Abstract
Alcanivorax is a hydrocarbonoclastic genus dominating oil spills worldwide. While its presence has been detected in oil-polluted seawaters, marine sediment and salt marshes under ambient pressure, its presence in deep-sea oil-contaminated environments is negligible. Recent laboratory studies highlighted the piezosensitive nature of some Alcanivorax species, whose growth yields are highly impacted by mild hydrostatic pressures (HPs). In the present study, osmotic stress was used as a tool to increase HP resistance in the type strain Alcanivorax borkumensis SK2. Control cultures grown under standard conditions of salinity and osmotic pressure with respect to seawater (35.6 ppt or 1136 mOsm kg-1, respectively) were compared with cultures subjected to hypo- and hyperosmosis (330 and 1720 mOsm kg-1, or 18 and 62 ppt in salinity, equivalent to brackish and brine waters, respectively), under atmospheric or increased HP (0.1 and 10 MPa). Osmotic stress had a remarkably positive impact on cell metabolic activity in terms of CO2 production (thus, oil bioremediation) and O2 respiration under hyperosmosis, as acclimation to high salinity enhanced cell activity under 10 MPa by a factor of 10. Both osmotic shocks significantly enhanced cell protection by reducing membrane damage under HP, with cell integrities close to 100% under hyposmosis. The latter was likely due to intracellular water-reclamation as no trace of the piezolyte ectoine was found, contrary to hyperosmosis. Notably, ectoine production was equivalent at 0.1 MPa in hyperosmosis-acclimated cells and at 10 MPa under isosmotic conditions. While stimulating cell metabolism and enhancing cell integrity, osmotic stress had always a negative impact on culture growth and performance. No net growth was observed during 4-days incubation tests, and CO2:O2 ratios and pH values indicated that culture performance in terms of hydrocarbon degradation was lowered by the effects of osmotic stress alone or combined with increased HP. These findings confirm the piezosensitive nature of A. borkumensis, which lacks proper resistance mechanisms to improve its metabolic efficiency under increased HP, thus explaining its limited role in oil-polluted deep-sea environments.
Keywords: osmolyte, piezolyte, hydrocarbons, oil, deep-sea, ectoine, Halomonas, petroleum
Introduction
Alcanivorax is a marine hydrocarbonoclastic genus, which dominates oil-polluted surface waters worldwide (Syutsubo et al., 2001; Hara et al., 2003; Yakimov et al., 2005). Its negligible presence in deep-sea areas contaminated with hydrocarbons (e.g., the Gulf of Mexico, following the deepwater Horizon (DWH) oil spill accident; Hazen et al., 2010; Valentine et al., 2010; Baelum et al., 2012; Mason et al., 2012; Gutierrez et al., 2013; Yergeau et al., 2015) supported the hypothesis that this genus might lack proper adaptation mechanisms to hydrostatic pressure (HP). Recent laboratory experiments on A. jadensis and A. dieselolei strains subjected to HP up to 10 MPa (equivalent to 1 km depth, approximately the depth of the oil plume formed after the DWH spill, Camilli et al., 2010) supported this hypothesis (Scoma et al., 2016). Similar tests on the type strain Alcanivorax borkumensis SK2 further refined these results, and indicated that the expression of all the genes related with the biosynthetic pathway of ectoine and its actual production per cell are enhanced under 10 MPa (Scoma et al., unpublished results). The mechanisms by which ectoine might offset HP remain unclear, as well as its possible role in supporting fitness, cell replication, or functionality.
Ectoine was first isolated from the halophilic phototrophic bacterium Ectothiorhodospira halochloris (Galinski et al., 1985) to which it owes its trivial name, and early after detected in several other microbes such as some Halomonadaceae (Wohlfarth et al., 1990), Bacterium Ba1, Vibrio costicola (Regev et al., 1990) and Brevibacterium linens (Bernard et al., 1993). Intracellular accumulation was noted to be proportional to an increase in osmolarity (Galinski et al., 1985) and could result from de novo synthesis using glutamate as precursor (Inbar and Lapidot, 1988) or uptake from the outer environment (Jebbar et al., 1992). Nonetheless, its intracellular concentration could be negatively regulated by supplying cells with other osmolytes or their precursors (e.g., glycine betaine, choline, proline, trehalose, and taurine) likely owing to ectoine energy-intensive de novo biosynthesis (Bernard et al., 1993). First evidence of its transcriptional regulation was found in Halomonas elongata, where a 3-gene cluster was identified (ectABC, Cánovas et al., 1997, 1998) and soon discovered to be evolutionary highly conserved and widespread (Louis and Galinski, 1997; Göller et al., 1998; Pflughoeft et al., 2003; Reshetnikov et al., 2006; Bursy et al., 2007; Saum and Müller, 2008). In recent years, other mechanisms have been found to account for the enhanced transcription of ectABC, such as high (Calderon et al., 2004) and low temperature (in Virgibacillus pantothenticus, Kuhlmann et al., 2008) and, as previously mentioned, HP (in A. borkumensis SK2, Scoma et al., unpublished results).
Intracellular accumulation of inorganic and organic compounds to counteract environmental osmotic stress is a widespread response in the microbial world (Sleator and Hill, 2002). Microorganisms can synthesize or take up such compounds from the environment to prevent cell disruption and/or other water-related stresses, which may co-occur with a change in osmolarity (e.g., oxidation, protein perturbation; Yancey, 2005). Although, much remains to be understood about the relationship among solute-accumulation, water retention and protein functionality, it appears that several osmolytes accumulated within the cell exert a protective function also with respect to other extreme conditions [e.g., high or low temperature and pH, toxic compounds (Wood, 1999; Welsh, 2000)]. This observation has been also reported for HP. Increased concentration of sodium chloride or sugars in several different microbes reduced the inactivation rate to high HP (>200 MPa; Oxen and Knorr, 1993; Palou et al., 1997; Molina-Höppner et al., 2004). The protective effect provided by osmotic stress response to enhanced HP may differently impact cell physiology and integrity. In Lactococcus lactis, supplying 0.5 M sucrose preserved metabolic activity and membrane integrity of cells exposed up to 600 MPa HP, whereas inorganic salts (4 M NaCl) preserved cell membrane integrity but failed to have a positive impact on cell metabolism (Molina-Höppner et al., 2004). In A. borkumensis SK2, application of 5 MPa essentially inactivated cultures supplied with n-dodecane, showing a net decrease in cell number and only 10% membrane integrity in the surviving cells (Scoma et al., unpublished results). However, incubation at 10 MPa re-established cell growth and nutrient uptake capacity, significantly enhanced cell integrity (∼25%) and resulted in a remarkable intracellular ectoine accumulation (Scoma et al., unpublished results). Another Alcanivorax species subjected to the same conditions (i.e., A. dieselolei, 10 MPa, n-dodecane as sole carbon source) showed much higher cell integrity levels at 10 MPa (∼70%) and no ectoine accumulation or gene expression upregulation (Scoma et al., 2016) suggesting a possible relationship between cell membrane integrity and intracellular ectoine levels at increased HP. In order to understand the physiological conditions triggering ectoine accumulation, in the present investigation different ectoine intracellular levels were stimulated by altering the physiological parameters known to affect its synthesis, that is, osmolarity and HP. A. borkumensis SK2 control cultures grown under standard isosmotic conditions similar to seawater were compared with cultures acclimated to altered osmotic conditions, the salinity of which resembled brackish or brine waters. All cultures were subjected to either 0.1 or 10 MPa HP. Results aimed primarily at testing whether shifts in extracellular osmolarity might protect Alcanivorax cells from the damaging effects exerted by enhanced HP and were discussed with respect to the response in ectoine accumulation.
Materials and Methods
Strain, Standard Medium, and Growth Conditions
Alcanivorax borkumensis SK2 was cultivated axenically in Schott glass bottles of 250 mL (operating volume 100 mL), without providing mixing, under ambient pressure (i.e., 0.1 MPa), using ONR7a medium (Dyksterhouse et al., 1995), initial pH 7.6, for 4–7 days at 20°C. Cultures were provided with 1% (v:v) n-dodecane (Sigma-Aldrich, Belgium) as sole carbon source (equivalent to about 7.5 g L-1) to imitate the conditions of an oil spill (high C/N ratio) as previously suggested for this strain (Sabirova et al., 2006).
