fruit development, a SQUAMOSA-class MADS-box transcription factor, has been demonstrated (Jaakola et al., 2010). However, there are indications that the regulation of anthocyanin biosynthesis might differ in genus Vaccinium compared with other species studied so far. In a recent study, white berry mutants of bog bilberry and bilberry deficient in anthocyanins were demonstrated to have a down-regulated MYBPA1-type transcription factor (Primetta et al., 2015), which has been indicated as the key regulator of proanthocyanidin biosynthesis in other fruit species. During recent years, several transcriptome and genome databases of Vaccinium berries have been published (Li X. et al., 2012; Rowland et al., 2012; Zifkin et al., 2012; Polashock et al., 2014; Gupta et al., 2015; Sun et al., 2015). From these databases, different families of transcription factors with potential roles in flavonoid biosynthesis have been identified. The databases will serve as an important tool in revealing signaling network involved in regulation of flavonoid biosynthesis and other metabolites in Vaccinium species.
Due to the high accumulation of anthocyanins in skin at ripening, carotenoids do not serve as the main pigments attracting seed dispersers in Vaccinium berries. However, among fruits Vaccinium berries can be considered as good sources of carotenoids, especially lutein and β-carotene (Marinova and Ribarova, 2007; Bunea et al., 2012; Lashmanova et al., 2012; Karppinen et al., 2016). Our recent study on carotenoid biosynthesis has shown that carotenoid content in bilberry fruit is modified during berry development with decreasing trend from small green berry toward ripening berries (Karppinen et al., 2016; Figure 1B). This trend is likely to reflect the variable roles of carotenoids during berry development and ripening. In unripe fruits, carotenoids are primarily involved in photosynthesis, whereas during ripening the carotenoid metabolism can turn toward enzymatic degradation to produce apocarotenoids, such as ABA and flavor compounds (McQuinn et al., 2015). Based on study in bilberry, transcriptional regulation of the both key biosynthetic and cleavage genes plays a role in the determination of carotenoid content during berry development and ripening (Karppinen et al., 2016). This indicates coordinately regulated interplay with ABA and carotenoid biosynthetic routes and, furthermore, anthocyanin biosynthesis at bilberry ripening.
Many berries accumulate carotenoid derived volatile flavor compounds at ripening (Beekwilder et al., 2008; García-Limones et al., 2008). However, reports concerning the regulation of formation of these compounds during development and ripening of Vaccinium berries are still scant (Rohloff et al., 2009; Gilbert et al., 2013). The aroma of ripe fruits is a complex combination of various flavor compounds, sugars and acids, and variations in these can be high even between the cultivars of the same species (El Hadi et al., 2013). Cultivar-specific differences in volatile profiles have been reported among Vaccinium species and highbush blueberry cultivars (Hirvi and Honkanen, 1983; Baloga et al., 1995; Horvat et al., 1996; Forney et al., 2012). The most critical volatiles for the blueberry aroma are considered to be linalool, trans-2-hexenol, trans-2-hexenal, hexanal, and 1-penten-3-ol, which show increasing trend in highbush blueberries toward fruit maturity (Du et al., 2011; Gilbert et al., 2013).
Fruits and berries are recognized as dietary sources of vitamins. Among berries, Vaccinium species have shown to be low or moderate sources of vitamin C with the levels of 0.1–27 mg 100 g-1 FW (Bushway et al., 1983; Klein, 2005; Walker et al., 2006; Brown et al., 2012). In bilberry, the levels of vitamin C have shown to be relatively stable during the berry development and ripening (Cocetta et al., 2012; Figure 1B), whereas more decrease during berry development was detected in highbush blueberry cultivars (Liu et al., 2015). Moreover, low to moderate levels of other vitamins are reported in Vaccinium fruits (Mazza, 2005; Chun et al., 2006). So far, studies on the upstream regulation of vitamin C biosynthesis during berry development in Vaccinium spp. species are lacking.
Environmental Regulation
Environmental factors have a substantial role in the regulation of secondary metabolism in fruits. In general, genetic background determines the secondary metabolite profile of species, whereas environmental factors can cause prominent qualitative and quantitative changes to the metabolite composition. In addition to temperature and light conditions, nutritional status, water balance, diseases and other stresses have been shown to affect the production of secondary metabolites in fruits and berries (Ferrandino and Lovisolo, 2014; Zoratti et al., 2014a; Koshita, 2015). The environmental effects on berry secondary metabolism have been studied widely also in genus Vaccinium (Table 1). Many studies have focused on the influence of growth conditions on the content of anthocyanins and other phenolic compounds in berries of both wild and cultivated species.
Table 1.
