Abstract
Objective. To determine whether immunological serum markers IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10 are elevated or decreased in patients compared with healthy controls. Methods. A complete search of the literature on this topic within the past 30 years was conducted across seven databases. Seventeen studies including 768 individuals were identified. Differences in serum marker levels between subjects and controls were pooled as MDs using the random-effects model. Results. The pooled MDs were higher in patients than in healthy controls for IFN-γ (MD 24.9, 95% CI 12.36–37.43), IL-17 (MD 28.92, 95% CI 17.44–40.40), IL-23 (MD 310.60, 95% CI 4.96–616.24), and TNF-α (MD 19.84, 95% CI 13.80–25.87). Pooled IL-4 (MD −13.5, 95% CI −17.74–−9.26) and IL-10 (MD −10.33, 95% CI −12.03–−8.63) levels were lower in patients. Conclusion. The pooled analyses suggest that levels of IFN-γ, IL-17, IL-23, and TNF-α are significantly elevated and that levels of IL-4 and IL-10 are significantly decreased in sera of patients with psoriasis vulgaris of blood-heat syndrome. Measuring progression of blood-heat syndrome of psoriasis vulgaris will require additional high-quality data, with a low risk of bias and adequate sample sizes, before and after antipsoriatic therapy.
1. Introduction
Psoriasis is a chronic immune-mediated skin disease that affects approximately 2–4% of the population in Western countries [1]. Patients with psoriasis may present with the pustular, guttate, arthritic, or erythrodermic variants and may have itching or painful lesions that negatively affect the quality of life. The understanding of the pathogenesis of psoriasis has improved, as has the understanding of the cellular components (mainly keratinocytes and T lymphocytes) involved in psoriasis, and the cytokines produced by the main Th lymphocyte subsets are now known to play a decisive role in pathogenesis [2]. Current evidence suggests that psoriasis is a T-cell-mediated disease driven at least in part by a positive feedback loop from activated T-cells to antigen-presenting cells (APCs) that is mediated by IFN-γ, IL-1, and tumor necrosis factor-α (TNF-α) [3, 4]. It has been shown that the Th1-Th2-Th17 balance is likely a key functional and genetic determinant of psoriasis [4].
However, current treatments remain unsatisfactory and burdensome, and they often do not meet patients' expectations [5]; thus, therapies collectively called alternative therapies are commonly used. One observational study revealed that 51% of patients with psoriasis opted to use alternative therapies [6]. Traditional Chinese medicine (TCM), one type of alternative therapy, has been used to treat human diseases for more than 2000 years in China. Over the course of history, physicians practicing TCM have accumulated a tremendous amount of knowledge and experience in treating psoriasis. TCM prescribes treatment for psoriasis vulgaris based on syndrome differentiation. According to TCM, the syndromes can be divided into three main categories: blood-heat syndrome, blood-stasis syndrome, and blood-dryness syndrome. Correspondingly, clearing heat and cooling blood, promoting blood circulation to dissipate blood stasis, and adding moisture to reduce blood dryness comprise the treatment principles for the three syndromes of psoriasis vulgaris [7, 8]. The distribution of the three syndromes has been shown to be closely correlated with disease stage: the blood-heat syndrome is the most common at the active stage; the blood-dryness syndrome is the most common at the resting and regressive stages; and the blood-stasis syndrome is the most common at the resting stage [9]. Several investigators have searched for immunological markers of blood-heat syndrome, not only in skin lesions, but also in the circulatory system, using them to measure disease severity or to quantify treatment response. However, the data on serum levels of immunological markers in patients compared with controls are contradictory; some authors have reported elevated levels, whereas others have reported conflicting results. The studies to date have used small sample sizes or have investigated different markers to assess immune status; moreover, measurement of immunological serum markers is often not their primary objective.
We performed a systematic review and meta-analysis to determine whether seven well-known immunological serum markers (IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10) are elevated or decreased in patients with psoriasis vulgaris of blood-heat syndrome compared with controls.
2. Materials and Methods
2.1. Eligibility Criteria
Inclusion and exclusion criteria were determined before the search was conducted. We included human studies comparing patients with psoriasis vulgaris of blood-heat syndrome with healthy controls, in which one or more of the following immunological markers was measured in the serum: IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10. If several studies reported results from the same study population, the most complete report was included. Case reports and letters were excluded.
2.2. Data Sources and Searches
To identify relevant psoriasis vulgaris of blood-heat syndrome studies that included immunological markers, three reviewers (X. L., Q. Q. X, and F. L. L.) systematically searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure database (CNKI), Chinese Scientific Journals Full-Text Database (CQVIP), Wanfang Data Knowledge Service Platform, and Chinese Biomedical Literature Service System (SINOMED). Papers published in English or Chinese and dated from January 1980 to May 2015 were included in this study.
The main descriptors adopted in the search strategy for primary studies were psoriasis, blood-heat syndrome, IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10.
The search strategy adopted in the MEDLINE database via PubMed, which was adapted for the other databases analyzed, is presented as follows: psoriasis[tw] AND blood-heat syndrome[tw] AND (interferon-γ[tw] OR IFN-gamma[tw] OR IFN-γ[tw] OR interleukin-4[tw] OR il-4[tw] OR interleukin-17[tw] OR il-17[tw] OR interleukin-23[tw] OR il-23[tw] OR interleukin-6[tw] OR il-6[tw] OR tumor necrosis factor∗[tw] OR tnf[tw] OR interleukin-10[tw] OR il-10[tw]) NOT (animals[mesh] NOT humans[mesh]) NOT (case reports[pt] OR letter[pt]).