Modified Media and Growth Conditions
Sea and ocean water are typically defined as saline waters, and have an average osmolarity of about 1000 mOsm kg-1 (Yancey, 2005) equivalent to a salinity of 35 ppt (or g kg-1) or, alternatively, 3.5% (Table 1). The large majority of the salinity in seawater is determined by NaCl (Yancey, 2005). The ONR7a medium is a standard medium to cultivate marine bacteria, has a salinity of 35.6 ppt, equivalent to 1136 mOsm kg-1 (Table 1) and is therefore isosmotic with respect to seawater (osmolarity was calculated as the sum of the osmotic pressure of all the single salts used to prepare the medium). In the present study, modified osmolarity was achieved by targeting the two most concentrated salts exerting the highest osmotic pressure in the ONR7a medium. The possibility of using proportional concentrations of the entire ONR7a medium was discarded, as altering the concentration of all the elements in seawater would have little effect on the osmolarity, while potentially impacting the whole microbial metabolism. The two most concentrated salts in ONR7a, accounting for about 90% of ONR7a osmotic pressure, are NaCl and MgCl2 (780 and 165 mOsm kg-1, respectively). Selection of another salt in combination with NaCl was necessary to impose a stronger hyposmotic effect to counteract HP by means of water influx into the cell, provided that a reduction of NaCl from 22.8 to 8.2 g L-1 would still yield an osmotic pressure of 638 mOsm kg-1 (vs. 1136 mOsm kg-1 in standard ONR7a). In moderately halophilic bacteria, several ions other than Na+ or Cl- are known to play a role in osmotic equilibrium, such as K+, Mg2+, Ca2+, and Mn2+ (Ventosa et al., 1998). In the ONR7a medium, MgCl2 was preferred over KCl because it brings a higher osmotic pressure in standard formulations (165 vs. 19.3 mOsm kg-1, respectively). NaCl and MgCl2 are normally provided in ONR7a at concentrations of 22.8 and 11.18 g L-1, respectively. Final concentrations under hyposmosis were 8.2 and 4.7 g L-1, while under hyperosmosis they were 44 and 21.5 g L-1, respectively. Hence, hypo-, iso-, and hyperosmosis exerted a different final osmotic pressure on bacterial cells equivalent to 330, 1136, and 1720 mOsm kg-1 (Table 1). In other words, the marine bacterium A. borkumensis SK2 was tested using very different salinities, equivalent to brackish, saline, and brine waters, respectively.
Table 1.
Overview of the different media salinities and osmotic pressures with respect to seawater.
| Culture conditions | Medium | Definition | Salinity |
NaCl | MgCl | Osmotic pressure | Reference | |
|---|---|---|---|---|---|---|---|---|
| ppt or g kg-1 | % | M | M | mOsm kg-1 | ||||
| Isosmosis | Seawater | Saline | 35.0 | 3.5 | 0.47 | 0.07 | 1000 | Yancey, 2005 |
| Isosmosis | ONR7a | Saline | 35.6 | 3.6 | 0.39 | 0.05 | 1136 | Dyksterhouse et al., 1995 |
| Hyposmosis | Modified ONR7a | Brackish | 18.0 | 1.8 | 0.03 | 0.02 | 330 | This work |
| Hyperosmosis | Modified ONR7a | Brine | 61.7 | 6.2 | 0.75 | 0.11 | 1720 | This work |
To achieve acclimation to a different salinity/osmolarity, SK2 cells were first incubated for 7–10 days using media at altered osmotic conditions. Then, 10% culture volume was withdrawn and re-incubated using the same modified ONR7a medium (either hypo- or hyperosmotic) for another 7–10 days of incubation. Cultures were considered acclimated to the new osmolarity after completing three full cycles of growth in their respective modified ONR7a. Later, acclimated cells were used in specific tests under ambient and increased HP. All other culture conditions were the same as for isosmosis.
Ambient and Increased HP Experiments
Cells were collected by centrifugation at 4000 rpm for 10 min at 4°C (Sorval RC5c PLUS, Beckman, Suarlée, Belgium), and resuspended in standard or modified ONR7a medium at an initial optical density (OD610) of 0.33 ± 0.02, corresponding to 3.6 ± 0.2 × 108 cells mL-1. Cells grown under isosmotic conditions were tested using standard ONR7a (isosmosis) or modified ONR7a exerting a different osmotic pressure (hyposmosis or hyperosmosis). Cells acclimated to a different osmolarities were tested using their respective medium (acclimated hyposmosis or hyperosmosis). None of the cultures was adapted to HP. Culture suspensions (3.5 mL) were transferred into sterile 10 mL syringes, and n-dodecane (C12) 1% (v:v) was supplied as the sole carbon source. The gas phase (equal to 6.5 mL) was constituted of air, which provided O2 to the cells during the subsequent incubation. Syringes were closed using a sterile three-way valve, and placed in a 1 L T316 stainless steel high-pressure reactor (HPR; Parr, USA). The reactor was filled with deionized water and HP was increased up to 10 MPa (equivalent to 1000 m below surface level) by pumping water with a high-pressure pump (HPLC pump series III, SSI, USA). Pressure was transmitted to the cultures through the piston of the syringe. Experiments at atmospheric pressure were run adjacent to the HP reactor. Control experiments were conducted using sterile syringes supplied only with sterile non-inoculated medium to check on CO2 and O2 levels in the headspace at both 0.1 and 10 MPa. Incubations were conducted in a temperature-controlled room at 20°C for 4 days. At the end of the experiments, HP was gently released and syringes set aside for 30 min before running biochemical analyses.
To assess the metabolic activity, efficiency, and oil bioremediation potential of A. borkumensis cultures, biomass growth, CO2 production, CO2:O2 ratios and pH decrease were measured at the end of the incubations. Cell membrane integrity was considered as a primary target for cell viability under stressing conditions, provided that both hydrocarbons (Heipieper and Martinez, 2010) and HP (Molina-Höppner et al., 2004) are known to impact this parameter, and its potential correlation with ectoine accumulation considered (Scoma et al., unpublished results).
Cell Counts and Integrity
Pressure-induced cell membrane damage analysis (Benito et al., 1999) and total cell count was done using flow cytometry: SYBR® Green I and Propidium Iodide were used in combination to discriminate cells with intact and damaged cytoplasmic membranes using the protocol described by De Roy et al. (2012). CO2 production and O2 respiration per cell were measured considering final cell numbers.
Chemical Analyses
O2 respiration and CO2 production rates were assessed by analyzing the headspace gas composition of syringes inoculated with strain SK2 cells as compared to sterile controls. The gas-phase was analyzed with a Compact GC-TCD (Global Analyser Solutions, Breda, The Netherlands), equipped with a Molsieve 5A pre-column and two channels (channel 1 for CH4, O2, H2 and N2 and channel 2 for CO2, N2O, and H2S). pH was measured using a pH meter (Herisau, Metrohm, Switzerland). Intracellular ectoine concentration was measured by collecting cells by centrifugation at 4°C (10000 g, 5 min), discarding the supernatant and storing pellets at -20°C. Later on, ectoine was extracted from pellets with an ethanol:water solution (4:1, v/v) for 30 min. Following centrifugation, the supernatant was evaporated overnight at 40°C and the residue dissolved in distilled water. Re-suspended ectoine in water was analyzed by HPLC using an Aminex HPX-87C column (Bio-Rad Laboratories, Hercules, CA, USA), with CaCl2 (5 mM) as eluent and detection at 210 nm (Onraedt et al., 2004).
Statistical Analysis
Results were expressed as mean values of experiments made in 10 independent replicates. Error bars in the graphs indicate a 95% confidence interval (95% CI) calculated using a Student t-test with a two-sided distribution. Statistical significance was assessed using a non-parametric test (Mann–Whitney test) which considered a two-sided distribution with 95% CI.
Results
Hyperosmosis Improves Cell Activity at Ambient and Increased HP
Alcanivorax borkumensis SK2 cells where incubated at different osmotic (hypo-, iso- or hyperosmosis, equivalent to 330, 1136, and 1720 mOsm kg-1 respectively) and HP (0.1 or 10 MPa) conditions to test whether the response to osmotic stress could prevent or mitigate the negative impact of HP on cell metabolism and culture performance.