Main responses of secondary metabolites to environmental effects in Vaccinium berries.
| Species | Metabolite | Experimental condition | Response | Reference |
|---|---|---|---|---|
| Vaccinium corymbosum (highbush blueberry) | Phenolic compounds | Year/season | Affects significantly the accumulation of total phenolic content and anthocyanins in different cultivars. | Connor et al., 2002b |
| Location | Affects significantly the accumulation of total phenolic content and anthocyanins in different cultivars. | Prior et al., 1998; Connor et al., 2002b; Spinardi et al., 2009; Jovančević et al., 2011; Može et al., 2011; Zoratti et al., 2015a | ||
| Light | Anthocyanin accumulation is dependent from high solar radiation. | Zoratti et al., 2015b | ||
| Temperature | The accumulation of anthocyanins is favored at 25°C compared to 30°C. Temperatures lower than 25°C retard ripening and anthocyanin accumulation. | Zoratti et al., 2015a,b | ||
| Post-harvest UV light | UV-B and UV-C increase accumulation of anthocyanins, flavonols, and phenolic acids. | Perkins-Veazie et al., 2008; Wang et al., 2009; Eichholz et al., 2011; Nguyen et al., 2014 | ||
| Volatile compounds | Year/season | 1-Hexenol, E2-hexanal, and hexanoic acid are the most variable compounds in six cultivars. | Gilbert et al., 2015 | |
| Location | Significant effect on volatile accumulation depending on the cultivar. | Du et al., 2011; Gilbert et al., 2015 | ||
| Post-harvest UV light | In cv. Bluecrop, UV-B increases the accumulation of terpenes, ketones, and aldehydes after 2 h of high irradiance whereas alcoholic compounds increased after 24 h. | Eichholz et al., 2011 | ||
| Post-harvest visible light | In cv. Scintilla, hexanal and trans-2-hexenal are increased after 8 h treatment under red and far-red light compared to white light. | Colquhoun et al., 2013 | ||
| V. myrtillus (bilberry) | Phenolic compounds | Year/season | Affects significantly anthocyanins in bilberry individuals grown in the same location. | Åkerström et al., 2010; Zoratti et al., 2015a |
| Location | The accumulation of anthocyanins increases progressively with increasing latitude and altitude. | Lätti et al., 2008; Rieger et al., 2008; Åkerström et al., 2010; Zoratti et al., 2015a,b | ||
| Light | High light increases content of anthocyanins, flavonols, hydroxycinnamic acids, and total phenolics. Blue, red, and far-red light increase the accumulation of anthocyanins and flavonols under controlled temperature conditions. | Jovančević et al., 2011; Zoratti et al., 2014b; Mikulic-Petkovsek et al., 2015 | ||
| Photoperiod | Photoperiod of 24 h increases the accumulation of phenolic compounds compared to 12 h day/night. | Uleberg et al., 2012 | ||
| Temperature | Higher levels of flavonols and hydroxycinnamic acids in 12°C vs. 18°C. Lower temperatures (10–15°C) favor the accumulation of delphinidins. | Uleberg et al., 2012; Zoratti et al., 2015a,b | ||
| V. macrocarpon (cranberry) | Phenolic compounds | Light | Visible light increases accumulation of anthocyanins. The highest increase was observed under red light wavelengths. | Zhou and Singh, 2002 |
| Post-harvest visible light | Increases accumulation of anthocyanins. | Zhou and Singh, 2004 |
Light conditions have a significant role in the flavonoid metabolism in fruits (Zoratti et al., 2014a), including Vaccinium berries, in which especially content and composition of anthocyanins is affected. However, the effect of light on the accumulation of flavonoids in Vaccinium berries seems to be regulated in a species-specific manner. Many of the wild Vaccinium berries, such as bilberry and lingonberry, grow in shaded habitats and do not require high light for induction of anthocyanin biosynthesis. In these berries, light conditions appear to have merely fine-tuning effects on flavonoid biosynthesis. Recently, it was reported that bilberries grown in sites with higher photosynthetic active radiation contained higher levels of anthocyanins, flavonols, hydroxycinnamic acids, and total phenolics (Mikulic-Petkovsek et al., 2015). The positive effect of light on total phenolics and anthocyanin was also apparent in bilberries grown under sunlight versus shadowed habitats in Montenegro (Jovančević et al., 2011). Although blueberries are also shade-adapted species they seem to require higher solar exposure for normal ripening and anthocyanin accumulation (Zoratti et al., 2015b). In a postharvest study, light had also positive effect on the accumulation of anthocyanins in cranberries (Zhou and Singh, 2004).
In addition to intensity, light effect can be transmitted through perception of other attributes, such as light quality and day length (Zoratti et al., 2014a). Longer days seem to be associated with more intense flavonoid production than shorter days (Jaakola and Hohtola, 2010; Mazur et al., 2014). In bilberry, the effect of photoperiod appears to be one reason for more rapid accumulation and higher concentrations of anthocyanins at northern latitudes compared to southern growth conditions (Uleberg et al., 2012; Table 1).