2.3. Study Selection
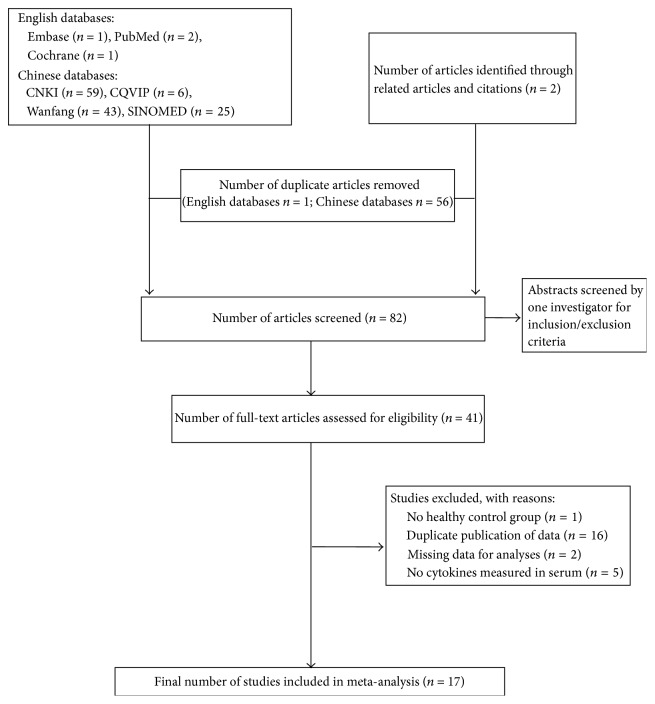
To determine eligibility for inclusion in the review, we screened all titles and abstracts for the following criteria: analyses comparing immunological marker profiles of patients with psoriasis vulgaris of blood-heat syndrome with those of control groups. There were no limitations on the study design, participant's age, gender, or nationality. The selection criteria for inclusion were as follows: (i) human-only studies; (ii) original data; (iii) a healthy control group; and (iv) provision of means and confidence intervals (CIs) for immunological serum markers. We identified 138 articles in the initial search (Figure 1). Through manual review of the citations from these articles, we identified 2 additional articles. After removing 57 duplicate articles and reading 82 individual abstracts, we identified 41 original studies that were eligible for inclusion criteria assessment. After reviewing the full text of these 48 studies, we excluded 24 articles for the following reasons: no healthy control group, duplicate publication of data, missing data from analyses, and no cytokines measured in serum. In the end, we selected 17 studies that met the inclusion criteria for this systematic review [10–26]. A flowchart of the search process is presented in Figure 1.
Figure 1.
Flowchart of literature search and study selection.
2.4. Data Extraction and Quality Assessment
Three reviewers independently collected descriptive data for each included study: (i) the first author; (ii) study characteristics (i.e., year, duration, country, and setting); (iii) characteristics of participants (i.e., mean age, male-to-female ratio, numbers of case and control subjects, duration, and mean PASI of cases); and (iv) outcome characteristics (i.e., the mean of immunological serum markers of psoriasis vulgaris blood-heat syndrome along with the standard deviation (SD) and whether results were from primary analysis of the study or were adjusted for comorbidities).
The Newcastle-Ottawa Scale [27] was used to assess study quality. It categorizes studies by three dimensions including selection, comparability, and exposure for case-control studies and selection, comparability, and outcome for cohort studies. Selection included four items, comparability included one item, and exposure included three items.
2.5. Data Synthesis and Analysis
The primary outcome was the identification of differences in mean serum levels of immunological markers between patients with psoriasis vulgaris of blood-heat syndrome and healthy controls for each study (Table 2). The degree of heterogeneity between studies was assessed using the I 2 test. An I 2 value > 50% was considered to indicate substantial heterogeneity. In this case, DerSimonian and Laird random-effects models were considered to compute the global MD. The between-study heterogeneity was not substantial (I 2 < 50%) and the fixed-effect model was suitable. Publication bias was investigated graphically by using funnel plots and was statistically assessed via Egger's regression. The methods and findings of the present review have been reported following the Meta-Analysis of Observational Studies in Epidemiology group guidelines and checklist [28]. The Cochrane Collaboration Software Review Manager 5.2 was used for meta-analysis (http://ims.cochrane.org/revman). Egger's regression was performed using STATA version 10.0 (STATA Corp., College Station, TX, USA).
Table 2.