Cell metabolism was highly stimulated by a change in osmolarity and/or HP (Figure 1). Provided that n-dodecane was supplied as sole carbon source, CO2 production was considered as an indirect measure of its oxidation by A. borkumensis. Under isosmotic conditions at 0.1 MPa, SK2 produced 3.1 ± 1.3 nmoles CO2 10-6 cells but faced a threefold reduction in activity when incubated at 10 MPa (Figure 1A). Transition to hyposmosis resulted into similar CO2 outputs at 0.1 and 10 MPa, which were of the same order as those observed under isosmotic conditions (∼1.5 nmoles CO2 10-6 cells, Figure 1A). Acclimation to hyposmosis at 0.1 MPa eventually resulted into a 10-fold increase (15.6 ± 4.8 nmoles CO2 10-6 cells, Figure 1A) while no effect could be observed at 10 MPa after acclimation. Thus, at ambient pressure acclimation to hyposmosis eventually enhanced cell metabolism, while a concomitant reduction in osmolarity and increase in HP prevented this effect (Figure 1A).
FIGURE 1.
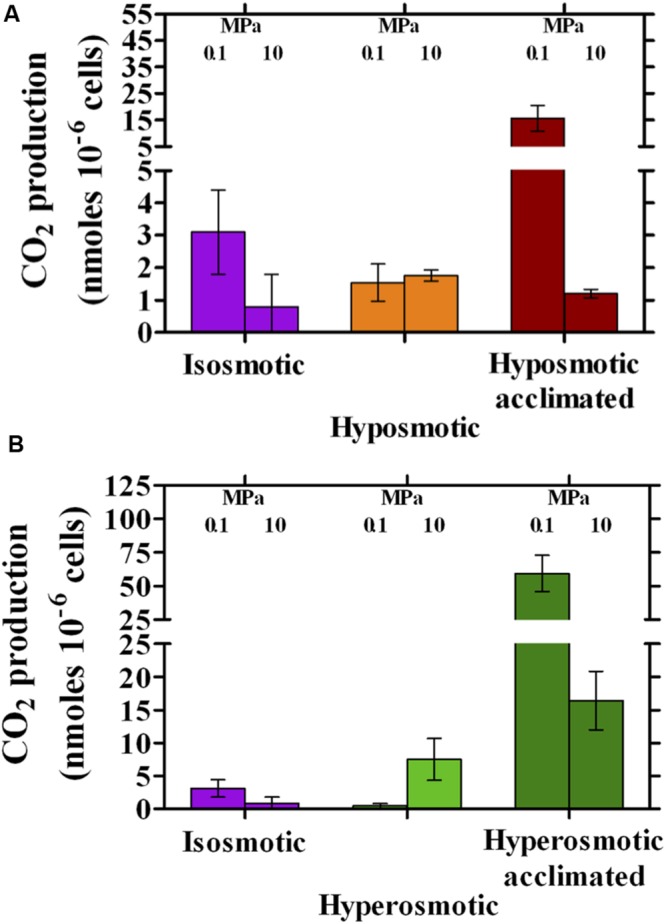
Production of CO2 per cell by Alcanivorax borkumensis SK2 cells subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Keys: isosmosis (purple), hyposmosis (orange), hyposmosis acclimated (red), hyperosmosis (light green), hyperosmosis acclimated (dark green). Bars indicate 95% confidence intervals.
Conversely, hyperosmosis generally enhanced CO2 production. At ambient pressure, transition to a hyperosmotic environment first showed a strong negative impact on CO2 production (from 3.1 ± 1.3 to 0.41 ± 0.38 nmoles CO2 10-6 cells, Figure 1B) while acclimation resulted into a notable increase (59 ± 13 nmoles CO2 10-6 cells, Figure 1B), which was the highest of all the tested conditions. A positive correlation between increased osmolarity and HP was also observed, as CO2 production at 10 MPa increased remarkably from iso- to hyperosmosis (from 0.8 ± 1.0 to 7.5 ± 3.2 nmoles CO2 10-6 cells, respectively, Figure 1B), with acclimation to hyperosmosis yielding a further improvement to 16.4 ± 4.5 nmoles CO2 10-6 cells (Figure 1B).
In agreement, O2 respiration was stimulated more by hyper- rather than hyposmosis (Figure 2). Under atmospheric pressure, hyposmosis slightly enhanced O2 respiration only after acclimation (Figure 2A). On the contrary, the concomitant application of increased HP and hyposmosis resulted into a prompt increase from 24 ± 39 to 161 ± 31 nmoles O2 10-6 cells, which remained constant also after acclimation (Figure 2A). With regard to hyperosmosis, at 0.1 MPa it increased O2 respiration by a factor of 5 (13, 59, and 296 nmoles O2 10-6 cells in iso-, hyper-, and hyperosmotic-acclimated cells, respectively, Figure 2B). Furthermore, the concomitant increase of both osmosis and HP boosted respiration by a factor of 50 (from 13 to ∼600 nmoles O2 10-6 cells), with acclimation not bringing further improvement (639 ± 197 nmoles O2 10-6 cells, Figure 2B). Hence, hyperosmosis induced a higher respiration activity with respect to hyposmosis. In particular, increased HP resulted in an increased O2 consumption by the cells in each treatment.
FIGURE 2.
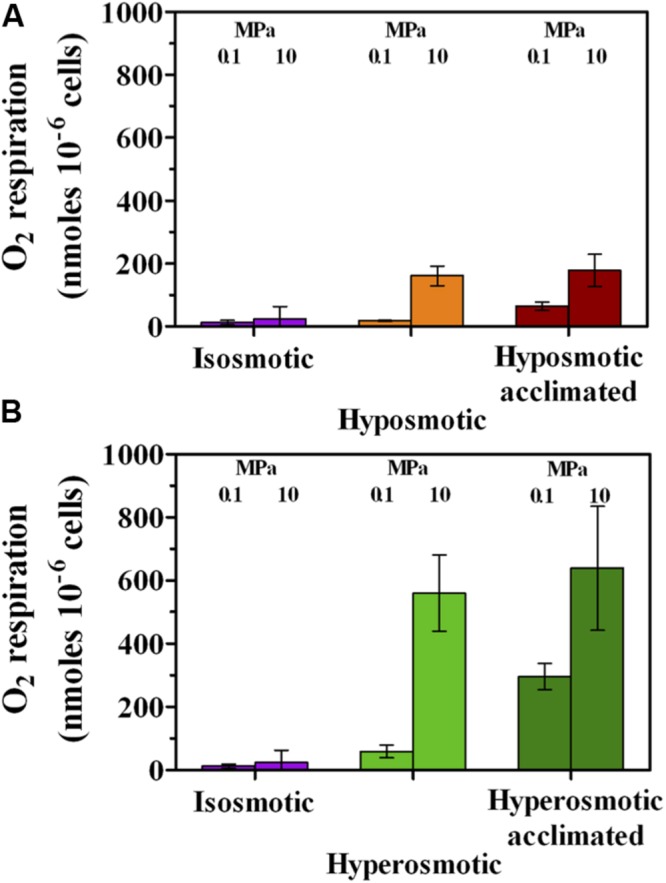
O2 respiration per cell by A. borkumensis SK2 cells subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Keys: isosmosis (purple), hyposmosis (orange), hyposmosis acclimated (red), hyperosmosis (light green), hyperosmosis acclimated (dark green). Bars indicate 95% confidence intervals.