Higher plants utilize multiple photoreceptors to detect different wavelengths of light from ultraviolet (UV)-B to far-red (Möglich et al., 2010; Casal, 2013). In a recent study, a short exposure to specific portions of light spectrum during the early development of bilberry fruit affected the final flavonoid profile in ripe berry (Zoratti et al., 2014b). Especially blue wavelengths increased the accumulation of more hydroxylated anthocyanins; delphinidins, petunidins and malvidins, but not cyanidins and peonidins. Earlier, short treatments with red wavelengths increased anthocyanin accumulation in cranberries compared to white light- or dark-treated berries (Zhou and Singh, 2002). Postharvest studies with UV-B and UV-C light induced anthocyanin accumulation in blueberries (Perkins-Veazie et al., 2008; Wang et al., 2009; Nguyen et al., 2014). However, the signaling pathway from different photoreceptors to flavonoid accumulation and induction of R2R3 MYB transcription factors is not well understood. It is generally accepted that CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) acts as a major center of light signaling directly interacting with photoreceptors (Jang et al., 2010; Galvão and Fankhauser, 2015). The MdCOP1 was shown to interact with MdMYB1, a positive regulator of anthocyanin biosynthesis, in apple (Li Y.Y. et al., 2012). A recent study in non-climacteric strawberry fruit revealed that light regulates anthocyanin biosynthesis and related R2R3 MYB transcription factors independently from ABA (Kadomura-Ishikawa et al., 2015). In accordance, additive effect on anthocyanin accumulation was observed under combined light and ABA treatments.
Temperature also affects the composition of secondary metabolites in fruits. In general, cooler temperatures favor biosynthesis of phenolic compounds and vitamin C (Lee and Kader, 2000; Koshita, 2015), whereas both lower and higher temperatures have been shown to decrease the carotenoid biosynthesis in tomatoes and other carotenoid accumulating fruits (Gross, 1991). In Vaccinium berries, the temperature effect has been most intensively studied in regards to formation of phenolic compounds. Many studies have concerned the optimal postharvest storage temperature for the stability of phenolic compounds in blueberries and cranberries (Wang and Stretch, 2001; Connor et al., 2002a; Schotsmans et al., 2007). Moreover, Uleberg et al. (2012) showed in a controlled experiment that bilberries produced higher levels of flavonols and hydroxycinnamic acids in 12°C than in 18°C, whereas contents of all anthocyanins, except delphinidin glycosides, were higher in 18°C. Zoratti et al. (2015b) compared the effect of light-temperature combinations contemporary on bilberry and highbush blueberry (cv. Brigitta Blue). For both species, lower temperatures favored the accumulation of anthocyanins in berries. In bilberry, decrease in temperature from 25 to 10°C increased the more hydroxylated forms of anthocyanins in ripening fruits. Similarly, a higher accumulation of anthocyanins was detected in blueberries ripened at 25°C compared to 30°C. However, temperatures below 25°C delayed the ripening of blueberries leading to a slight decrease in all anthocyanins (Zoratti et al., 2015a,b).
Genotype × environment (G×E) interaction related with the formation of secondary metabolites has been studied in many Vaccinium species. Connor et al. (2002b) reported significant variation in anthocyanin content among highbush blueberry cultivars across different locations in US, as well as within years in each location indicating a considerable G×E interaction in regulation of anthocyanin content. The G×E interaction was observed also in bilberries affecting especially to accumulation of anthocyanins in relation to differences in latitude and altitude, in which the variation of climatic factors such as temperature, day length, and spectral composition of sunlight are closely correlated (Zoratti et al., 2015a,b). Especially latitude has been shown to influence the accumulation of anthocyanins in Vaccinium berries, as a clear increasing trend in anthocyanin content toward north has been reported for North European populations of both bilberry and bog bilberry (Lätti et al., 2008, 2010; Åkerström et al., 2010). Bilberries of the northernmost clones contained not only higher yields of anthocyanins but also a higher proportion of delphinidins whereas more cyanidins accumulated in the berries grown in southern latitudes.
In Vaccinium berries, only few studies on the production of secondary metabolites have specifically focused on the effect of increasing altitudes, which are characterized by progressive decrease in temperature and increase in the intensity of visible light. In Northern Italy, higher levels of anthocyanins and ascorbic acid were found in blueberries grown at 600 m a.s.l. compared with 450 m a.s.l. (Spinardi et al., 2009). The same trend in anthocyanin accumulation in bilberries and blueberries was detected along an altitudinal gradient in the Alps of Italy (Zoratti et al., 2015a) as well as in accumulation of anthocyanins and total phenolics in bilberries grown in different altitudes in Montenegro (Jovančević et al., 2011). In the study of Zoratti et al. (2015a), six natural bilberry populations between 1166 and 1829 m a.s.l. showed a clear positive trend in anthocyanin accumulation with increasing elevation, in a 2-year study. In the same study, highbush blueberries showed variation in the anthocyanin accumulation in relation to growth location at different altitude levels, although it resulted to be mostly dependent on the season and particularly temperature. Seasonal differences might explain the results of a 2-year study in Austria (Rieger et al., 2008), where decreasing bilberry anthocyanin contents were found along with increasing altitude (from 800 to 1500 m a.s.l.).
Moreover, environmental factors affect other metabolites in Vaccinium berries. In blueberry, G×E interaction was detected in the accumulation of volatile compounds of blueberry aroma profile. Eichholz et al. (2011) and Colquhoun et al. (2013) reported that the accumulation of volatile compounds is affected by light quality, especially UV and red/far-red wavelengths (Table 1). The variation of triterpenoid compounds has been studied in bilberry and lingonberry (Szakiel et al., 2012a,b). In lingonberry, dependence of the metabolite levels on geographical origin was detected and considered to be related to length of the growing season and thickness of snow cover.
