Abstract
Stroke is an important health issue corresponding to the second cause of mortality and first cause of severe disability with no effective treatments after the first hours of onset. Regenerative approaches such as cell therapy provide an increase in endogenous brain structural plasticity but they are not enough to promote a complete recovery. Tissue engineering has recently aroused a major interesting development of biomaterials for use into the central nervous system. Many biomaterials have been engineered based on natural compounds, synthetic compounds, or a mix of both with the aim of providing polymers with specific properties. The mechanical properties of biomaterials can be exquisitely regulated forming polymers with different stiffness, modifiable physical state that polymerizes in situ, or small particles encapsulating cells or growth factors. The choice of biomaterial compounds should be adapted for the different applications, structure target, and delay of administration. Biocompatibilities with embedded cells and with the host tissue and biodegradation rate must be considerate. In this paper, we review the different applications of biomaterials combined with cell therapy in ischemic stroke and we explore specific features such as choice of biomaterial compounds and physical and mechanical properties concerning the recent studies in experimental stroke.
1. Introduction
The stroke is a major public health issue in the world due to aging populations and the socioeconomic burden of neurovascular disorders. It corresponds to the one of the leading causes of death and severe disability in adults worldwide. Ischemic stroke is the most common type of stroke corresponding to 85% of all strokes [1]. Pathophysiology of ischemic stroke involves a complex and dynamic process which is not limited to neurons but involves all brain cells and extracellular matrix (ECM) in a “glioneurovascular niche” that interacts with the peripheral immune system. Stroke patients could benefit from reperfusion therapies up to 6 h after ischemic stroke onset [2]. After these first hours, there is no effective treatment available besides rehabilitation [3].
Development of innovating therapies using stem cells or trophic factors can enhance brain remodeling; this process is crucial and success requires a pathophysiological viewpoint [4]. These approaches also have the advantage of action over an extended therapeutic time-window after stroke and thereby might be effective in more patients than those helped by current acute strategies such as thrombolysis and thrombectomy. Cell-based therapy has been proposed as a potential source of new cells to replace lost cells due to central nervous system injury, as well as a source of trophic molecules to minimize damage and promote recovery [5, 6].
Stem/progenitor cell transplantation improves recovery after stroke in rodent models [7]. Nevertheless, there are two main limits concerning clinical translation in cell transplantation in stroke [8].
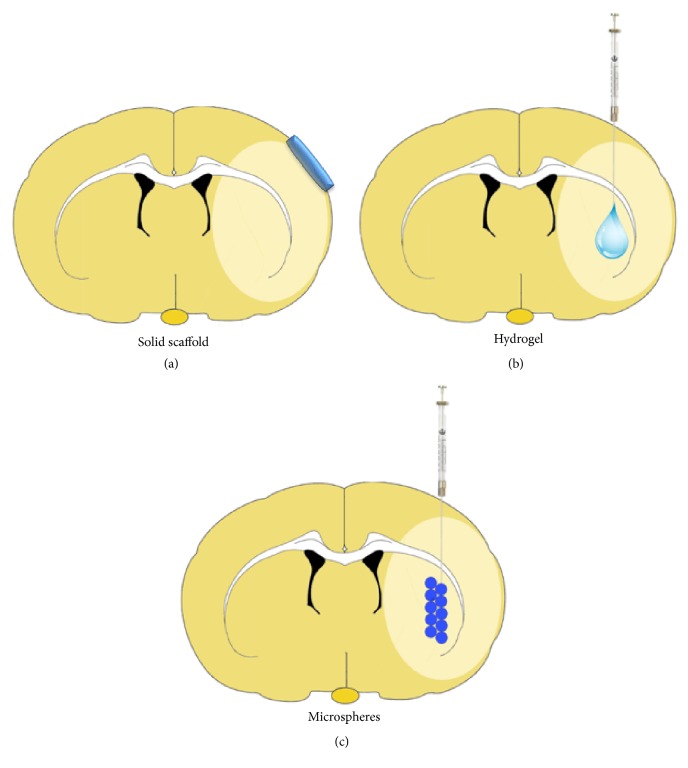
Firstly, when the stem/progenitor cells are systemically administrated, this requires the administration of a high number of cells and only a few amounts of cells achieve the brain [9]. An alternative way is the intracerebral (IC) administration of cells directly into the brain parenchyma and/or into the lesion cavity [10] (Figure 1). This location is a compartmentalized area of lost tissue that has undergone necrosis and can allow a large volume injection, and it is directly adjacent to peri-infarct zone [11], site of greatest neuroplasticity after stroke [12].
Figure 1.
Schematic illustration of different biomaterial applications on ischemic brain. (a) Solid brain scaffolds for surface application and gradual liberation of cells, drugs, or growth factors. (b) Injectable hydrogel, in liquid phase with an in situ gelation. (c) Microspheres for gradual intracerebral delivery.
The second point concerning cell administration is the important cell death observed after IC graft. After stroke, within the infarct cavity, a very important loss of ECM in addition to neuronal and glial cell loss is noted. This cavity is filled by extracellular fluid and proteins from leakage of plasma proteins [13]. This damaged area is a hostile environment for cell transplantations resulting in a severe loss of grafted cells [14, 15].