Summary of immunological markers of blood-heat syndrome.
| Source | Markers used ELISA measurement methods, mean (SD) | ||||||
|---|---|---|---|---|---|---|---|
| IFN-γ (pg/mL) | IL-4 (pg/mL) | IL-17 (pg/mL) | IL-23 (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) | IL-10 (pg/mL) | |
| Cao et al. [10] | Control 47.44 (21.46); psoriasis 91.20 (48.37) |
Control 47.41 (14.26); psoriasis 32.73 (7.90) |
Control 49.15 (31.18); psoriasis 60.30 (48.08) |
||||
|
| |||||||
| Chen et al. [11] | Control 46.00 (0.67); psoriasis 86.48 (46.44) |
Control 48.45 (13.63); psoriasis 32.52 (8.13) |
|||||
|
| |||||||
| Chen and Yang [23] | Control 20.70 (13.20); psoriasis 81.40 (15.10) |
Control 201.00 (78.00); psoriasis 529.00 (202.00) |
|||||
|
| |||||||
| Fan et al. [12] | Control 2.85 (0.64); psoriasis 7.32 (2.01) |
Control 814.93 (372.51); psoriasis 1491.80 (464.36) |
Control 109.32 (7.22); psoriasis 418.05 (108.83) |
||||
|
| |||||||
| He et al. [13] | Control 30.07 (3.36); psoriasis 11.29 (3.70) |
Control 7.40 (1.56); psoriasis 30.58 (7.14) |
|||||
|
| |||||||
| Hu and Yang [24] | Control 8.72 (2.04); psoriasis 24.10 (8.13) |
Control 30.21 (3.40); psoriasis 19.89 (1.19) |
Control 74.30 (24.14); psoriasis 130.26 (21.99) |
||||
|
| |||||||
| Li [14] | Control 69.16 (29.99); psoriasis 98.68 (49.65) |
Control 86.65 (41.26); psoriasis 131.24 (41.24) |
Control 85.73 (27.96); psoriasis 105.60 (35.27) |
||||
|
| |||||||
| Li [15] | Control 6.43 (0.33); psoriasis 15.29 (0.49) |
Control 18.25 (1.44); psoriasis 6.51 (0.97) |
|||||
|
| |||||||
| Lin et al. [16] | Control 8.72 (2.06); psoriasis 24.10 (7.84) |
Control 21.27 (4.80); psoriasis 8.59 (4.97) |
|||||
|
| |||||||
| Liu [17] | Control 10.86 (5.41); psoriasis 14.19 (6.84) |
Control 67.65 (17.01); psoriasis 63.69 (20.23) |
Control 46.89 (9.13); psoriasis 55.43 (13.76) |
Control 24.39 (7.57); psoriasis 16.84 (6.74) |
|||
|
| |||||||
| Liu et al. [18] | Control 6.24 (1.66); psoriasis 23.37 (2.40) |
Control 6.95 (0.83); psoriasis 23.12 (1.89) |
|||||
|
| |||||||
| Sun and Zho [19] | Control 8.63 (0.88); psoriasis 11.37 (2.80) |
Control 21.65 (1.53); psoriasis 39.44 (6.11) |
|||||
|
| |||||||
| Wu et al. [25] | Control 46.69 (11.43); psoriasis 114.60 (19.78) |
Control 24.39 (2.27); psoriasis 14.14 (1.62) |
|||||
|
| |||||||
| Zhang [20] | Control 7.37 (0.85); psoriasis 26.67 (17.78) |
||||||
|
| |||||||
| Zhang et al. [21] | Control 8.59 (2.59); psoriasis 11.50 (4.48) |
Control 78.20 (43.00); psoriasis 239.06 (159.11) |
Control 6.5 (3.1); psoriasis 12.8 (7.4) |
||||
|
| |||||||
| Zhou [22] | Control 71.43 (45.90); psoriasis 244.41 (133.25) |
Control 5.9 (3.4); psoriasis 11.3 (7.8) |
|||||
|
| |||||||
| Zhou and Wang [26] | Control 33.34 (4.50); psoriasis 96.14 (12.29) |
Control 25.91 (3.69); psoriasis 12.88 (2.66) |
Control 8.09 (1.06); psoriasis 32.64 (6.23) |
Control 21.96 (3.16); psoriasis 12.77 (2.70) |
|||
ELISA, enzyme-linked immunosorbent assay; SD, standard deviation; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
3. Results
3.1. Study Selection
Of a total of 139 titles, the full text of 41 potentially relevant studies was reviewed to confirm their eligibility. Among these 41 studies, 24 were excluded, including one with no healthy control group, 23 with duplicate publication of data, two with missing data for analyses, and five with no measurement of cytokines in serum. In total, 17 trials met the inclusion criteria (Figure 1).
3.2. Study Characteristics
The 17 selected studies included data on 768 individuals (443 patients with psoriasis vulgaris of blood-heat syndrome and 325 healthy controls). All of these seventeen studies were conducted in China; sixteen were published in Chinese and one was published in English. The age and male-to-female ratios of patients with psoriasis vulgaris of blood-heat syndrome and healthy controls were comparable (Table 1). In total, 59% of the studies reported a Psoriasis Area and Severity Index (PASI). Of these, 90% of the patients were from studies reporting a mean PASI > 10, indicating that the majority of the studies included patients with severe disease. The reported Newcastle-Ottawa Scale scores were between 3 and 7, as shown in Table 1. Specifically, fifteen of the studies were deemed as medium-quality (4 to 6 stars), one [20] was deemed as poor-quality (<4 stars), and another [26] was deemed as high-quality (7 or >7 stars).
Table 1.