Future Prospects
Vaccinium berries are among economically the most important fleshy berry fruits worldwide, and the interest in utilization of both cultivated and wild berries of the genus has been showing an increasing trend. The studies reviewed here show that environmental factors can modify the content and composition of secondary metabolites in Vaccinium berries, which is important to consider when using these berries in industrial applications. The recent and upcoming data from transcriptome and genome databases along with more accurate tools for metabolite and metabolomics analyses are opening a new era in studies concerning regulation of secondary metabolism in Vaccinium species. New methods allow more in depth studies at species and cultivar level and they will increase our understanding on the role of complicated G×E interactions in the regulation of formation of the health-beneficial secondary compounds.
Author Contributions
All authors (KK, LZ, NN, HH, and LJ) have participated in preparation of the manuscript and have accepted the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the Finnish Cultural Foundation, Niemi Foundation and Osk. Huttunen Foundation to KK, and Centre for International Mobility (CIMO, Finland) to NN. For the photographs in Figure 1A, we thank Ilkka Jaakola (V. corymbosum, V. myrtillus, V. vitis-idaea, and V. uliginosum) and Dr. Marge Starast (V. macrocarpon).
References
- Åkerström A., Jaakola L., Bång U., Jäderlund A. (2010). Effects of latitude-related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (bilberries). J. Agric. Food Chem. 58 11939–11945. 10.1021/jf102407n [DOI] [PubMed] [Google Scholar]
- Ancillotti C., Ciofi L., Pucci D., Sagona E., Giordani E., Biricolti S., et al. (2016). Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 204 176–184. 10.1016/j.foodchem.2016.02.106 [DOI] [PubMed] [Google Scholar]
- Baloga D. W., Vorsa N., Lawter L. (1995). “Dynamic headspace gas chromatography-mass spectrometry analysis of volatile flavor compounds from wild diploid blueberry species,” in Fruit Flavors: Biogenesis, Characterization and Authentication. ACS Symposium Series 596, eds Rousseff R. L., Leahy M. M. (Oxford: Oxford University Press; ), 235–247. [Google Scholar]
- Beekwilder J., van der Meer I. M., Simic A., Uitdewilligen J., van Arkel J., de Vos R. C. H., et al. (2008). Metabolism of carotenoids and apocarotenoids during ripening of raspberry fruit. Biofactors 34 57–66. [PubMed] [Google Scholar]
- Blumberg J. B., Camesano T. A., Cassidy A., Kris-Etherton P., Howell A., Manach C., et al. (2013). Cranberries and their bioactive constituents in human health. Adv. Nutr. 4 618–632. 10.3945/an.113.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. N., Turi C. E., Shipley P. R., Murch S. J. (2012). Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med. 78 630–640. 10.1055/s-0031-1298239 [DOI] [PubMed] [Google Scholar]
- Bunea A., Ruginǎ D., Pintea A., Andrei S., Bunea C., Pop R., et al. (2012). Carotenoid and fatty acid profiles of bilberries and cultivated blueberries from Romania. Chem. Pap. 66 935–939. 10.2478/s11696-012-0162-2 [DOI] [Google Scholar]
- Bushway R. J., Mc Gann D. F., Cook W. P., Bushway A. A. (1983). Mineral and vitamin content of lowbush blueberries (Vaccinium angustifolium Ait.). J. Food Sci. 48:1878 10.1111/j.1365-2621.1983.tb05109.x [DOI] [Google Scholar]
- Casal J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Ann. Rev. Plant Biol. 64 403–427. 10.1146/annurev-arplant-050312-120221 [DOI] [PubMed] [Google Scholar]
- Castrejón A. D. R., Eichholz I., Rohn S., Kroh L. W., Huyskens-Keil S. (2008). Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 109 564–572. 10.1016/j.foodchem.2008.01.007 [DOI] [Google Scholar]
- Česonienė L., Daubaras R., Jasutienė I., Miliauskienė I., Zych M. (2015). Investigations of anthocyanins, organic acids, and sugars show great variability in nutritional and medicinal value of European cranberry (Vaccinium oxococcus) fruit. J. Appl. Bot. Food Qual. 88 295–299. 10.5073/JABFQ.2015.088.042 [DOI] [Google Scholar]
- Chun J., Lee J., Ye L., Exler J., Eitenmiller R. R. (2006). Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 19 196–204. 10.1016/j.jfca.2005.08.001 [DOI] [Google Scholar]
- Cocetta G., Karppinen K., Suokas M., Hohtola A., Häggman H., Spinardi A., et al. (2012). Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. J. Plant Physiol. 169 1059–1065. 10.1016/j.jplph.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Colquhoun T. A., Schwieterman M. L., Gilbert J. L., Jaworski E. A., Langer K. M., Jones C. R., et al. (2013). Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Technol. 86 37–44. 10.1016/j.postharvbio.2013.06.013 [DOI] [Google Scholar]
- Connor A. M., Luby J. J., Hancock J. F., Berkheimer S., Hanson E. J. (2002a). Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 50 893–898. 10.1021/jf011212y [DOI] [PubMed] [Google Scholar]