Recent advances in tissue engineering have produced applications that may provide solutions to the problem of transplanted cell death and damage associated with the transplant [11]. Biopolymer hydrogels have been projected to promote cell survival and engraftment (Figure 1).
Currently, biomaterials researchers are seeking to optimize injectable hydrogels by combining cell seeding with the incorporation of growth factors or tracers. The use of biomaterials to improve benefit of cell therapy after stroke must be carefully investigated in experimental studies prior to transferring this promising procedure to clinical trials.
In this paper, we aim to review the different applications of biomaterials after ischemic brain lesion and to explore specific features such as the choice of biomaterial compounds, physical and mechanical properties, biocompatibilities, and degradation regarding recent studies in experimental stroke (Table 1).
Table 1.
Examples of biomaterials applications in experimental stroke.
| Cells/growth factors | Species/stroke model | Biomaterial | Outcomes | References |
|---|---|---|---|---|
| hRecombinant osteopontin | Rats tMCAO | Gelatin type A microspheres | ↓ of infarct volume neurological deficits | Jin et al. 2014 [122] |
|
| ||||
| rBMSCs | Rats pMCAO | N-Isopropyl- acrylamide polymer sheets | Improvement of motor function | Ito et al. 2014 [87] |
|
| ||||
| Pegylated EGF and EPO | Mice focal ischemia endothelin-1 | PEG microparticles PLGA nanoparticles dispersed in a (HAMC) hydrogel |
↓ of inflammation, ↓ of infarct volume | Wang et al. 2013 [99] |
|
| ||||
| hNSC | Rats tMCAO | VEGF-PLGA microparticles | Neovascularization, angiogenesis | Bible et al. 2012 [123] |
|
| ||||
| iPS-NPCs | Mice cortical photothrombotic | HA, acrylate | ↑ of differentiation to neuroblast | Lam et al. 2014 [95] |
|
| ||||
| hNSC | Rats tMCAO | Xenogeneic (ECM) bioscaffold | Formation of de novo tissue | Bible et al. 2012 [121] |
|
| ||||
| ONO-1301 | Rats tMCAO | Subcutaneous (PLGA) microspheres | Neuroprotection and ↓ side effects compared to OA | Hazekawa et al. 2012 [81] |
|
| ||||
| HMGB1 | Rats tMCAO | Gelatin microspheres | ↓ infarct volume | Jin et al. 2011 [124] |
|
| ||||
| EGF | Mice focal ischemia endothelin-1 | PEG microparticles dispersed in a (HAMC) hydrogel | ↑ neural stem/progenitor cells | Cooke et al. 2011 [125] |
|
| ||||
| NSC | Rats tMCAO | Collagen type I matrix | ↑ synapses and functional recovery | Yu et al. 2010 [57] |
|
| ||||
| hVEGF | Rats tMCAO | Alginate hydrogel | ↓ infarct volume ↓ functional deficits |
Emerich et al. 2010 [55] |
MCAo p or t, permanent or transient middle cerebral artery occlusion; BMSCs, bone marrow stromal cells; EGF, epidermal growth factor; EPO, erythropoietin; SC, stem cells; PEG, polyethylene glycol; HAMC, hyaluronan methylcellulose; h, human; NSC, neural stem cells; VEGF, vascular endothelial growth factor, iPS, induced pluripotent stem; HA, hyaluronic acid; NPCs, neural pluripotent cells; PLGA, poly lactic-co-glycolic acid; OA, oral administration; HMGB1, high-mobility group box 1 protein; ECM, extracellular matrix.
Stem Cell in Stroke Repair. The benefits of exogenous cell-based strategies include their potential to rescue damaged brain tissue by simultaneously promoting endogenous neuroprotection and neural repair (including neurogenesis, angiogenesis, oligodendrogliogenesis, axonal sprouting, and synaptogenesis) [6]. Additionally, these cells could act in synergy with endogenous stem cells. The different cell sources and types were recently reviewed by Jendelová et al. [16].
Currently, we distinguish two main strategies of cell therapy: (1) a paracrine trophic support using “peripheral” stem or stromal cells and (2) a direct neural replacement using neural stem/progenitor cells or mature cells such as neurons. The route, dose, and timing for cell transplantation after stroke are still debated, depending on the chosen cell product and the expected therapeutic effect.
Direct replacement of injured neurons (“homotopic” repair) has been suggested after neural stem cells (NSC) IC administration [17] or intra-arterial (IA) injection [18]. These results were demonstrated by using induced pluripotent stem cells (iPSC) derived neurons [19], bone marrow stromal cells (BMSCs) [20], or embryonic stem cells (ESC) derived mesenchymal stem cells (MSC) injections [21]. However, only a few grafted cells can be expected to express neuronal markers, and long-term graft survival is relatively poor [22–26]. Moreover, despite possible integration of grafted NSCs [27–29] into the host circuitry, functional recovery occurs, too early to be caused by newly formed neurons and synapses.