Characteristics of the included studies and NOS quality assessment.
|
Author (pub. year) |
Study setting | Study period MM/YY–MM/YY | Psoriasis, blood heat syndrome | Healthy control | NOS quality assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean age, years, mean (SD) | Males (%) | Mean PASI (SD) | Duration, years, mean (SD) | N | Mean age, years, mean (SD) | Males (%) | Selection | Comparability | Exposure/outcome | Overall star rating |
|||
| Cao et al. (2014) [10] | China | 07/2011–04/2012 | 16 | 46.36 (13.04) | 63.33 | 10.35 (5.84) | NR | 16 | 27.31 (1.40) | 56.67 | ++ | +++ | 5 | |
| Chen et al. (2014) [11] | China | 09/2011–04/2012 | 15 | 46.20 (13.97) | 53.33 | 15.8 (8.60) | 3.26 (2.98) | 16 | 42.31 (13.61) | 53.33 | + | ++ | +++ | 6 |
| Chen and Yang (2012) [23] | China | 01/2010–12/2010 | 17 | 31.00 (1.50) | 52.94 | 24.21 (7.03) | 7–10 | 15 | 30.00 (0.50) | 53.33 | + | ++ | +++ | 6 |
| Fan et al. (2015) [12] | China | 11/2010–10/2011 | 30 | 37.50 (7.50) | 53.33 | 11.05 (9.10) | 0–30 | 10 | 40.29 (9.91) | 70.00 | + | ++ | +++ | 6 |
| He et al. (2015) [13] | China | 09/2012–09/2013 | 40 | 35.92 (12.91) | 62.50 | 18.619 (3.403) | 5.53 (5.25) | 20 | 33.30 (10.96) | 60.00 | + | ++ | +++ | 6 |
| Hu and Yang (2015) [24] | China | NR | 30 | NR | 54.44 | NR | NR | 30 | Match | 50.00 | + | ++ | +++ | 6 |
| Li (2010) [14] | China | 03/2009–03/2010 | 30 | 41.59 (14.73) | 70.59 | 10.99 (11.62) | NR | 18 | NR | NR | + | +++ | 4 | |
| Li (2011) [15] | China | 04/2009–08/2010 | 24 | NR | NR | NR | NR | 20 | Match | Match | ++ | +++ | 5 | |
| Lin et al. (2008) [16] | China | 10/2005–03/2006 | 30 | 33.13 (11.10) | 70.00 | 10.78 (3.51) | 10.47 (9.02) | 20 | Match | Match | + | ++ | +++ | 6 |
| Liu (2011) [17] | China | 10/2009–10/2010 | 20 | 42.45 (13.55) | 65.00 | 12.80 (4.30) | 11.98 (10.23) | 20 | 37.05 (14.16) | 40.00 | ++ | +++ | 5 | |
| Liu et al. (2012) [18] | China | 03/2010–12/2010 | 20 | 34.11 | 60.00 | NR | NR | 10 | 30.50 | 50.00 | ++ | +++ | 5 | |
| Sun and Zhao (2010) [19] | China | 10/2008–02/2010 | 30 | 33.00 (13.57) | 43.33 | 21.42 (3.06) | 6.70 (5.24) | 18 | 31.72 (12.06) | 44.44 | + | ++ | +++ | 6 |
| Wu et al. (2013) [25] | China | NR | 30 | NR | NR | NR | NR | 30 | NR | NR | + | +++ | 4 | |
| Zhang (2003) [20] | China | 05/2001–01/2003 | 20 | NR | NR | NR | NR | 10 | NR | NR | +++ | 3 | ||
| Zhang et al. (2010) [21] | China | 06/2006–05/2009 | 47 | Match | 56.00 | NR | 16.67–34 | 31 | 41.50 (9.20) | 67.742 | ++ | +++ | 5 | |
| Zhou (2007) [22] | China | 06/2006–03/2007 | 14 | Match | Match | NR | NR | 31 | 41.50 | 64.50 | ++ | +++ | 5 | |
| Zhou and Wang (2011) [26] | China | 10/2009–04/2011 | 30 | 32.21 (8.33) | 70.00 | 8.92 (2.07) | 16.67–32 | 10 | Match | Match | ++ | ++ | +++ | 7 |
PASI, Psoriasis Area and Severity Index; NR, not reported; NOS, Newcastle-Ottawa Scale; NOS quality assessment: a star system was used to allow a semiquantitative assessment of study quality. A study could be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars could be awarded for comparability. The NOS ranges from zero to nine stars. We consider those that achieve seven or more stars as high-quality studies, those that achieve four to six stars as medium-quality studies, and those that achieve fewer than four stars as poor-quality studies.