- Connor A. M., Luby J. J., Tong C. B. S., Finn C. E., Hancock J. F. (2002b). Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hort. Sci. 127 89–97. [Google Scholar]
- Du X., Plotto A., Song M., Olmstead J., Rouseff R. (2011). Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. J. Agric. Food Chem. 59 8347–8357. 10.1021/jf201184m [DOI] [PubMed] [Google Scholar]
- Eichholz I., Huyskens-Keil S., Keller A., Ulrich D., Kroh L. W., Rohn S. (2011). UV-B-induced changes of volatile metabolites and phenolic compounds in blueberries (Vaccinium corymbosum L.). Food Chem. 126 60–64. 10.1016/j.foodchem.2010.10.071 [DOI] [Google Scholar]
- El Hadi M. A. M., Zhang F. J., Wu F. F., Zhou C. H., Tao J. (2013). Advances in fruit aroma volatile research. Molecules 18 8200–8229. 10.3390/molecules18078200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandino A., Lovisolo C. (2014). Abiotic stress effects on grapevine (Vitis vinifera L.): focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 103 138–147. 10.1016/j.envexpbot.2013.10.012 [DOI] [Google Scholar]
- Ferreyra M. L. F., Rius S. P., Casati P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3:222 10.3389/fpls.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney C. F., Kalt W., Vander Kloet S. P. (2012). Comparison of berry composition of selected Vaccinium species (Ericaceae) with Gaylussacia dumosa. Botany 90 355–363. 10.1139/B11-098 [DOI] [Google Scholar]
- Galvão V. C., Fankhauser C. (2015). Sensing the light environment in plants: photoreceptors and early signaling steps. Curr. Opinion Neurobiol. 34 46–53. 10.1016/j.conb.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Gapper N. E., McQuinn R. P., Giovannoni J. J. (2013). Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 82 575–591. 10.1007/s11103-013-0050-3 [DOI] [PubMed] [Google Scholar]
- García-Limones C., Schnäbele K., Blanco-Portales R., Bellido M. L., Caballero J. L., Schwab W., et al. (2008). Functional characterization of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. J. Agric. Food Chem. 56 9277–9285. 10.1021/jf801096t [DOI] [PubMed] [Google Scholar]
- Gibson L., Rupasinghe H. P. V., Forney C. F., Eaton L. (2013). Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2 216–229. 10.3390/antiox2040216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. L., Guthart M. J., Gezan S. A., de Carvalho M. P., Schwieterman M. L., Colquhoun T. A., et al. (2015). Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLoS ONE 10:e0138494 10.1371/journal.pone.0138494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. L., Schwieterman M. L., Colquhoun T. A., Clark D. G., Olmstead J. W. (2013). Potential for increasing southern highbush blueberry flavor acceptance by breeding for major volatile components. Hortscience 48 835–843. [Google Scholar]
- Grace M. H., Esposito D., Dunlap K. L., Lila M. A. (2014). Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 62 4007–4017. 10.1021/jf403810y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. (1991). Pigments in Vegetables. Chlorophylls and Carotenoids. New York, NY: Springer Science + Business Media. [Google Scholar]
- Gupta V., Estrada A. D., Blakley I., Reid R., Patel K., Meyer M. D., et al. (2015). RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. Gigascience 4:5 10.1186/s13742-015-0046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J. B. (1997). “Phytochemistry of fruits and vegetables: an ecological overview,” in Phytochemisty of Fruits and Vegetables, eds Tomás-Barberán F. A., Robins R. J. (New York, NY: Oxford University Press; ), 335–367. [Google Scholar]
- He J., Giusti M. M. (2010). Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 1 163–187. 10.1146/annurev.food.080708.100754 [DOI] [PubMed] [Google Scholar]
- Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V. (2011). Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 62 2465–2483. 10.1093/jxb/erq442 [DOI] [PubMed] [Google Scholar]
- Hirvi T., Honkanen E. (1983). The aroma of blueberries. J. Sci. Food Agric. 34 992–996. 10.1002/jsfa.2740340916 [DOI] [Google Scholar]
- Horvat R. J., Schlotzhauer W. S., Chortyk O. T., Nottingham S. F., Payne J. A. (1996). Comparison of volatile compounds from rabbiteye blueberry (Vaccinium ashei) and deerberry (V. stamineum) during maturation. J. Essent. Oil Res. 8 645–648. 10.1080/10412905.1996.9701033 [DOI] [Google Scholar]
- Jaakola L., Hohtola A. (2010). Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 33 1239–1247. 10.1111/j.1365-3040.2010.02154.x [DOI] [PubMed] [Google Scholar]