Hyposmosis Improves Cell Integrity at Ambient and Increased HP
Changes in osmosis and HP with respect to standard conditions had a remarkable impact on cell membrane integrity (Figure 3). Increase to 10 MPa at isosmotic conditions resulted in a reduced fraction of intact cells (from ∼50 to ∼25%, Figure 3A). When growing cells in a medium with reduced osmolarity membrane integrity approached 100%, irrespective of the HP applied (Figure 3A), with acclimation slightly reducing these values (Figure 3A). At increased osmolarity, no difference was observed at 0.1 MPa even after acclimation (Figure 3B), while cell integrity improved significantly when concomitantly increasing both osmolarity and HP, values slightly decreasing after acclimation. As a whole, transition from isosmosis to both altered osmotic conditions conferred enhanced cell integrity under HP (p < 0.0001) and, notably, resulted into a higher intact cell number in the most unfavorable condition (i.e., 10 rather than 0.1 MPa). Hence, hyposmosis conferred resistance to HP through structurally resistant cells (Figure 3A) with low activity (Figures 1A and 2A), while hyperosmosis counteracted HP by remarkably enhancing metabolic activity (Figures 1B and 2B) while increasing cell integrity to a lower extent (Figure 3B).
FIGURE 3.
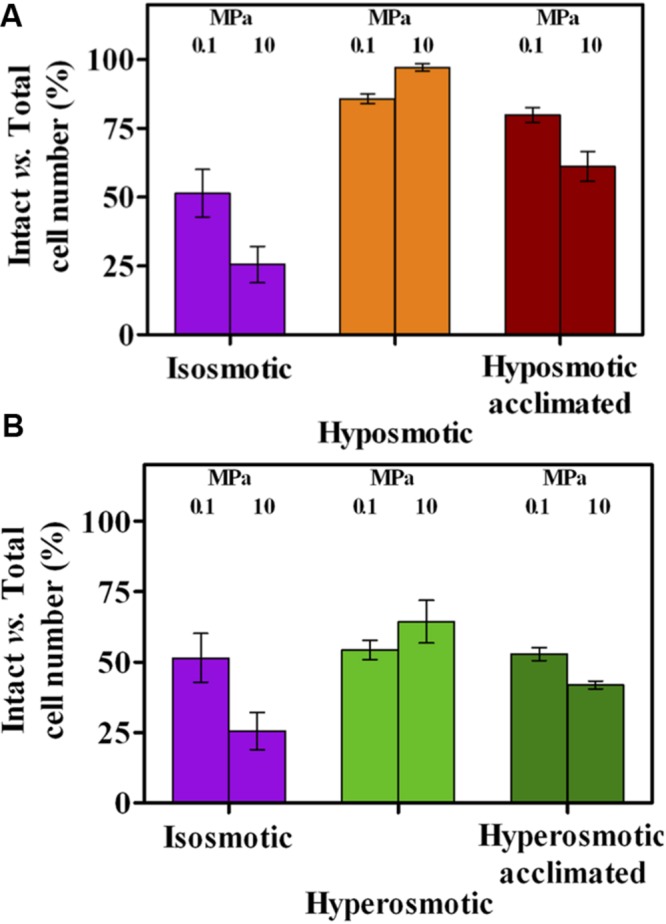
Relative number of intact cells over total cell count in A. borkumensis SK2 cells subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Keys: isosmosis (purple), hyposmosis (orange), hyposmosis acclimated (red), hyperosmosis (light green), hyperosmosis acclimated (dark green). Bars indicate 95% confidence intervals.
Production of the piezolyte ectoine in SK2 cells was substantially different. Under isosmotic conditions, minor amounts of ectoine were produced, with a sharp increase as HP was brought to 10 MPa (Figure 4). No trace of ectoine could be detected irrespective of the applied HP or acclimation under hyposmosis (data not shown, detection limit 4 mg L-1), suggesting that the remarkable cell integrity (Figure 3A) likely relied on a water-reclamation effect. However, cells adapted to hyposmosis did not lose their capacity to produce ectoine, as 4-days incubations of these cells under HP and hyperosmosis did yield some ectoine (Supplementary Figure S1). On the other hand, hyperosmosis at 0.1 MPa led to an increase in the amount of ectoine per cell only by means of acclimation (from 0.5 to 3.5 fmoles ectoine cell-1, Figure 4), while a different trend was noted with the concomitant increase of osmolarity and HP. As a matter of fact, the highest value was observed in isosmosis by only increasing HP to 10 MPa (3.5 ± 1.1 fmoles cell-1, Figure 4). The concomitant increase of osmolarity and HP actually reduced ectoine production, which further slightly increased with cell acclimation to high osmolarity (0.98 ± 0.03 and 1.63 ± 0.08 fmoles cell-1, respectively, Figure 4). This result suggests that A. borkumensis cells may use ion transport to counteract HP effects, rather than synthesizing ectoine de novo. Further, it should be noted that an increase to 10 MPa HP under isosmosis induced the same ectoine accumulation as after acclimation to hyperosmosis at ambient pressure (Figure 4).
FIGURE 4.
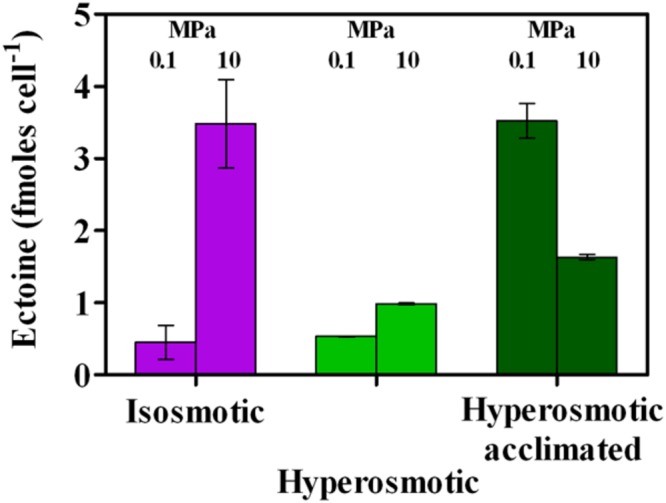
Ectoine production per cell in A. borkumensis SK2 cells subjected to hyperosmotic conditions under different hydrostatic pressures (0.1 or 10 MPa) as compared to standard isosmotic conditions. Keys: isosmosis (purple), hyperosmosis (light green), hyperosmosis acclimated (dark green). Bars indicate 95% confidence intervals.
Osmolarity Shocks Impair Culture Performance
Standard conditions of osmosis and HP (isosmosis using the standard ONR7a medium and 0.1 MPa) ideal to A. borkumensis SK2 (Yakimov et al., 1998) resulted into a sustained cell replication, which significantly (p < 0.05) decreased when cultures were grown under a HP of 10 MPa (Figures 5A,B). Incubation at altered osmotic conditions had a detrimental effect on culture growth, as a net decrease in cell number was always observed irrespective of the HP applied (up to -314 × 106 cells mL-1; Figures 5A,B). Acclimation to different osmolarities yielded similar results, with cultures acclimated to hyperosmosis being the most affected (-375 × 106 cells mL-1; Figure 5B).
FIGURE 5.
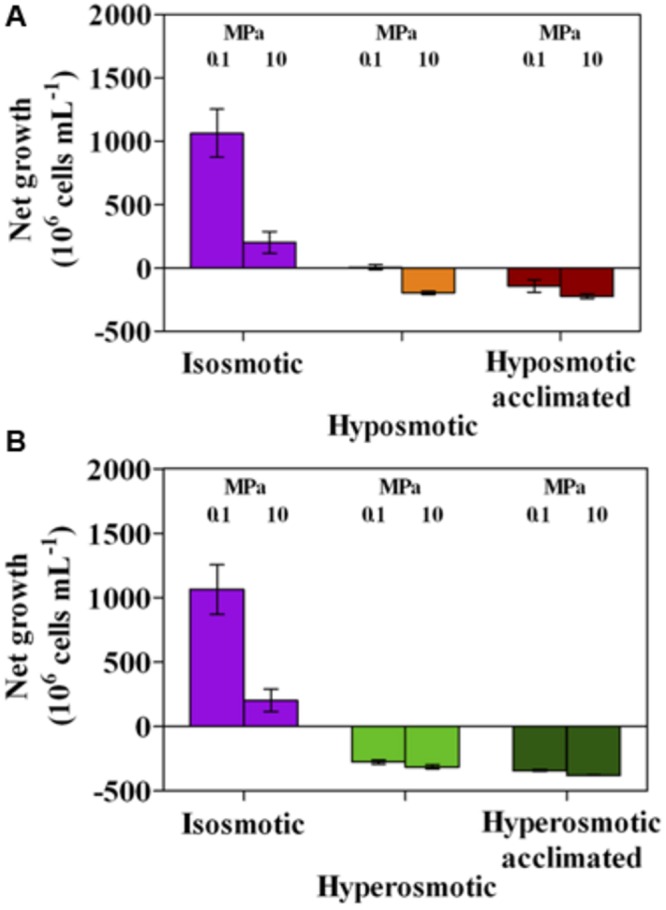
Net growth in A. borkumensis SK2 cultures subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Keys: isosmosis (purple), hyposmosis (orange), hyposmosis acclimated (red), hyperosmosis (light green), hyperosmosis acclimated (dark green). Bars indicate 95% confidence intervals.