3.3. Outcomes
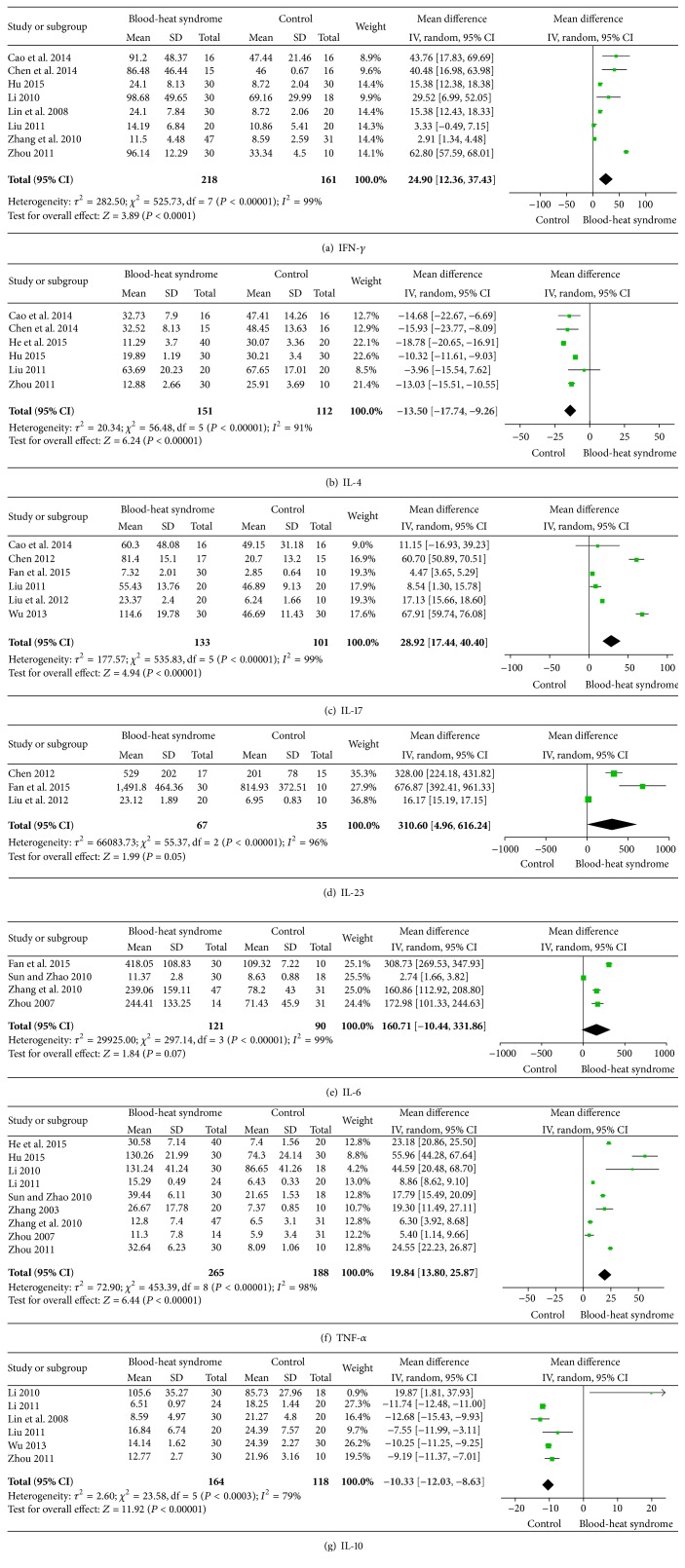
Meta-analysis of the results for all markers, including IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10, for subjects with psoriasis vulgaris of blood-heat syndrome revealed significant between-study heterogeneity (I 2 > 75%) with random-effects modeling. The MD for studies analyzing IFN-γ was 24.9 (95% CI 12.36–37.43), indicating a significant difference in serum IFN-γ between the 218 patients with psoriasis vulgaris of blood-heat syndrome and 161 controls (Figure 2(a)). Six studies reported plasma IL-4 levels, in 151 patients and 112 controls. Figure 2(b) shows that a significantly lower level of IL-4 was observed in patients with psoriasis vulgaris of blood-heat syndrome, with a pooled MD of −13.5 (95% CI −17.74–−9.26). Six studies (including 133 patients and 101 controls) showed an elevated MD for IL-17 (28.92, 95% CI 17.44–40.40) (Figure 2(c)). The mean IL-23 across studies was significantly elevated in the 67 patients compared with the 35 controls (MD 310.60; 95% CI 4.96–616.24) (Figure 2(d)). Pooling of IL-6 levels resulted in a small, positive but not statistically significant MD between 121 patients with psoriasis vulgaris of blood-heat syndrome and 90 healthy controls (160.71; 95% CI −10.44–331.86) (Figure 2(e)). In total, nine articles including 265 patients and 188 controls showed a significantly elevated MD for TNF-α between psoriasis and controls (MD 19.84, 95% CI 13.80–25.87) (Figure 2(f)). The combined MD for IL-10 was decreased in 164 patients compared with 118 controls (MD −10.33, 95% CI −12.03–−8.63) (Figure 2(g)).
Figure 2.
Meta-analysis of serum IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10 levels in patients with psoriasis vulgaris of blood-heat syndrome. The mean difference (MD) in IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10 levels of patients with psoriasis compared with controls. The point estimate (center of each green square) and statistical size (proportional area of the square) are represented. Horizontal lines indicate 95% confidence intervals. The subtotal and total MDs (diamonds) were calculated using random-effects models.
3.4. Assessment of Publication Bias
The funnel plots for IFN-γ, IL-4, IL-17, IL-23, IL-6, TNF-α, and IL-10 showed evidence of asymmetry (Figure S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/9503652; see MOOSE checklist).
Egger's test confirmed the presence of publication bias for IFN-γ (9.29, 95% CI 2.30–16.28), IL-4 (−17.74, 95% CI −28.06–−7.42), IL-17 (10.90, 95% CI 2.53–19.28), IL-23 (8.54, 95% CI 1.68–15.42), TNF-α (9.78, 95% CI 5.74–13.83), and IL-10 (−14.61, 95% CI −23.40–−5.81). There appeared to be no publication bias for IL-6 (7.06, 95% CI −1.88–16.01).