- Jaakola L., Määttä K., Pirttilä A. M., Törrönen R., Kärenlampi S., Hohtola A. (2002). Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 130 729–739. 10.1104/pp.006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola L., Poole M., Jones M. O., Kämäräinen-Karppinen T., Koskimäki J. J., Hohtola A., et al. (2010). A SQUAMOSA MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol. 153 1619–1629. 10.1104/pp.110.158279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I. C., Henriques R., Seo H. S., Nagatani A., Chua N. H. (2010). Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22 2370–2383. 10.1105/tpc.109.072520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. F., Chai Y. M., Li C. L., Lu D., Luo J. J., Qin L., et al. (2011). Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 157 188–199. 10.1104/pp.111.177311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovančević M., Balijagić J., Menković N., Šavikin K., Zdunić G., Janković T., et al. (2011). Analysis of phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plants Res. 5 910–914. [Google Scholar]
- Kadomura-Ishikawa Y., Miyawaki K., Takahashi A., Masuda T., Noji S. (2015). Light and abscisic acid independently regulated FaMYB10 in Fragaria × ananassa fruit. Planta 241 953–965. 10.1007/s00425-014-2228-6 [DOI] [PubMed] [Google Scholar]
- Karppinen K., Hirvelä E., Nevala T., Sipari N., Suokas M., Jaakola L. (2013). Changes in the abscisic acid levels and related gene expression during fruit development and ripening in bilberry (Vaccinium myrtillus L.). Phytochemistry 95 127–134. 10.1016/j.phytochem.2013.06.023 [DOI] [PubMed] [Google Scholar]
- Karppinen K., Zoratti L., Sarala M., Carvalho E., Hirsimäki J., Mentula H., et al. (2016). Carotenoid metabolism during bilberry (Vaccinium myrtillus L.) fruit development under different light conditions is regulated by biosynthesis and degradation. BMC Plant Biol. 16:95 10.1186/s12870-016-0785-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. A. (2005). “Cranberry (Vaccinium macrocarpon) Aiton,” in Encyclopedia of Dietary Supplements, eds Coates P. M., Blackman M. R., Cragg G. M., Levine M., Moss J., White J. D. (New York, NY: Marcel Dekker; ), 143–149. 10.1081/E-EDS2-130002026 [DOI] [Google Scholar]
- Kolehmainen M., Mykkänen O., Kirjavainen P. V., Leppänen T., Moilanen E., Adriaens M., et al. (2012). Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 56 1501–1510. 10.1002/mnfr.201200195 [DOI] [PubMed] [Google Scholar]
- Koshita Y. (2015). “Effect of temperature on fruit color development,” in Abiotic Stress Biology in Horticultural Plants, eds Kanayama Y., Kochetov A. (Berlin: Springer; ), 47–58. [Google Scholar]
- Lashmanova K. A., Kuzivanova O. A., Dymova O. V. (2012). Northern berries as a source of carotenoids. Acta Biochim. Pol. 59 133–134. [PubMed] [Google Scholar]
- Lätti A. K., Jaakola L., Riihinen K. R., Kainulainen P. S. (2010). Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland. J. Agric. Food Chem. 58 427–433. 10.1021/jf903033m [DOI] [PubMed] [Google Scholar]
- Lätti A. K., Riihinen K. R., Jaakola L. (2011). Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium × intermedium Ruthe). Phytochemistry 72 810–815. 10.1016/j.phytochem.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Lätti A. K., Riihinen K. R., Kainulainen P. S. (2008). Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 56 190–196. 10.1021/jf072857m [DOI] [PubMed] [Google Scholar]
- Lee J., Finn C. E. (2012). Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: anthocyanin and free amino acid composition. J. Funct. Foods 4 213–218. 10.1016/j.jff.2011.10.007 [DOI] [Google Scholar]
- Lee S. K., Kader A. A. (2000). Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 20 207–220. 10.1016/S0925-5214(00)00133-2 [DOI] [Google Scholar]
- Li C., Jia H., Chai Y., Shen Y. (2011). Abscisic acid perception and signaling transduction in strawberry. Plant Signal. Behav. 6 1950–1953. 10.4161/psb.6.12.18024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sun H., Pei J., Dong Y., Wang F., Chen H., et al. (2012). De novo sequencing and comparative analysis of the blueberry transcriptome to discover putative genes related to antioxidants. Gene 511 54–61. 10.1016/j.gene.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Mao K., Zhao C., Zhao X. Y., Zhang H. L., Shu H. R., et al. (2012). MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 160 1011–1022. 10.1104/pp.112.199703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Wang L., Gu L., Zhao W., Su H., Cheng X. (2015). Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 188 399–405. 10.1016/j.foodchem.2015.05.036 [DOI] [PubMed] [Google Scholar]
- Lohachoompol V., Mulholland M., Srzednicki G., Craske J. (2008). Determination of anthocyanins in various cultivars of highbush and rabbiteye blueberries. Food Chem. 111 249–254. 10.1016/j.foodchem.2008.03.067 [DOI] [Google Scholar]
- Luo H., Dai S., Ren J., Zhang C., Ding Y., Li Z., et al. (2014). The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant Growth Regul. 33 373–383. 10.1007/s00344-013-9388-7 [DOI] [Google Scholar]