Degradation of n-dodecane leading to different CO2 production values was consistent with the general decrease in pH value in all conditions (Figure 6), as different acidification levels were the combined result of reduced final cell numbers (Figure 5) and CO2 production per cell (Figure 1). Isosmosis and ambient pressure resulted in the lowest pH value (5.95, Figures 6A,B), meaning that cells were very active in oil degradation leading to CO2 production. In agreement with the observed high activity of cells exposed to hyperosmosis (Figure 1B), the pH value was generally lower when osmolarity was increased (6.43 vs. 6.74 at 0.1 MPa in hyper vs. hypo-osmotic cultures, Figures 6A,B). Enhanced acidification was not observed in cultures incubated under HP. The final pH in these cultures was always higher than their respective incubation test carried out at 0.1 MPa (6.86 vs. 7.36 at 10 MPa in hyper vs. hypo-osmotic cultures, Figures 6A,B), although it can be assumed that under increased HP cells experienced somewhat lower pH values than that measured after decompression.
FIGURE 6.
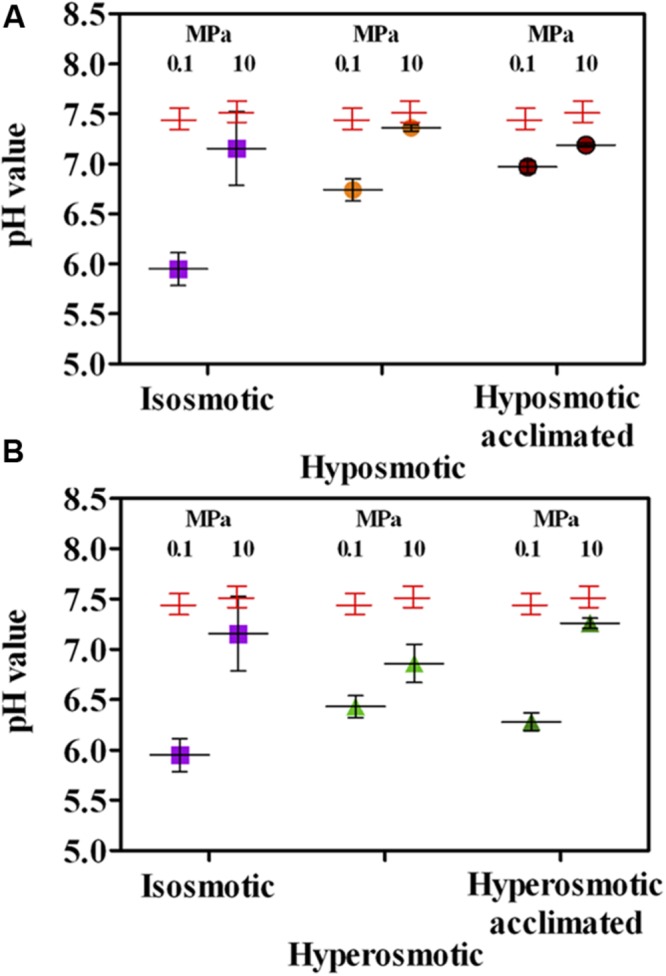
Changes in pH value in A. borkumensis SK2 cells subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Keys: isosmosis (purple squares), hyposmosis (orange circles), hyposmosis acclimated (red circles), hyperosmosis (light green triangles), hyperosmosis acclimated (dark green triangles). Red crosses indicate pH in sterile controls. Bars indicate 95% confidence intervals.
Reduced oil degradation by cultures subjected to osmotic and HP was reflected by a reduced molar ratio between CO2 produced and O2 respired, compared to the stoichiometric CO2:O2 ratio for n-dodecane oxidation equal to 0.649 (i.e., at least 18.5 moles of O2 are needed to oxidize 1 mole of n-dodecane, yielding 12 moles of CO2). The best efficiency was observed in isosmosis at ambient pressure (0.407, Figures 7A,B). Almost all the other degradation efficiencies decreased by a factor of 10 to ∼0.05 or lower, excepting for cultures acclimated to both altered osmotic conditions under ambient pressure, where CO2:O2 ratio reached ∼0.21 (Figures 7A,B). Hence, the effects exerted by changes in osmosis under HP on cell metabolism and integrity (Figures 1–4) were not reflected in improved culture performance in terms of cell growth (Figure 5) or substrate degradation efficiency (Figures 6 and 7).
FIGURE 7.
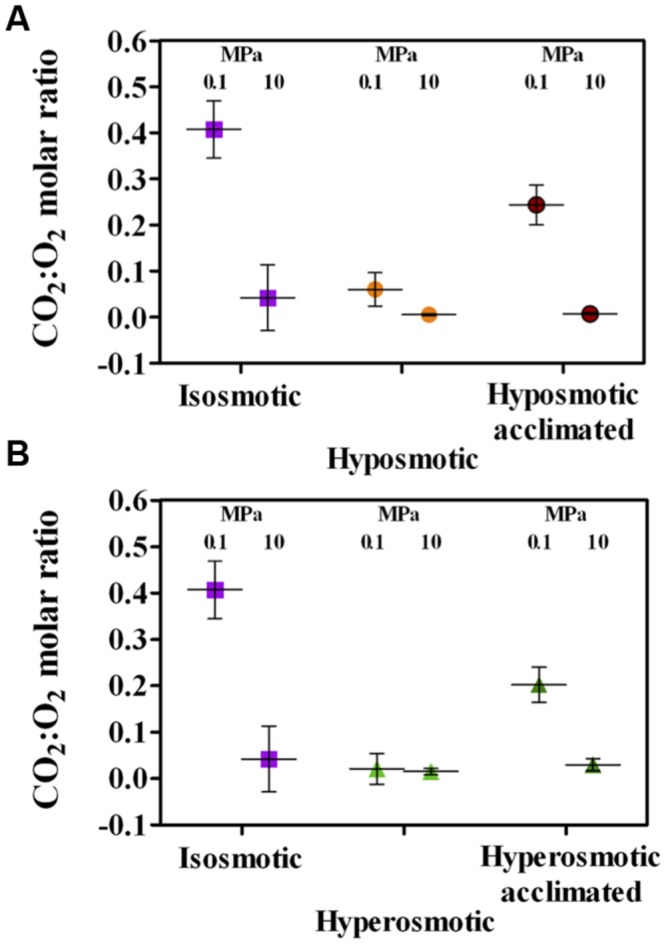
Molar ratio between CO2 production and O2 respiration in A. borkumensis SK2 cultures subjected to hyposmotic (A) or hyperosmotic (B) conditions under different hydrostatic pressures (0.1 or 10 MPa), as compared to standard isosmotic conditions. Note that stoichiometric mineralization of n-dodecane would yield a CO2:O2 ratio equal to 0.649. Keys: isosmosis (purple squares), hyposmosis (orange circles), hyposmosis acclimated (red circles), hyperosmosis (light green triangles), hyperosmosis acclimated (dark green triangles). Bars indicate 95% confidence intervals.