The effects of cell therapies on poststroke vasculogenesis and angiogenesis seem to be crucial. IC injection of endothelial cells can improve vasculogenesis linked to neurogenesis via vascular endothelial growth factor (VEGF) release mechanisms [30]. Proangiogenic effects were also observed early after MSC injection contributing to VEGF-induced angiogenesis [31], after injection of NSC [32, 33], endothelial progenitor (EP) [34], or cord-blood mononuclear cells CD34+ [35]. Moreover, EP, MSC, or NSC can also facilitate protection or restoration of the blood-brain barrier after stroke [33, 36, 37].
Another important effect of cell therapy is enhancement of glial remodeling and limitations in anterograde degeneration [38–40]. For example, intravenous (IV) injection of MSC has beneficial effects on both poststroke glial remodeling and axonal remyelination [41]. It also increases glial cell-derived neurotrophic factor (GDNF) levels, creating a hospitable environment for neural repair and neuroblast migration from the subventricular zone (SVZ) [42].
Additionally, cell therapies can limit host cell death through antiapoptotic and immunomodulatory mechanisms. Although MSCs are known to attenuate microglia and leukocyte inflammatory responses after stroke [43–45], some immunomodulatory properties were also observed for cord-blood cells [46] or NSC [47, 48], which can both influence splenic inflammatory responses after stroke [49].
2. Biomaterials as Cell Scaffold to Enhance Cell Graft
An important cell death is reported after IC graft into the damaged area [14, 15]. The use of “carrier” scaffolds is particularly relevant for injections into the stroke cavity at a chronic stage, avoiding a deleterious injection into the adjacent brain tissue where important recovery processes may be underway.
Enhancing the graft survival after IC injection is the common aim of several ongoing experimental strategies. Advances in regenerative medicine are increasingly providing new opportunities to repair damaged tissue by using biomaterials to enhance cell graft. Biomaterials are materials specially developed for use in tissues with the minimum of biological response to the foreign body. Furthermore, biomaterial seems to improve graft cell survival, proliferation, migration, and differentiation, protecting grafted cells from immune response and thus improving cell therapy effects.
A study using matrix gel scaffolding associated with human ESC neuronal precursor cells (NPCs) administrated 3 weeks after an experimental ischemic stroke in rats demonstrated beneficial effects induced by biomaterial coadministration. The effects include cell survival and neuronal differentiation, reduction of infarct volume, and improvement of functional outcome [50].
Biomaterials improve cell survival even if these cells are administrated in the intact brain adjacent to the lesion. A study using a thermoreversible gelation polymer (TGP) as scaffold in MSCs transplantation demonstrated that the association of MSC-TGP significantly improved cell survival [51]. The fate of transplanted MSC was examined 8 weeks after transplantation with immunohistochemistry. The majority of cells were positive for both NeuN and MAP2 [51].
Zhong et al. tested the effects of a Hyaluronan-Heparin-Collagen based hydrogel in cell protection in vitro [11]. Stem cell survival was tested under conditions of growth factor and nutritional support and under conditions of stress induced by growth factor and nutrition withdrawal to mimic the initial transplant state. In stem cell cultures with nutrient and growth factor support, the hydrogel modestly but significantly increased survival. In stem cell cultures without such support, the hydrogel substantially increased the survival [11]. Furthermore, they demonstrated that this hydrogel was able to improve the survival of NPCs into the brain cavity after stroke. Additionally, the authors reported a reduction of inflammatory cells infiltration into the graft. Active microglia/macrophages infiltrating the cell engraftment were significantly decreased with hydrogel [11].
Such as described below (see “Interest of Biomaterials in Cell Therapies”), the inflammatory response is an important step of healing process. Nevertheless, it is recognized that a reduced inflammatory response can result in a more favorable outcome.
Biomaterials alone are able to modulate the inflammatory response. In a cortical brain damage model, a three percent HA gel was coated onto the lesion for the experimental groups and normal saline solutions for the control groups. The results from immunohistological analysis put in evidence a significant reduction of the number of GFAP+ cells [52].
The ultimate goal of stroke treatment is the functional recovery. Identifying behavioral deficits in animal models of stroke is essential for potential translational applications [53]. As we noted, regenerative approaches such as cell therapy and administration of trophic factors provide an increase in endogenous brain structural plasticity and motor remapping after ischemia [54]. The use of biomaterials may enhance these functional effects. Emerich et al. have demonstrated that alginate hydrogel used as implant for sustained release of VEGF promotes functional and structural protection from ischemic damage after transient ischemia [55]. The group treated with VEGF-Hydrogel had an important decrease (about 80%) in lesion volume evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Behavioral analysis using motor asymmetry and neurologic scores demonstrated that recovery is improved by the association of hydrogel-VEGF compared to VEGF alone [55]. Similarly, Guan et al. demonstrated that human MSCs transplanted with collagen scaffolds in a model of brain injury present better outcomes compared to MSC alone [56]. Collagen scaffolds increased the retention of MSC in the lesion site and limited its distribution at the transplanted region resulting in better functional recovery during 4 weeks after transplantation [56]. Another study assessed the combination of NSC and collagen type-I administrated 24 hours after stroke and showed an improvement of the structural and functional recovery [57]. In this study, rats were submitted to a transient ischemia and received a graft of a brain scaffold of collagen type-I seeded with NSC. The evaluation by microscopy showed that, 30 days after transplantation, NSC-collagen group presented new synapses and better functional recovery, while at this time point collagen has been completely degraded [57].