- Määttä-Riihinen K. R., Kähkönen M. P., Törrönen A. R., Heinonen I. M. (2005). Catechins and procyanidins in berries of Vaccinium species and their antioxidant activity. J. Agric. Food Chem. 53 8485–8491. 10.1021/jf050408l [DOI] [PubMed] [Google Scholar]
- Määttä-Riihinen K. R., Kamal-Eldin A., Mattila P. H., Gonzáles-Paramás A. M., Törrönen A. R. (2004). Distribution and contents of phenolic compounds in eighteen Skandinavian berry species. J. Agric. Food Chem. 52 4477–4486. 10.1021/jf049595y [DOI] [PubMed] [Google Scholar]
- Marinova D., Ribarova F. (2007). HPLC determination of carotenoids in Bulgarian berries. J. Food Composit. Anal. 20 370–374. 10.1016/j.jfca.2006.09.007 [DOI] [Google Scholar]
- Mazur S. P., Sønsteby A., Wold A. B., Foito A., Freitag S., Verrall S., et al. (2014). Post-flowering photoperiod has marked effects on fruit chemical composition in red raspberry (Rubus idaeus). Ann. Appl. Biol. 165 454–465. 10.1111/aab.12153 [DOI] [Google Scholar]
- Mazza G. (2005). Compositional and functional properties of saskatoon berry and blueberry. Int. J. Fruit Sci. 5 101–120. 10.1300/J492v05n03_10 [DOI] [Google Scholar]
- McAtee P., Karim S., Schaffer R., David K. (2013). A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 4:79 10.3389/fpls.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuinn R. P., Giovannoni J. J., Pogson B. J. (2015). More than meets the eye: from carotenoid biosynthesis, to new insights into apocarotenoid signaling. Curr. Opin. Plant Biol. 27 172–179. 10.1016/j.pbi.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. (2015). A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 95 776–785. 10.1002/jsfa.6897 [DOI] [PubMed] [Google Scholar]
- Möglich A., Yang X., Ayers R. A., Moffat K. (2010). Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61 21–47. 10.1146/annurev-arplant-042809-112259 [DOI] [PubMed] [Google Scholar]
- Može S., Polak T., Gašperlin L., Koron D., Vanzo A., Poklar Ulrih N., et al. (2011). Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 59 6998–7004. 10.1021/jf200765n [DOI] [PubMed] [Google Scholar]
- Nguyen C. T. T., Kim J., Yoo K. S., Lim S., Lee E. J. (2014). Effect of prestorage UV-A, -B, and –C radiation on fruit quality and anthocyanin of ‘Duke’ blueberries during cold storage. J. Agric Food Chem. 62 12144–12151. 10.1021/jf504366x [DOI] [PubMed] [Google Scholar]
- Nile S. H., Park S. W. (2014). Edible berries: bioactive components and their effect on human health. Nutrition 30 134–144. 10.1016/j.nut.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Norberto S., Silva S., Meireles M., Faria A., Pintado M., Calhau C. (2013). Blueberry anthocyanins in health promotion: a metabolic overview. J. Funct. Foods 5 1518–1528. 10.1016/j.jff.2013.08.015 [DOI] [Google Scholar]
- Osorio S., Scossa F., Fernie A. R. (2013). Molecular regulation of fruit ripening. Front. Plant Sci. 4:198 10.3389/fpls.2013.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. (2014). Blueberry as functional food and dietary supplement: the natural way to ensure holistic health. Med. J. Nutri. Metab. 7 133–143. 10.3233/MNM-140013 [DOI] [Google Scholar]
- Perkins-Veazie P., Collins J. K., Howard L. (2008). Blueberry fruit response to postharvest application of ultravioted radiation. Postharvest Biol. Technol. 47 280–285. 10.1016/j.postharvbio.2007.08.002 [DOI] [Google Scholar]
- Polashock J., Zelzion E., Fajardo D., Zalapa J., Georgi L., Bhattacharya D., et al. (2014). The American cranberry: first insights into the whole genome of a species adapted to bog habitat. BMC Plant Biol. 14:165 10.1186/1471-2229-14-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polashock J. J., Griesbach R. J., Sullivan R. F., Vorsa N. (2002). Cloning of a cDNA encoding the cranberry dihydroflavonol-4-reductase (DFR) and expression in transgenic tobacco. Plant Sci. 163 241–251. 10.1016/S0168-9452(02)00087-0 [DOI] [Google Scholar]
- Primetta A. K., Karppinen K., Riihinen K. R., Jaakola L. (2015). Metabolic and molecular analyses of white mutant Vaccinium berries show down-regulation of MYBPA1-type R2R3 MYB regulatory factor. Planta 242 631–643. 10.1007/s00425-015-2363-8 [DOI] [PubMed] [Google Scholar]
- Prior R. L., Cao G., Martin A., Sofic E., McEwen J., O’Brien C., et al. (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 46 2686–2693. 10.1021/jf980145d [DOI] [Google Scholar]
- Rieger G., Müller M., Guttenberger H., Bucar F. (2008). Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 56 9080–9086. 10.1021/jf801104e [DOI] [PubMed] [Google Scholar]
- Rodrigues-Mateos A., Cifuentes-Gomez T., Tabatabaee S., Lecras C., Spencer J. P. E. (2012). Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 60 5772–5778. 10.1021/jf203812w [DOI] [PubMed] [Google Scholar]
- Rohloff J., Nestby R., Nes A., Martinussen I. (2009). Volatile profiles of European blueberry: few major players, but complex aroma patterns. Latvian J. Agron. 12 98–103. [Google Scholar]