Discussion
Improved microbial tolerance to HP consistent with a high resistance to other extreme conditions has been widely reported in the literature, especially with respect to food preservation technology (De Angelis and Gobbetti, 2004). HP-resistant Escherichia coli cells were found to be also more resistant to low pH, mild heat, oxidation, and osmotic stress compared to HP-sensitive E. coli strains (Benito et al., 1999). Preincubation of Lactobacillus sanfranciscensis under increased salinity, low temperature or pH improved its tolerance to HP (Scheyhing et al., 2004). Similarly, HP inactivation of Lactococcus lactis was less effective when cells were concomitantly exposed to increased osmolarity (Molina-Höppner et al., 2004). Synergistic effects between these parameters (e.g., pH, temperature, HP) are typically found in bacteria inhabiting environmental niches such as deep-sea hydrothermal vents (Kaye and Baross, 2004). The reasons for this interdependency rely on the fact that at extreme levels some of these conditions may impose a similar physicochemical stress on cell metabolism. HP influences pHi by enhancing the dissociation of weak organic acids and increasing the permeability of the cytoplasmic membrane, which limits the level of pH homeostasis (De Angelis and Gobbetti, 2004), and may resemble the impact of extreme extracellular pH. Bacteria have evolved a number of strategies to counteract the low cell membrane fluidity due to cold temperatures (Chintalapati et al., 2004), a condition also imposed by an increase in HP (Bartlett, 2002). However, the main effect of HP application is volume change, meaning that increased HP accelerates any reaction that entails a reduction in volume, while the opposite is true for a positive volume change (Abe, 2007). This typically impairs cell division and replication (Bartlett, 2002; Abe, 2007) and the activity of several multi-component protein complexes (Oger and Jebbar, 2010), where assemblage of different subunits yields an increase in volume, as in the case of ribosomes and protein translation (Schwarz and Landau, 1972). Water stress- as that following a change in osmolarity in the environment- exerts similar effects on cell metabolism. Hyperosmosis results in a net water efflux from the cell, with detrimental loss of cell turgor pressure and cell shrinkage, while a reduced environmental osmolarity leads to a massive influx of water into the cell (Csonka, 1989). In both cases, cells have to quickly react to prevent cell lysis. Rapid shifts in cellular pressure and volume will impact protein–protein and protein–ligand interactions (Heremans, 1982) and compromise enzyme functionality.
In A. borkumensis SK2, osmolarity shocks could activate some resistance mechanisms against HP stress, particularly at hyperosmosis where an increased cell metabolism was observed (Figures 1B and 2B), while hyposmosis only impacted O2 respiration capacity (Figure 2A). The most remarkable effect was exerted on cell integrity, which increased under HP in both altered osmotic conditions with respect to standard isosmosis under HP (Figures 3A,B). A positive correlation between reduced cell damage, cell viability and HP resistance has been previously suggested (Benito et al., 1999; Pagán and Mackey, 2000). As it stands, hyposmosis counteracted HP by conferring a high membrane integrity but low activity, while hyperosmosis conferred resistance to HP by strongly improving cell metabolism and enhancing cell integrity to a lower extent.
How do cells counterbalance a shift in osmolarity? Common strategies to maintain homeostasis are intracellular accumulation of organic compounds (by the bacterial class of “osmoconformers”) and/or ion transport (by the “osmoregulators”), being the choice between the two based on evolutionary and, particularly, energetic aspects (Oren, 1999). Cell membranes are permeable to water, and rely on efflux or influx/production of such osmolytes to counterbalance water stress. The average concentration of salts in seawater brings about 1000 mOsm kg-1 (mainly due to NaCl), which is 2–3 times more than that found in most cells (∼300–400 mOsm kg-1; Yancey, 2005). In the present study, isosmotic conditions were equivalent to 1136 mOsm kg-1, while hypo- and hyperosmotic were 330 and 1720 mOsm kg-1, respectively (Table 1). Hence, A. borkumensis SK2 cells grown in hypo-, iso-, and hyperosmosis experienced salinities equivalent to brackish, saline and brine waters, respectively (Table 1). Strain SK2 was isolated from sea water and sediment samples collected near the Isle of Borkum (North Sea), under ambient pressure. It grows at salinities comparable to other heterotrophic marine halophilic γ-Proteobacteria with which it shares many traits such as Marinobacter, Oceanospirillum, and Halomonas (between 3 and 10% NaCl, Yakimov et al., 1998). For instance, the hydrocarbonoclastic Marinobacter and Alcanivorax genera were detected in oil mousses (ambient pressure) 1 year after the DWH spill, but their relative abundance was reduced in salt marshes (Liu and Liu, 2013). Hence, the present hyperosmotic condition was within the living boundaries for SK2, while hyposmosis must have exerted a stronger stress. Conversely, Alcanivorax presence was low and not correlated with hydrocarbon levels in DWH sediment samples (Kimes et al., 2013) and its contribution in the DWH oil plume at deep-sea considered negligible (Gutierrez et al., 2013). Together with low temperature (Gutierrez et al., 2013), HP has been proposed as a major driver to explain Alcanivorax absence in the deep sea (Scoma et al., 2016). Recent findings on strain SK2 subjected to 10 MPa in standard marine media reported a remarkable accumulation of ectoine, a cyclic, amino acid derivate organic osmolyte which has now been proposed as a piezolyte, as it also accumulates as a result of increased HP (Scoma et al., unpublished results). The capacity of several organic osmolytes to engage in unique reactions exerting a protective effect on cells in ways other than via osmotic-based strategies supported the hypothesis that some solutes might in fact have a role also in counteracting HP. Previous examples are N-trimethylamine oxide (TMAO; reviewed by Yancey, 2005) and probably β-hydroxybutyrate (Martin et al., 2002).
In the present study, estimates of ectoine accumulation on a cell dry weight (CDW) basis were of the same order of the highest found in literature at ambient pressure (up to 0.5 gectoine/gCDW, Supplementary Table S1). However, owe to a net decrease in cell number (Figure 5), ectoine final concentration in the broth medium was rather low (<0.14 g L-1, Supplementary Table S1). The highest (comparable) productivities were stimulated by high HP or salinity, that is isosmosis at 10 MPa or hypersomosis at ambient pressure (Figure 4), although cells exposed to HP were incubated for 4 days while acclimation to hyperosmosis lasted for ∼30 days.
The mechanisms by which ectoine might offset HP are unknown, hence its classification as compatible, counteracting or compensatory solute remains unclear (Yancey, 2005). The complete lack of ectoine production consistent with a decreased metabolic activity in hyposmosis indicate that this piezolyte might be involved to some extent in maintaining functional cellular processes. However, its concentration (Figure 4) was not linearly related with any cell metabolic activity evaluated in this study (Figures 1, 2, 6, and 7). In halophilic bacteria such as Alcanivorax ectoine functions as a water-reclaimer preventing dehydration has been widely assessed, owe to its capacity to form water structures in its proximity (da Costa et al., 1998). In non-halophilic streptomycetes it was also observed to protect antibiotic-producing cells from their own products (da Costa et al., 1998). Possibly, ectoine functionality as a piezolyte may still be to act as water-reclaimer just as osmolytes (reviewed in da Costa et al., 1998; Yancey, 2005). In this respect, it must be noted that the water-reclaming, ectoine-free cell membrane protection to HP observed in hyposmosis was almost complete (Figure 3). This hypothesis needs further experimental evidence. Notably, concomitant application of high osmolarity and HP did not sum up to yield even higher ectoine productivities as compared to isosmosis at 10 MPa but resulted into lower ectoine concentrations (Figure 4). This suggests that, when possible (e.g., in hyperosmosis), in A. borkumensis SK2 ion transport may be concomitantly used as an energy-efficient mechanism to counteract HP (Oren, 1999). Under isosmosis cells may have no other strategy to counteract HP other than synthesizing ectoine, while environmental hyperosmosis may still be regulated by the cell through ion transport. Notably, the energy-intensive ectoine production (Bernard et al., 1993) does not bring any growth advantage to SK2 under HP (Figure 5). The lack of a linear ectoine accumulation following osmotic shocks suggests that resistance to HP through an unbalanced osmolarity is achieved through different strategies, which may compensate each other to some extent. Nonetheless, ectoine synthesis under HP can be predicted in other halophiles closely related to Alcanivorax and possessing the ectABC gene cluster such as Halomonas.