2.1. Interest of Biomaterials in Cell Therapies
Some minutes after blood flow interruption and energetic deprivation, a cascade of cellular and molecular mechanisms are activated resulting in cell death.
Inflammation is initiated by necrosis and tissue injury through the recognition of damage associated molecular patterns [58]. The process of activation of inflammatory response is currently incompletely understood [59]. Inflammation subserves a number of biological functions and can have both positive and negative consequences [60]. This process is necessary to remove necrotic and apoptotic cells and cleaved extracellular matrix molecules and to initiate angiogenesis and tissue repair [61]. However, exacerbates and chronic inflammation lead to the formation of inhospitable environment for regeneration and cell grafting, resulting in further cell death.
The ischemic lesion promotes changes in extracellular environment such as ECM. The ECM is a three-dimensional, noncellular structure composed of collagens, elastin, proteoglycans (including hyaluronan), and noncollagenous glycoproteins [62] in healthy conditions. ECM macromolecules are bioactive and modulate cellular events such as adhesion, migration, proliferation, differentiation, and survival [63]. During brain ischemia, the basement membranes of blood-brain barrier are degraded and new ECM proteins are deposited in brain parenchyma, either by secretion from activated glia or by leakage of plasma proteins, such as fibrinogen [64]. The significance and consequences of these changes may vary with the time point after injury [13].
Remodeling and repair of brain parenchyma are influenced by ECM composition. Stroke induces alterations in ECM such as increase of proteoglycans [65], inhibition of neurite outgrowth by astrocytic activation [66], and upregulation of matrix-metalloproteinases (MMPs) [62]. These mechanisms can contribute negatively to endogenous remodeling and to the host response to cell therapy.
To enhance structural and functional recovery after stroke, biomaterials protecting grafted cells and/or supporting repair processes such as ECM substitute are currently in development and could be a promising neurorestorative approach.
3. Biomaterials Components
Biomaterials are based on natural or on synthetic compounds used alone or in mixtures, providing a polymer with different properties [67]. The choice of biomaterial is of importance because it can influence biomaterial effectiveness and the response of host tissue. Natural polymers such as hyaluronan, chitosan, and collagen are advantageous because they have already been used in clinical applications as injectable hydrogels such as lubrifiants, wound sealants, viscosupplements, or filling agents in esthetic medicine [68, 69].
On the other hand, synthetic hydrogels can be engineered to more accurately mimic the physical and mechanical characteristics of ECM [70]. The advantage of synthetic biomaterials is the ability to tightly control the polymerization, degradation, and biocompatibility of hydrogel. Synthetic hydrogels are better chemically defined and in most cases are biologically inert, reducing potential immune reaction into the brain [70]. In this section, we explore some components used in recent studies in experimental stroke.
3.1. Chitosan
Biomaterial can be produced from chitosan, a natural polysaccharide produced by deacetylation of chitin from crustacean shells [71]. Chitosan-based biomaterials have been used in different applications such as corneal wound healing [72], peripheral nerve injury [73], and mechanical brain injury [74]. In a recent study, chitosan hydrogel coadministrated with ESC-derived endothelial cells showed a positive effect by presenting a high cell survival and minimal cytotoxicity in vitro [75]. When this chitosan-based hydrogel encapsulating mixed adult and endothelial cells and containing VEGF was implanted into a mouse model of hindlimb ischemia, it induced neovascularization through vasculogenesis and angiogenesis. It also led to recovery of blood flow in ischemic hindlimbs [75]. Ding et al. have demonstrated a most pronounced neuroprotective effect mediated by acetyl-11-keto-β-boswellic acid (AKBA) loaded in O-carboxymethyl chitosan nanoparticles (NPs) when compared to the AKBA only in a rat model of ischemic stroke [76]. The combination AKBA+NPs promoted a functional improvement by reducing infarct volume and apoptosis [76]. However, chitosan-based biomaterials present some disadvantages such as a fast biodegradation in situ. Additionally, the compatibility of chitosan with physiological medium depends on the preparation method. Residual proteins could indeed cause allergic reactions [71].