- Rowland L. J., Alkharouf N., Darwish O., Ogden E. L., Polashock J. J., Bassil N. V., et al. (2012). Generation and analysis of blueberry transcriptome sequences from leaves, developing fruit, and flower buds from cold acclimation through deacclimation. BMC Plant Biol. 12:46 10.1186/1471-2229-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotsmans W., Molan A., MacKay B. (2007). Controlled atmosphere storage of rabbiteye blueberries enhances postharvest quality aspects. Postharvest Biol. Technol. 44 277–285. 10.1016/j.postharvbio.2006.12.009 [DOI] [Google Scholar]
- Seymour G. B., Østergaard L., Chapman N. H., Knapp S., Martin C. (2013). Fruit development and ripening. Annu. Rev. Plant Biol. 64 219–241. 10.1146/annurev-arplant-050312-120057 [DOI] [PubMed] [Google Scholar]
- Shen X., Zhao K., Liu L., Zhang K., Yuan H., Liao X., et al. (2014). A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 55 862–880. 10.1093/pcp/pcu013 [DOI] [PubMed] [Google Scholar]
- Spinardi A., Mignani I., Folini L., Beghi R. (2009). Quality and nutraceutical content of blueberries (Vaccinium corymbosum) grown at two different altitudes (450 and 650 m above sea level). Acta Hort. 810 817–822. 10.17660/ActaHortic.2009.810.108 [DOI] [Google Scholar]
- Sun H., Liu Y., Gai Y., Geng J., Chen L., Liu H., et al. (2015). De novo sequencing and analysis of the cranberry fruit transcriptome to identify putative genes involved in flavonoid biosynthesis, transport and regulation. BMC Genomics 16:652 10.1186/s12864-015-1842-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajdek A., Borowska E. J. (2008). Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods Hum. Nutr. 63 147–156. 10.1007/s11130-008-0097-5 [DOI] [PubMed] [Google Scholar]
- Szakiel A., Pkaczkowski C., Huttunen S. (2012a). Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 60 11839–11849. 10.1021/jf3046895 [DOI] [PubMed] [Google Scholar]
- Szakiel A., Pkaczkowski C., Koivuniemi H., Huttunen S. (2012b). Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 60 4994–5002. 10.1021/jf300375b [DOI] [PubMed] [Google Scholar]
- Uleberg E., Rohloff J., Jaakola L., Trôst K., Junttila O., Häggman H., et al. (2012). Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 60 10406–10414. 10.1021/jf302924m [DOI] [PubMed] [Google Scholar]
- Vvedenskaya I. O., Vorsa N. (2004). Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait. Plant Sci. 167 1043–1054. 10.1016/j.plantsci.2004.06.001 [DOI] [Google Scholar]
- Walker P. G., Gordon S. L., Brennan R. M., Hancock R. D. (2006). A high-throughput monolithic HPLC method for rapid vitamin C phenotyping of berry fruit. Phytochem. Anal. 17 284–290. 10.1002/pca.916 [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Chen C. T., Wang S. Y. (2009). Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 117 426–431. 10.1016/j.foodchem.2009.04.037 [DOI] [Google Scholar]
- Wang S. Y., Stretch A. W. (2001). Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem. 49 969–974. 10.1021/jf001206m [DOI] [PubMed] [Google Scholar]
- Wheeler S., Loveys B., Ford C., Davies C. (2009). The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 15 195–204. 10.1111/j.1755-0238.2008.00045.x [DOI] [Google Scholar]
- Xu W., Dubos C., Lepiniec L. (2015). Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 20 176–185. 10.1016/j.tplants.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Singh B. R. (2002). Red light stimulates flowering and anthocyanin biosynthesis in American cranberry. Plant Growth Regul. 38 165–171. 10.1023/A:1021322418740 [DOI] [Google Scholar]
- Zhou Y., Singh B. R. (2004). Effect of light on anthocyanin levels in submerged, harvested cranberry fruit. J. Biomed. Biotechnol. 5 259–263. 10.1155/S1110724304403027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifkin M., Jin A., Ozga J. A., Zaharia L. I., Schernthaner J. P., Gesell A., et al. (2012). Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 158 200–224. 10.1104/pp.111.180950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti L., Jaakola L., Häggman H., Giongo L. (2015a). Anthocyanin profile in berries of wild and cultivated Vaccinium spp. along altitudinal gradients in the Alps. J. Agric. Food Chem. 63 8641–8650. 10.1021/acs.jafc.5b02833 [DOI] [PubMed] [Google Scholar]
- Zoratti L., Jaakola L., Häggman H., Giongo L. (2015b). Modification of sunlight radiation through colored photo-selective nets affects anthocyanin profile in Vaccinium spp. berries. PLoS ONE 10:e0135935 10.1371/journal.pone.0135935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti L., Karppinen K., Luengo Escobar A., Häggman H., Jaakola L. (2014a). Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 5:534 10.3389/fpls.2014.00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti L., Sarala M., Carvalho E., Karppinen K., Martens S., Giongo L., et al. (2014b). Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Biol. 14:377 10.1186/s12870-014-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]