Notwithstanding the positive effects observed on cell activity and integrity, osmotic shocks always negatively impacted culture performance at HP. No net culture growth was observed during the 4-days incubation test (Figures 5A,B), and growth was only observed by growing cultures for 7–10 days (as during the acclimation phase). Medium acidification resulting from CO2 production was severely impacted (Figures 6A,B), and mirrored in the reduced efficiency in n-dodecane utilization (Figures 7A,B), which dropped to ∼0.05 CO2:O2 ratio under HP irrespective of the osmolarity applied. These results confirm the piezosensitive nature of A. borkumensis SK2, a model organism for hydrocarbonoclastic bacteria, which is highly impaired by a mild increase in HP (Scoma et al., unpublished results). While dominating bacterial blooms in oil-polluted surface waters worldwide (Yakimov et al., 2005), in deep-sea contaminated waters A. borkumensis presence is negligible (Hazen et al., 2010; Valentine et al., 2010; Baelum et al., 2012; Mason et al., 2012; Yergeau et al., 2015). Osmotic shocks may partially cope with HP stressing effects, but their main outcome is to support survival rather than growth, highlighting the lack of proper HP adaptation mechanism in A. borkumensis SK2. Similar responses might be descriptive of other piezosensitive bacteria in the water column, and contribute to shape microbial communities in oil-contaminated deep-sea environments.
Author Contributions
AS conceived the project, designed and performed the experiments, analyzed the data, and wrote the paper. NB conceived, supervised, and funded the project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge Ms. Maria Elena Antinori for her technical assistance. We thank Dr. Jan Arends, Dr. Jo De Vrieze, Dr. Massimo Marzorati, and Dr. Ralph Lindeboom for the fruitful scientific discussion.
Footnotes
Funding. This work was funded by FP-7 project Kill Spill (No. 312139, “Integrated Biotechnological Solutions for Combating Marine Oil Spills”).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00729
References
- Abe F. (2007). Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: perspectives from piezophysiology. Biosci. Biotechnol. Biochem. 71 2347–2357. 10.1271/bbb.70015 [DOI] [PubMed] [Google Scholar]
- Baelum J., Borglin S., Chakraborty R., Fortney J. L., Lamendella R., Mason O. U., et al. (2012). Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 14 2405–2416. 10.1111/j.1462-2920.2012.02780.x [DOI] [PubMed] [Google Scholar]
- Bartlett D. H. (2002). Pressure effects on in vivo microbial processes. Biochim. et Biophys. Act 1595 367–381. 10.1016/S0167-4838(01)00357-0 [DOI] [PubMed] [Google Scholar]
- Benito A., Ventoura G., Casadei M., Robinson T., Mackey B. (1999). Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl. Environ. Microbiol. 65 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard T., Jebbar M., Rassouli Y., Himdi-Kabbab S., Hamelin J., Blanco C. (1993). Ectoine accumulation and osmotic regulation in Brevibacterium linens. J. Gen. Microbiol. 139 129–136. 10.1099/00221287-139-1-129 [DOI] [Google Scholar]
- Bestvater T., Louis P., Galinski E. A. (2008). Heterologous ectoine production in Escherichia coli: by-passing the metabolic bottle-neck. Saline Syst. 4:12 10.1186/1746-1448-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursy J., Pierik A. J., Pica N., Bremer E. (2007). Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 282 31147–31155. 10.1186/1746-1448-4-12 [DOI] [PubMed] [Google Scholar]
- Calderon M. I., Vargas C., Rojo F., Iglesias-Guerra F., Csonka L. N., Ventosa A., et al. (2004). Complex regulation of the synthesis of the compatible solute ectoine in the halophilic bacterium Chromohalobacter salexigens DSM 3043T. Microbiology 150 3051–3063. 10.1074/jbc.M704023200 [DOI] [PubMed] [Google Scholar]
- Camilli R., Reddy C. M., Yoerger D. R., Van Mooy B. A., Jakuba M. V., Kinsey J. C., et al. (2010). Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science 330 201–204. 10.1099/mic.0.27122-0 [DOI] [PubMed] [Google Scholar]
- Cánovas D., Vargas C., Calderón M. I., Ventosa A., Nieto J. J. (1998). Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst. Appl. Microbiol. 21 487–497. 10.1126/science.1195223 [DOI] [PubMed] [Google Scholar]
- Cánovas D., Vargas C., Iglesias-Guerra F., Csonka L. N., Rhodes D., Ventosa A., et al. (1997). Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J. Biol. Chem. 272 25794–25801. 10.1016/S0723-2020(98)80060-X [DOI] [PubMed] [Google Scholar]
- Chintalapati S., Kiran M. D., Shivaji S. (2004). Role of membrane lipid fatty acids in cold adaptation. Cell. Mol. Biol. (Noisy-le-grand) 50 631–642. 10.1074/jbc.272.41.25794 [DOI] [PubMed] [Google Scholar]
- Csonka L. N. (1989). Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53 121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa M. S., Santos H., Galinski E. A. (1998). “An overview of the role and diversity of compatible solutes in Bacteria and Archaea,” in Biotechnology of Extremophiles ed. Antranikian G. (Berlin: Springer; ) 117–153. [DOI] [PubMed] [Google Scholar]
- Davis D., Eisen G. (1973). Bacterial Physiology: Microbiology. Hagerstown, MD: Harper and Row. [Google Scholar]
- De Angelis M., Gobbetti M. (2004). Environmental stress responses in Lactobacillus: a review. Proteomics 4 106–122. 10.1002/pmic.200300497 [DOI] [PubMed] [Google Scholar]
- De Roy K., Clement L., Thas O., Wang Y., Boon N. (2012). Flow cytometry for fast microbial community fingerprinting. Water Res. 46 907–919. 10.1016/j.watres.2011.11.076 [DOI] [PubMed] [Google Scholar]
- Dyksterhouse S. E., Gray J. P., Herwig R. P., Lara J. C., Staley J. T. (1995). Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 45 116–123. 10.1099/00207713-45-1-116 [DOI] [PubMed] [Google Scholar]
- Galinski E. A., Pfeiffer H. P., Trüper H. G. (1985). 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. a novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 149 135–139. 10.1111/j.1432-1033.1985.tb08903.x [DOI] [PubMed] [Google Scholar]
- Göller K., Ofer A., Galinski E. A. (1998). Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol. Lett. 161 293–300. 10.1016/S0378-1097(98)00086-X [DOI] [PubMed] [Google Scholar]
- Gutierrez T., Singleton D. R., Berry D., Yang T., Aitken M. D., Teske A. (2013). Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 7 2091–2104. 10.1038/ismej.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán H., Van-Thuoc D., Martín J., Hatti-Kaul R., Quillaguamán J. (2009). A process for the production of ectoine and poly (3-hydroxybutyrate) by Halomonas boliviensis. Appl. Microbiol. Biotechnol. 84 1069–1077. 10.1007/s00253-009-2036-2 [DOI] [PubMed] [Google Scholar]
- Hara A., Syutsubo K., Harayama S. (2003). Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation. Environ. Microbiol. 5 746–753. 10.1046/j.1468-2920.2003.00468.x [DOI] [PubMed] [Google Scholar]
- Hazen T. C., Dubinsky E. A., DeSantis T. Z., Andersen G. L., Piceno Y. M., Singh N., et al. (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330 204–208. 10.1126/science.1195979 [DOI] [PubMed] [Google Scholar]
- Heipieper H. J., Martinez P. M. (2010). Handbook of Hydrocarbon and Lipid Microbiology. Heidelberg: Springer. [Google Scholar]
- Heremans K. (1982). High pressure effects on proteins and other biomolecules. Annu. Rev. Biophys. Bioeng. 11 1–21. 10.1146/annurev.bb.11.060182.000245 [DOI] [PubMed] [Google Scholar]
- Inbar L., Lapidot A. (1988). The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus. Use of 13C-and 15N-labeled L-glutamate and 13C and 15N NMR spectroscopy. J. Biol. Chem. 263 16014–16022. [PubMed] [Google Scholar]