3.2. Hyaluronic Acid (HA)
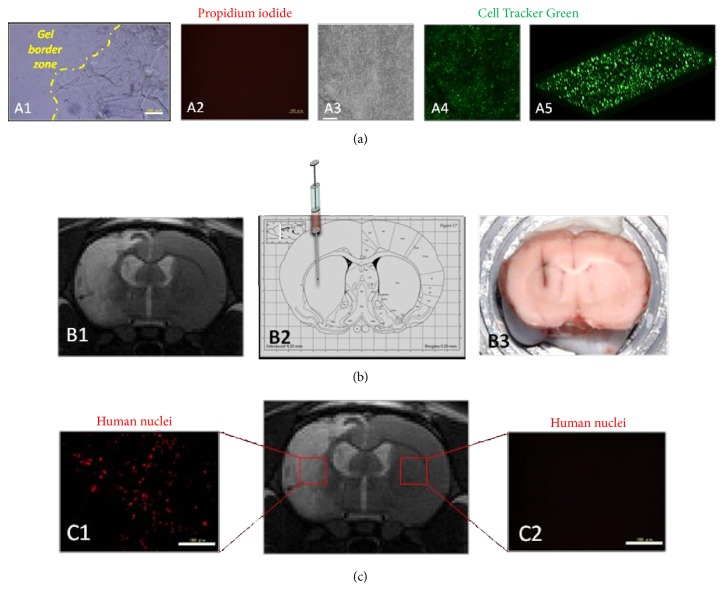
Another promising material is HA, an abundant glycosaminoglycan in the brain ECM [13, 62, 63]. HA is a linear polymer composed of the repeating disaccharide unit of D-glucuronic acid and N-acetyl-D-glucosamine. This polysaccharide plays a key role in many biological processes such as stabilizing the ECM, regulating cell adhesion and motility, and mediating cell proliferation and differentiation [77]. Liang et al. reported an increase in engrafted cells' survival and proliferation of three different cell lines (C17.2 cells, human neural progenitor cells (ReNcells), and human glial-restricted precursors) into a HA-gelatin-polyethylene glycol diacrylate (PEGDA) gel, although a mild inflammatory response towards the implanted hydrogel was observed [8]. As an example, we report here that the same HA hydrogel can be used for MSC transplantation after experimental stroke (Figure 2).
Figure 2.
Different experimental steps for intracerebral graft of cell-biomaterial after stroke. Scale bar = 100 μm. (a) In vitro biocompatibility: after mixing human mesenchymal stem cells (MSC) within hyaluronic acid (HA) hydrogel (Hystem HP, Sigma: hyaluronan+polyethylene glycol diacrylate), MSC survived into the gel during several days in culture (A1) without cell death (A2, propidium iodide cell dead assay). Cell survival and spreading into the HA gel were assessed in one-week culture (A3) using confocal microscopy and confocal microscopy stacks and viable cell labelling (A4 and A5, Cell Tracker Green CMFDA, Life). (b) Intracerebral transplantation: one week after experimental ischemic stroke in rat, magnetic resonance imaging was used to determine the injection site into the stroke cavity near plastic areas surrounding the lesion (B1). Coordinates for stereotactic injection were defined using anatomic atlas (Watson-Paxinos) (B2). By histology, the stereotactic tract can be macroscopically observed (B3, crysostat section). (c) In vivo biocompatibility and effects: ex vivo brain immunohistology demonstrated cell survival into the graft site such as human MSC identification in stroke lesion (C1, human-specific monoclonal antibody to nuclear antigen, MAB1281, 1/1,000, Chemicon) without cell migration in contralateral hemisphere (C2). Additional experiments must be done to assess long-term cell differentiations and host integration, hydrogel biodegradation, local inflammatory response, and behavior recovery effects.
3.3. Poly(Latic-co-glycolic Acid) (PLGA)
PLGA is one of most commonly used biodegradable synthetic polymers for three-dimensional (3D) scaffolds in tissue engineering [78]. The advantage of synthetic polymers is a high control of degradation rate and mechanical properties [79]. PLGA is biocompatible and has been investigated to increase cell survival. NSCs grafted into PLGA slices of 2 mm depth were viable after 14 days of culture [80]. PLGA can be used to produce microspheres for a gradual delivery of cells or drugs [81–83]. Bible et al. optimized the conditions for cell attachment in order to preserve the MHP36 cell line properties in PLGA microspheres [84]. In this experiment, 100–200 μm PLGA microparticles that were modified with poly(allylamine) via plasma polymerization of allylamine and further coated with plasma-derived fibronectin were administrated into the lesion cavity (two weeks after stroke). They demonstrated a primitive de novo tissue formation within 7 days [84]. Another interesting study using PLGA microspheres showed that a single subcutaneous administration of ONO-1301 (a long-acting prostacyclin agonist) in PLGA microspheres was able to improve poststroke recovery, edema, and infarct volume in rats [81].
There are some concerns that PLGA degrades into acidic by-products within the brain that may exacerbate inflammation and secondary damage after brain injuries [79]. The less explored poly-ε-caprolactone (PCL) polymers might be a safer alternative. PCL induced a lower inflammatory response than PLGA, as demonstrated by lower activated macrophages and glial fibrillary acidic protein (GFAP) expression [85].
4. Mechanical and Physical Properties
Biomaterials can be produced using various types of polymerization and take on distinct forms such as solid scaffolds, hydrogels, or micro/nanoparticles. The choice of biomaterial stiffness depends on the administration site target (brain surface or parenchyma), delay of release intended (gradual or immediate), and the therapeutic goal (Figure 1).
Solid scaffolds require surgery to implant and thus are more suitable for surface application [86, 87]. In situ gelling hydrogels and particles can typically be delivered in a minimally invasive manner using a syringe without the need of open surgery [58]. Hydrogel micro/nanoparticles are also suited for protein, gene, and drug delivery [76, 88, 89]. Hydrogels can be used to graft cells and to provide a microenvironment that can be tuned, promoting cell survival and improving function [8, 11, 57] (Figure 1).