4. Discussion
4.1. Summary of Evidence
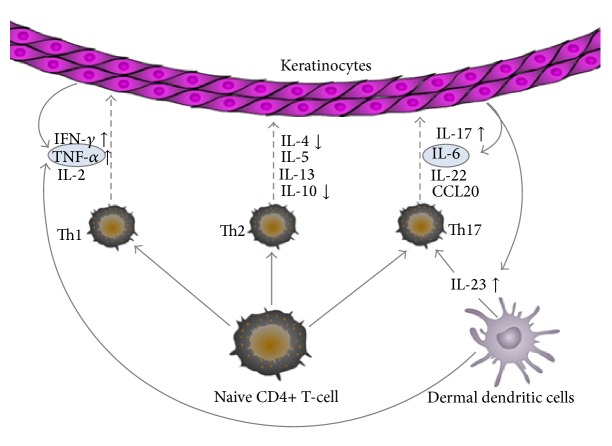
This review involved a systematic assessment of mainly Chinese-sourced studies reporting immunological serum markers in patients with psoriasis vulgaris of blood-heat syndrome compared to healthy controls; 17 studies were identified for systematic review and meta-analysis. Although most studies used small sample sizes, analysis of the pooled data showed an elevated MD of serum IFN-γ, IL-17, IL-23, and TNF-α in patients with psoriasis vulgaris of blood-heat syndrome, when compared to the control groups. Pooled IL-4 and IL-10 levels were significantly lower in patients than in controls, but pooled IL-6 levels were not significantly elevated. A simplified model depicting the role of the immunological markers of blood-heat syndrome is presented in Figure 3.
Figure 3.
A simplified model depicting the role of the immunological markers with psoriasis vulgaris of blood-heat syndrome in this meta-analysis.
4.2. Possible Rationales
According to TCM, patients with psoriasis vulgaris present with one of three syndromes: blood-heat syndrome (53.8%), blood-dryness syndrome (27.4%), or blood-stasis syndrome (18.1%) [9]. At the initiation of the active stage, psoriasis vulgaris usually manifests as blood-heat syndrome; later it may be ameliorated or be converted to blood-dryness/blood-stasis syndrome. The clinical efficacy of TCM, including internal and external applications, in treating psoriasis vulgaris of blood-heat syndrome has been confirmed by a number of previous studies [29–32]. Specifically, the treatment principles of clearing heat and cooling blood are believed to have a positive influence on various pathogenic mechanisms observed in psoriasis because of their anti-inflammatory and antiangiogenic effects, as well as their potential to adjust the Th1/Th2 equilibrium and to change the cytokine balance [15, 26].
Each T-cell subset produces distinct cytokine expression profiles that influence cell fate specification. These different cytokines, released within an inflammatory context, may contribute to psoriasis susceptibility and pathogenesis. Th1 cells develop in the presence of IL-12 and mainly produce IFN-γ, IL-2, and lymphotoxin. Th2 cells differentiate in the presence of IL-4 and produce IL-4, IL-5, and IL-13. In the presence of IL-6 and IL-23, Th17 cells are characterized by their capacity to generate cytokines such as IL-6, IL-17, IL-22, and CCL20 [33, 34]. It has been shown that serum levels of IFN-γ are much higher in patients with psoriasis than in controls and were correlated with the PASI score (psoriasis activity and severity index), whereas levels of Th2 cytokines (IL-4 and IL-10) were reported to be lower [35, 36]. An important cytokine that is generated by Th17 cells, IL-17F, shows significantly higher mRNA expression in lesional skin, and serum levels of the IL-17F protein were substantially increased in a psoriasis(-like) mouse model as well [37–39]. The cytokines IL-6 and TNF-α, which are produced by keratinocytes, play an important role in the activation of innate immunity through activation of dendritic and T-cells [40].
4.3. Limitations of This Review
Our analysis has some limitations. First, we were unable to measure the progression of psoriasis vulgaris of blood-heat syndrome using serum markers in pretherapeutic patients, and only three/four eligible observation studies that reported serum IL-23/IL-6 levels were reviewed. We used two different methods to assess publication bias, and, based on the method used, we found some extent of publication bias. The distorting effects of publication and location bias on systematic reviews and meta-analyses have been well documented [41]. Although we are confident that our search strategy enabled us to locate all relevant studies, a certain degree of uncertainty nevertheless remains. Furthermore, the 17 included studies were mainly observational and consisted of small numbers of patients with psoriasis vulgaris of blood-heat syndrome; more high-quality studies, with a low risk of bias and adequate sample sizes, are required to fully clarify the effects.
5. Conclusion
In summary, patients with psoriasis vulgaris of blood-heat syndrome show significantly elevated levels of IFN-γ, IL-17, IL-23, and TNF-α and decreased levels of IL-4 and IL-10. To investigate the clinical relevance of these findings, a review summarizing the evidence on the effect of clearing heat and cooling blood therapy on markers of immunology would be useful.
Supplementary Material
The methods and findings of the present review have been reported following the MOOSE Checklist. Funnel plots identifying publication bias for all studied outcomes were included in this MOOSE Checklist.