- Jebbar M., Talibart R., Gloux K., Bernard T., Blanco C. (1992). Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J. Bacteriol. 174 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J. Z., Baross J. A. (2004). Synchronous effects of temperature, hydrostatic pressure, and salinity on growth, phospholipid profiles, and protein patterns of four Halomonas species isolated from deep-sea hydrothermal-vent and sea surface environments. Appl. Environ. Microbiol. 70 6220–6229. 10.1128/AEM.70.10.6220-6229.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes N. E., Callaghan A. V., Aktas D. F., Smith W. L., Sunner J., Golding B., et al. (2013). Metagenomic analysis and metabolite profiling of deep-sea sediments from the Gulf of Mexico following the Deepwater Horizon oil spill. Front. Microbiol. 4:50 10.3389/fmicb.2013.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann A. U., Bursy J., Gimpel S., Hoffmann T., Bremer E. (2008). Synthesis of the compatible solute ectoine in Virgibacillus pantothenticus is triggered by high salinity and low growth temperature. Appl. Environ. Microbiol. 74 4560–4563. 10.1128/AEM.00492-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Liu J. (2013). Evaluating bacterial community structures in oil collected from the sea surface and sediment in the northern Gulf of Mexico after the Deepwater Horizon oil spill. Microbiologyopen 2 492–504. 10.1002/mbo3.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loferer-Krößbacher M., Klima J., Psenner R. (1998). Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl. Environ. Microbiol. 64 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Galinski E. A. (1997). Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143 1141–1149. 10.1099/00221287-143-4-1141 [DOI] [PubMed] [Google Scholar]
- Martin D., Bartlett D. H., Roberts M. F. (2002). Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles 6 507–514. 10.1007/s00792-002-0288-1 [DOI] [PubMed] [Google Scholar]
- Mason O. U., Hazen T. C., Borglin S., Chain P. S., Dubinsky E. A., Fortney J. L., et al. (2012). Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 6 1715–1727. 10.1038/ismej.2012.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Höppner A., Doster W., Vogel R. F., Gänzle M. G. (2004). Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high-pressure treatments. Appl. Environ. Microbiol. 70 2013–2020. 10.1128/AEM.70.4.2013-2020.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Wang Y. B. (2001). Accumulation of ectoine in the halotolerant Brevibacterium sp. JCM 6894. J. Bosci. Bioeng. 91 288–293. 10.1016/S1389-1723(01)80136-5 [DOI] [PubMed] [Google Scholar]
- Oger P. M., Jebbar M. (2010). The many ways of coping with pressure. Res. Microbiol. 161 799–809. 10.1016/j.resmic.2010.09.017 [DOI] [PubMed] [Google Scholar]
- Onraedt A., De Muynck C., Walcarius B., Soetaert W., Vandamme E. (2004). Ectoine accumulation in Brevibacterium epidermis. Biotechnol. Lett. 26 1481–1485. 10.1023/B:BILE.0000044448.86907.e4 [DOI] [PubMed] [Google Scholar]
- Onraedt A. E., Walcarius B. A., Soetaert W. K., Vandamme E. J. (2005). Optimization of ectoine synthesis through fed-batch fermentation of Brevibacterium epidermis. Biotechnol. Prog. 21 1206–1212. 10.1021/bp0500967 [DOI] [PubMed] [Google Scholar]
- Oren A. (1999). Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxen P., Knorr D. (1993). Baroprotective effects of high solute concentrations against inactivation of Rhodotorula rubra. LWT-Food Sci. Technol. 26 220–223. 10.1006/fstl.1993.1048 [DOI] [Google Scholar]
- Pagán R., Mackey B. (2000). Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential-and stationary-phase cells and variation among strains. Appl. Environ. Microbiol. 66 2829–2834. 10.1128/AEM.66.7.2829-2834.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou E., Lopez-Malo A., Barbosa-Canovas G. V., Welti-Chanes J., Swanson B. G. (1997). Effect of water activity on high hydrostatic pressure inhibition of Zygosaccharomyces bailii. Lett. Appl. Microbiol. 24 417–420. [Google Scholar]
- Pflughoeft K. J., Kierek K., Watnick P. I. (2003). Role of ectoine in Vibrio cholerae osmoadaptation. Appl. Environ. Microbiol. 69 5919–5927. 10.1128/AEM.69.10.5919-5927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev R., Peri I., Gilboa H., Avi-Dor Y. (1990). 13C NMR study of the interrelation between synthesis and uptake of compatible solutes in two moderately halophilic eubacteria: bacterium Ba1 and Vibro costicola. Arch. Biochem. Biophys. 278 106–112. 10.1016/0003-9861(90)90237-S [DOI] [PubMed] [Google Scholar]
- Reshetnikov A. S., Khmelenina V. N., Trotsenko Y. A. (2006). Characterization of the ectoine biosynthesis genes of haloalkalotolerant obligate methanotroph Methylomicrobium alcaliphilum 20Z. Arch. Microbiol. 184 286–297. 10.1007/s00203-005-0042-z [DOI] [PubMed] [Google Scholar]
- Sabirova J. S., Ferrer M., Regenhardt D., Timmis K. N., Golyshin P. N. (2006). Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J. Bacteriol. 188 3763–3773. 10.1128/JB.00072-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saum S. H., Müller V. (2008). Growth phase - dependent switch in osmolyte strategy in a moderate halophile: ectoine is a minor osmolyte but major stationary phase solute in Halobacillus halophilus. Environ. Microbiol. 10 716–726. 10.1111/j.1462-2920.2007.01494.x [DOI] [PubMed] [Google Scholar]
- Scheyhing C. H., Hörmann S., Ehrmann M. A., Vogel R. F. (2004). Barotolerance is inducible by preincubation under hydrostatic pressure, cold-, osmotic- and acid- stress conditions in Lactobacillus sanfranciscensis DSM 20451T. Lett. Appl. Microbiol. 39 284–289. 10.1111/j.1472-765X.2004.01578.x [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Landau J. V. (1972). Inhibition of cell-free protein synthesis by hydrostatic pressure. J. Bacteriol. 112 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoma A., Barbato M., Hernandez-Sanabria E., Mapelli F., Daffonchio D., Borin S., et al. (2016). Microbial oil-degradation under mild hydrostatic pressure (10 MPa): which pathways are impacted in piezosensitive hydrocarbonoclastic bacteria? Sci. Rep. 6:23526 10.1038/srep23526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleator R. D., Hill C. (2002). Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26 49–71. 10.1111/j.1574-6976.2002.tb00598.x [DOI] [PubMed] [Google Scholar]
- Syutsubo K., Kishira H., Harayama S. (2001). Development of specific oligonucleotide probes for the identification and in situ detection of hydrocarbon-degrading Alcanivorax strains. Environ. Microbiol. 3 371–379. 10.1046/j.1462-2920.2001.00204.x [DOI] [PubMed] [Google Scholar]
- Valentine D. L., Kessler J. D., Redmond M. C., Mendes S. D., Heintz M. B., Farwell C., et al. (2010). Propane respiration jump-starts microbial response to a deep oil spill. Science 330 208–211. 10.1126/science.1196830 [DOI] [PubMed] [Google Scholar]
- Ventosa A., Nieto J. J., Oren A. (1998). Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62 504–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D. T. (2000). Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 24 263–290. 10.1111/j.1574-6976.2000.tb00542.x [DOI] [PubMed] [Google Scholar]
- Wohlfarth A., Severin J., Galinski E. A. (1990). The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. Microbiology 136 705–712. 10.1099/00221287-136-4-705 [DOI] [Google Scholar]
- Wood J. M. (1999). Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63 230–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimov M. M., Denaro R., Genovese M., Cappello S., D’Auria G., Chernikova T. N., et al. (2005). Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ. Microbiol. 7 1426–1441. 10.1111/j.1462-5822.2005.00829.x [DOI] [PubMed] [Google Scholar]
- Yakimov M. M., Golyshin P. N., Lang S., Moore E. R., Abraham W. R., Lünsdorf H., et al. (1998). Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 48 339–348. 10.1099/00207713-48-2-339 [DOI] [PubMed] [Google Scholar]
- Yancey P. H. (2005). Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208 2819–2830. 10.1242/jeb.01730 [DOI] [PubMed] [Google Scholar]
- Yergeau E., Maynard C., Sanschagrin S., Champagne J., Juck D., Lee K., et al. (2015). Microbial community composition, functions, and activities in the gulf of Mexico 1 year after the deepwater horizon accident. Appl. Environ. Microbiol. 81 5855–5866. 10.1128/AEM.01470-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. H., Lang Y. J., Nagata S. (2009). Efficient production of ectoine using ectoine-excreting strain. Extremophiles 13 717–724. 10.1007/s00792-009-0262-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