4.1. Brain Scaffolds
In a recent study, Hwang et al. used a model of corticectomy to monitor in a noninvasive way by bioluminescence the behavior of stem cells embedded within poly-L-lactic acid (PLLA) scaffold [86]. Human NSCs expressing enhanced firefly luciferase were implanted into the ablated area with or without a PLLA scaffold. They have demonstrated that NSC survived over 14 days compared with 8 days for the nonencapsulated cells [86]. The mechanical strength or stiffness of a hydrogel is named compressive modulus measured in kPa. Solid scaffolds can be projected to present compressive moduli that stimulate cell survival and proliferation [90].
4.2. Injectable Hydrogels
Hydrogels are 3D cross-linked networks of water-soluble polymers [91]. Hydrogel polymers can absorb a high water content up to 99%, due to their hydrophilic nature [70], and can be engineered in a variety of physical forms, including a liquid state for in situ cross-linking [8]. They have excellent nutrient and oxygen permeability, allowing cell survival in the scaffold [92]. The most important advantage of this kind of biomaterial is that hydrogels form in situ [79], allowing an administration with a minimal invasiveness by injection [8, 11, 68]. The cross-linking process can be induced by temperature [93], pH [94], or addition of a synthetic cross-linker such as PEGDA for HA hydrogel. Besides, hydrogels possess elastic properties that are similar to those of brain tissue. Hydrogels injected in liquid phase usually present low compressive moduli after polymerization, which promotes a stem cell differentiation toward neural lineages [90].
Lam et al. assessed the effect of cell therapy by administrating neural progenitor cells derived from iPSC (iPSC-NPC) into the infarct cavity of mice submitted to a cortical photothrombotic stroke. iPSC-NPCs were encapsulated in a HA hydrogel matrix or in PBS [95]. The combination (hydrogel + iPSC-NPC) was able to promote differentiation of the neural progenitor cells to neuroblasts. Despite this good result, it did not increase cell survival one week after transplantation [95]. The hydrogels used in this study were synthesized to contain the adhesion peptide and were cross-linked with either matrix-metalloproteinase (MMP) degradable peptides or non-MMP degradable peptides. The hydrogel was specifically engineered to have a compressive modulus of ~3 kPa because that is the approximate stiffness of the brain [96]. In a recent study, Massensini et al. assessed rheological properties and gelation at body temperature of a biological hydrogel produced from porcine urinary bladder ECM [97]. They performed an efficient MRI-guided injection with drainage of fluid from the cavity to assess in situ hydrogel formation and ECM retention at different concentrations. The concentrations superior than 3 mg/mL polymerized within stroke cavity, whereas lower concentrations remained in liquid phase permeating the peri-infarct area.
A downside to hydrogels is that cell migration and outgrowth are often poor due to its weak mechanical structure [79]. Moreover, biodegradation rate is hard to control [98] and must be carefully investigated in the future (see Section 5).
4.3. Microencapsulation
Biomaterials can also be used to encapsulate molecules, cells, cell aggregates, or drugs with the aim of promoting a gradual liberation [88, 99] or graft protection. Molecules or cells encapsulation can be automatized to provide a large number of implantable “active” capsules. This could be an alternative to intracerebroventricular injection through the catheter/osmotic minipump systems. This strategy provides a gradual release and a sufficient penetration of growth factors in brain tissue [99]. Nakaguchi et al. have demonstrated an increase in the endogenous neurogenesis in the SVZ of adult mice induced by IC administration of growth factors: insulin-like grow factor 1 and hepatocyte growth factor encapsulated into gelatin hydrogel microspheres [100]. For NSC grafting, Skop et al. optimized multifunctional and biocompatible chitosan-based films and microspheres. Heparin was covalently cross-linked to the chitosan scaffolds which bound fibroblast growth factor-2 (FGF-2) and sustain survival and growth of NSC [101].
4.4. Neural Networks as Potential Strategy
In general way, cell replacement strategies do not take account of the complexity of the brain. Indeed, the cerebral abilities are linked to highly complex connections established between specialized neuroanatomical regions. Replacing lost neurons and extracellular environment does not warrant the restoration of this complex network of axonal tracts. Focusing on this question, alternative biomaterials have been developed with the aim to restore long-distance axonal connections. Replacing lost neurons and extracellular environment does not warrant the restoration of this complex network of axonal tracts. Focusing on this question, alternative biomaterials have been developed with the aim of restoring long-distance axonal connections.
A recent strategy in neural tissue engineering involves the development and application of “living scaffolds,” which are defined as constructs with a controlled, often heterogeneous, and anisotropic 3D cell architecture and biomaterial composition [102]. This living cellular-biomaterial scaffold presents a new form to implant biomaterials and cells. These living scaffolds are able to orientate, give support to, and aid regenerating cells and/or processes (e.g., axons), mimicking crucial aspects of developmental path finding [102]. The cells constitute the “living” component of scaffolds.