Acknowledgments
This study was supported by a grant from the National Science Foundation (NSFC) of China (81273764 and 81473682 to Bin Li and 81302971 to Xin Li). It was also supported by grants from the Shanghai Science and Technology Committee (nos. 12401903500 and 14401970200 to Bin Li and 14401972703 and 16QA1403800 to Xin Li) and the Shanghai Health Bureau Project (2010Y133, 2011XY004, XYQ2013073, ZY3-CCCX-1-1008, and ZY3-CCCX-3-3033).
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Xin Li and Qing-qing Xiao are equal contributors.
References
- 1.Kurd S. K., Gelfand J. M. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. Journal of the American Academy of Dermatology. 2009;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perera G. K., Di Meglio P., Nestle F. O. Psoriasis. Annual Review of Pathology: Mechanisms of Disease. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 3.Büchau A. S., Gallo R. L. Innate immunity and antimicrobial defense systems in psoriasis. Clinics in Dermatology. 2007;25(6):616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder J. T., Bruce A. T., Gudjonsson J. E., et al. Molecular dissection of psoriasis: integrating genetics and biology. Journal of Investigative Dermatology. 2010;130(5):1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 5.Bartosińska J. P., Pietrzak A., Szepietowski J., Dreiher J., Maciejewski R., Chodorowska G. Traditional Chinese medicine herbs—are they safe for psoriatic patients? Folia Histochemica et Cytobiologica. 2011;49(2):201–205. doi: 10.5603/fhc.2011.0027. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer A. B., Jr., Feldman S. R., Rapp S. R., Reboussin D. M., Exum M. L., Clark A. R. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58(3):216–220. [PubMed] [Google Scholar]
- 7.Koo J., Arain S. Traditional chinese medicine for the treatment of dermatologic disorders. Archives of Dermatology. 1998;134(11):1388–1393. doi: 10.1001/archderm.134.11.1388. [DOI] [PubMed] [Google Scholar]
- 8.Tse T. W. Use of common Chinese herbs in the treatment of psoriasis. Clinical and Experimental Dermatology. 2003;28(5):469–475. doi: 10.1046/j.1365-2230.2003.01322.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G.-Z., Wang J.-S., Wang P., et al. Distribution and development of the TCM syndromes in psoriasis vulgaris. Journal of Traditional Chinese Medicine. 2009;29(3):195–200. doi: 10.1016/S0254-6272(09)60064-9. [DOI] [PubMed] [Google Scholar]
- 10.Cao X. X., Xu R., Li F. L., et al. The expression of Th1/Th2/Th17 in peripheral blood of the patients with blood-heat syndrome of psoriasis. China Journal of Leprosy and Skin Diseases. 2014;30(9):524–526. [Google Scholar]
- 11.Chen J., Cao X.-X., Xu R., et al. Research on different expressions of peripheral blood Th1/Th2 cells in psoriasis patients of blood heat syndrome and of blood stasis syndrome. Chinese Journal of Integrated Traditional and Western Medicine. 2014;34(1):46–50. [PubMed] [Google Scholar]
- 12.Fan B., Li X., Ze K., et al. Expression of T-helper 17 cells and signal transducers in patients with psoriasis vulgaris of blood-heat syndrome and blood-stasis syndrome. Chinese Journal of Integrative Medicine. 2015;21(1):10–16. doi: 10.1007/s11655-014-1769-7. [DOI] [PubMed] [Google Scholar]
- 13.He S. M., Zhang H. Y., Liu T. F., et al. Observation the efficacy of Qingre Liangxue Jiedu Decoction in the treatment of psoriasis vulgaris and the influence to TNF-alpha and IL-4 in peripheral blood. Clinical Journal of Traditional Chinese Medicine. 2015;27(1):68–71. [Google Scholar]
- 14.Li J. W. Evaluation of the clinical efficacy and mechanism of of Liangxue Qianyang therapy in patients with blood heat type psoriasis vulgaris [Ph.D. thesis] Shanghai University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 15.Li X. R. Effect of Qingre Lishi Yin in treatment of psoriasis patients of blood-heat syndrome type and its impact on peripheral Th1/Th2 equilibrium [M.S. thesis] Shandong University of Traditional Chinese Medicine; 2011. [Google Scholar]
- 16.Lin Y., Sun H., Liu Y. P., Zou Y. D. Effects of Xiaoyin decoction on the serum levels of interferon-γ and interleukin-10 in patients with psoriasis vulgaris. Chinese Journal of Dermatovenereology of Integrated Traditional and Western Medicine. 2008;7(2):76–79. [Google Scholar]
- 17.Liu J. Effects of the TCM blood-regulating method on cytokine Th1, Th2 & Th17 of common psoriasis patients in different periods [M.S. thesis] Shanghai University of Traditional Chinese Medicine; 2011. [Google Scholar]
- 18.Liu T. F., Zhang H. Y., Liu X. P., et al. Ze Qi granule treatment on diffentent TCM syndrome types of PV patients effect on IL-23/Th 17 cell axis. The Chinese Journal of Dermatovenereology. 2012;26(5):442–444. [Google Scholar]
- 19.Sun H., Zhao F. Q. Clinical effect of Xiaoyin mixture on treating psoriasis vulgaris and detection of correlative serum cytokines. Chinese Journal of Dermatovenereology of Integrated Traditional and Western Medicine. 2010;9(6):358–360. [Google Scholar]