A very interesting study of Struzyna et al. [103] using microtissue engineering neural networks for reconstituting the architecture of axonal tracts demonstrates that this approach is effective in promoting survival at least one month and additionally they detected neurite penetration and synapse formation [103]. In this referred study, the microtube was constructed based on an agarose hydrogel and the interior containing extracellular matrix proteins and cerebral cortical neurons and was implanted in healthy rat brain. This very encouraging result presents a great potential for neuroregenerative therapy and may ultimately facilitate functional recovery if it could be transposed/overlapped in stroke models in the future.
5. Biomaterials Degradation
Many materials formulated for tissue engineering and/or the release of therapeutics are designed to be biodegradable (or bioresorbable) to reduce the complications of tissue scarring and glia tumor formation from permanent implants [70]. Thus, it is necessary to determine the biodegradability of materials in vitro and in vivo [104]. Independent of their composition (cross-linking reagents and the functional group of HA derivatives), HA hydrogels have variable degradation rate. By example, in Hahn et al., the authors demonstrated that HA hydrogels prepared with three different cross-linking reagents have variable degradation test results [105]. Indeed, adipic acid dihydrazide grafted HA (HA-ADH), methacrylated HA (HA-MA), thiolated HA (HA-SH) were compared and according to in vitro degradation tests, HA-SH hydrogel was degraded very fast, compared to HA-ADH and HA-MA hydrogels and HA-ADH hydrogel was degraded slightly faster than HA-MA hydrogel. Moreover, when HA-MA hydrogels and HA-SH hydrogels are implanted in the back of rats, HA-SH hydrogel was in vivo degraded completely only in 2 weeks, whereas HA-MA hydrogels were degraded only partially even in 29 days. There was no adverse effect during the in vivo tests.
6. Biocompatibilities with Therapeutic Cells and Host Tissue
Brain is mostly isolated from the periphery by the blood-barrier. It has a similar but slightly different response to tissue damage and foreign materials [70]. The use of biomaterials, such as hydrogels, as neural cell delivery devices is becoming more common in areas of research such as stroke, traumatic brain injury, and spinal cord injury.
When reviewing the available research, there is some ambiguity in the type of materials used and results are often at odds. Hydrogels must be designed to be biocompatible with the implanted cells [106] and with the tissue environment. In vitro cultures of embedded cells to assess cell compatibility and functionality must be done prior to in vivo graft (Figure 1). 3D cultures can be useful to precise cell location and cell distribution into a gel.
In vivo, a wide variety of synthetic polymers have been shown to be biocompatible in the body, such as polyesters and acrylates [107–110]. Natural polymers, such as poly(amino acids) and HA, have been modified to form biocompatible hydrogels. Polymeric hydrogels placed into a fimbria-fornix lesion cavity promote fiber (re)growth in morphological study in the rat [111–114]. This biocompatibility refers to the histocompatibility of an implanted hydrogel and the local and systemic response of the host which includes the inflammatory and immune reaction of the brain [70]. Implanted biomaterials promote a foreign body response. This inflammatory response presents a variable level which varies depending on the material choice and the site of implantation [115]. After implantation, a biomaterial acquires a layer of host proteins that is associated with the surface chemistry of material [58].
Brain tissue engineering in the postinjury brain represents a promising option for cell replacement and rescue, providing a cell scaffold for transplanted or resident cells. But a number of natural biomaterials have intrinsic anti-inflammatory properties, including HA and chitosan [116]. Thus, they are suitable as carriers for anti-inflammatory therapeutics. However, synthetic materials are also capable of acting in an anti-inflammatory way. Zhong et al. have demonstrated a beneficial effect of a hyaluronan-heparin-collagen hydrogel by promoting the survival of ES-NPCs and by reducing inflammatory infiltration of the graft with the hydrogel transplant [11]. However, further optimization of hydrogel compositions is warranted to avoid possible inflammatory responses such as those observed in immunocompetent mouse brain 2 weeks after IC injection of a HA hydrogel preseeded with human NSCs or glial precursors [8]. Indeed, HA degradation is facilitated in inflammation and injury by the production of reactive oxygen and nitrogen species [77, 117]. HA is degraded in vivo by hyaluronidases (HAases) into shorter fragments. However, the extent of HA degradation that occurs under pathological conditions may be greatly enhanced.
7. Imaging of Biomaterials Engraftment
Clinical studies can benefit from noninvasive methods to assess brain stroke. Experimental studies also have used noninvasive imaging techniques to monitor grafted cells distribution and their effects on brain tissue [118]. Several imaging techniques such as MRI [31], positron emission tomography (PET) [119], and nuclear imaging [9] have been used to track transplanted cells in vivo. Imaging modalities with precise anatomical information like MRI can be used to evaluate the lesion size and extension and to precisely guide biomaterial administration. Furthermore, recent advances using multiparametric MRI enable longitudinal monitoring of vascular remodeling [7, 120] and brain function by using functional MRI.
Bible et al., for example, have demonstrated that NSCs coadministrated with ECM bioscaffold produced from porcine brain and urinary bladder promote the formation of de novo tissue in the lesion cavity and repair processes after ischemic stroke evaluated by MRI [121]. Noninvasive imaging by MRI was used to guide the administration of biomaterials in a similar study [97].