- 20.Zhang C. H. Clinical study on TULing decoction in treating acute psoriasis vulgaris and detection of TNF-α and IL-8 [M.S. thesis] Jinan, China: Shandong University of Traditional Chinese Medicine; 2003. [Google Scholar]
- 21.Zhang L., Liu X., Wang L. H., Zhao J. X., Li P., Wang J. S. Effect of blood-treating prescriptions on serum IFN-γ, IL-6, and TNF-α of the psoriasis patients with different TCM syndromes. Journal of Traditional Chinese Medicine. 2010;51(12):1083–1085, 1092. [Google Scholar]
- 22.Zhou D. M. Clinical Research of Treatment of Patients with Psoriasis Vulgaris Based on Blood Syndrome Differentiation. Beijing University of Chinese Medicine; 2007. [Google Scholar]
- 23.Chen J. G., Yang Z. B. Studies on interleukin-17 and interleukin-23 in treatment of patients with psoriasis vulgaris based on blood syndrome differentiation. Chinese Journal of Dermatology. 2012;45(2):140–141. [Google Scholar]
- 24.Hu X. Y., Yang W. X. The correlation between serum TNF-α, IFN-γ and IL-4 and the syndrome of Traditional Chinese Medicine in psoriasis vulgaris. Journal of Military Surgeon in in Southwest China. 2015;17(2):156–158. [Google Scholar]
- 25.Wu J., Wang W. Q., Wu A. W. Research on the imbalance of Th17/Treg cells in psoriasis patients of blood heat syndrome and blood stasis syndrome. China Journal of Leprosy and Skin Diseases. 2013;29(7):446–448. [Google Scholar]
- 26.Zhou G. J., Wang W. Q. Evaluation of the clinical efficacy of Xiaoyin decoction in the treatment of the patients with blood-heat syndrome of psoriasis and its influence on Th1/Th2 in peripheral blood. Fujian Journal of Traditional Chinese Medicine. 2011;42(6):1–3. [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. The Journal of the American Medical Association. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L.-X., Bai Y.-P., Song P.-H., You L.-P., Yang D.-Q. Effect of Chinese herbal medicine combined with acitretin capsule in treating psoriasis of blood-heat syndrome type. Chinese Journal of Integrative Medicine. 2009;15(2):141–144. doi: 10.1007/s11655-009-0145-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhou N., Bai Y. P., Man X. H., et al. Effect of new Pulian Ointment (sic) in treating psoriasis of blood-heat syndrome: a randomized controlled trial. Chinese Journal of Integrative Medicine. 2009;15(6):409–414. doi: 10.1007/s11655-009-0409-0. [DOI] [PubMed] [Google Scholar]
- 31.Qiu S., Tan S., Zhang J., Liu P., Ran L., Lei X. Effect of Liangxue Huoxue Tang on serum levels of TNF-α, IFN-γ and IL-6 in psoriasis of blood-heat type. Journal of Traditional Chinese Medicine. 2005;25(4):292–295. [PubMed] [Google Scholar]
- 32.Dai Y.-J., Li Y.-Y., Zeng H.-M., et al. Effect of Yinxieling decoction on PASI, TNF-α and IL-8 in patients with psoriasis vulgaris. Asian Pacific Journal of Tropical Medicine. 2014;7(8):668–670. doi: 10.1016/s1995-7645(14)60113-9. [DOI] [PubMed] [Google Scholar]
- 33.Ghoreschi K., Laurence A., Yang X.-P., Hirahara K., O'Shea J. J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends in Immunology. 2011;32(9):395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Elios M. M., Del Prete G., Amedei A. Targeting IL-23 in human diseases. Expert Opinion on Therapeutic Targets. 2010;14(7):759–774. doi: 10.1517/14728222.2010.497143. [DOI] [PubMed] [Google Scholar]
- 35.Ekman A.-K., Sigurdardottir G., Carlström M., Kartul N., Jenmalm M. C., Enerbäck C. Systemically elevated Th1-, Th2- and Th17-associated chemokines in psoriasis vulgaris before and after ultraviolet B treatment. Acta Dermato-Venereologica. 2013;93(5):527–531. doi: 10.2340/00015555-1545. [DOI] [PubMed] [Google Scholar]
- 36.Schlaak J. F., Buslau M., Jochum W., et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. Journal of Investigative Dermatology. 1994;102(2):145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 37.Lowes M. A., Kikuchi T., Fuentes-Duculan J., et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. Journal of Investigative Dermatology. 2008;128(5):1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 38.Ma H.-L., Liang S., Li J., et al. IL-22 is required for Th17 cell–mediated pathology in a mouse model of psoriasis-like skin inflammation. The Journal of Clinical Investigation. 2008;118(2):597–607. doi: 10.1172/jci33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson N. J., Boniface K., Chan J. R., et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunology. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 40.Nestle F. O., Kaplan D. H., Barker J. Mechanisms of disease: Psoriasis. New England Journal of Medicine. 2009;361(5):444–509. doi: 10.1056/nejmra0804595. [DOI] [PubMed] [Google Scholar]
- 41.Egger M., Davey Smith G. Meta-analysis: bias in location and selection of studies. British Medical Journal. 1998;316(7124):61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The methods and findings of the present review have been reported following the MOOSE Checklist. Funnel plots identifying publication bias for all studied outcomes were included in this MOOSE Checklist.