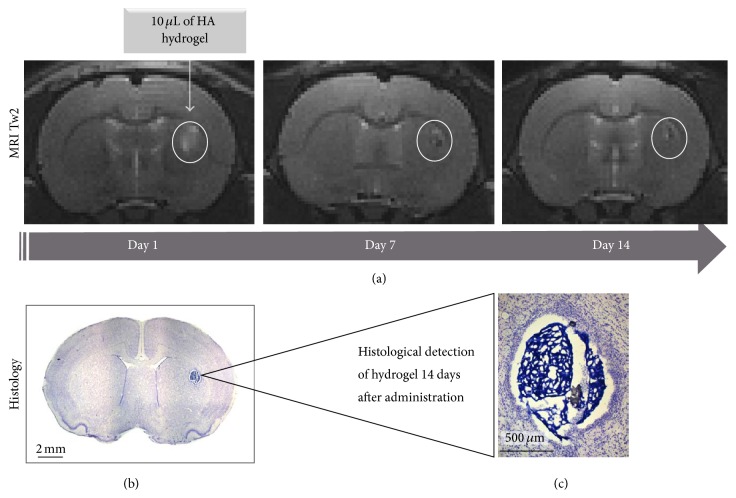
Noninvasive evaluations are a powerful tool for determining the efficacy of the combination biomaterials with cell therapy, allowing a validation of biomaterial application by a correlation of in vivo images and histological findings (Figure 3).
Figure 3.
Representative images of in vivo and ex vivo detection of hyaluronan-acid (HA) hydrogel. (a)Magnetic Resonance Imaging (MRI) weighed in T2, hydrogel detected at different time points (days one, seven, and fourteen after administration). (b) Cresyl violet staining of HA hydrogel acquired two weeks after administration, noted in (×2) (b) and (c) (x10) magnification. These images demonstrate efficient local gel formation instead of liquid diffusion which would be due to a delayed polymerization after infusion and the in vivo stability of HA hydrogel.
8. Conclusion
The use of biomaterials for stroke therapy provides a promising avenue for cell transplantation, especially in the brain where the regenerative properties can be limited.
However, the choice of biomaterial compounds and properties must be adapted, according to biocompatibilities with embedded cells and host tissue and to biodegradation rate. Thus, collaborations between bioengineering researchers and neuroscientists are required to validate and optimize preclinical experiments. Purification of biomaterials is imperative for safe use in humans. Therefore, it requires rigorous tests of cytotoxicity. Important aspects such as reproducibility, correlation with behavioral outcomes, and a long-term assessment of biomaterials degradation should be considered before clinical translation.
Hydrogels could be used to enhance cell transplantation benefit in patients by stereotactic injection of liquid hydrogel with in situ polymerization, or by surgical graft of stiffer cell-biomaterial layers, for example, during a hemicraniectomy for large stroke or during subsequent reparative cranioplasty. An appropriate follow-up with a noninvasive brain imaging to track hydrogel degradation and brain remodeling is strongly indicated.
Abbreviations
- ADH:
Adipic acid dihydrazide
- AKBA:
Acetyl-11-keto-β boswellic acid
- BBB:
Blood-brain barrier
- BMSC:
Bone marrow stromal cells
- CCL-2:
Chemokine ligand 2
- ECM:
Extracellular matrix
- EGF:
Epidermal growth factor
- EP:
Endothelial progenitor
- EPO:
Erythropoietin
- ESC:
Embryonic stem cells
- FGF-2:
Fibroblast growth factor type 2
- GDNF:
Glial cell-derived neurotrophic factor
- GFAP:
Glial fibrillary acidic protein
- h:
Human
- HA:
Hyaluronic acid
- HAMC:
Hyaluronan methylcellulose
- HGF:
Hepatocyte growth factor
- HMGB1:
High-mobility group box 1 protein
- HMW:
High molecular weight
- IA:
Intra-arterial
- IC:
Intracerebral
- IGF1:
Insulin-like growth factor 1
- IL-12:
Interleukin-12
- iNOS:
Inducible nitric oxide synthase
- iPSC:
Induced pluripotent stem cells
- IV:
Intravenous
- kPA:
Kilopascals
- LMW:
Low molecular weight
- MA:
Methacrylated
- MCAo:
Middle cerebral artery occlusion
- MIP-1α:
Macrophage inflammatory protein
- MSC:
Mesenchymal stem cells
- NPC:
Neural progenitor cell
- NSC:
Neural stem cells
- PBS:
Phosphate buffered saline
- PEG:
Polyethylene glycol
- PEGDA:
Polyethylene glycol diacrylate
- PLC:
Poly-ε-caprolactone
- PLD:
Poly-D-lysine
- PLLA:
Poly-L-lactic acid
- SC:
Stem cells
- SH:
Thiolated
- SVZ:
Subventricular zone
- TNF-α:
Tumoral necrosis factor
- TTC:
2,3,5-Triphenyltetrazolium chloride
- VEGF:
Vascular endothelial growth factor.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Beal C. C. Gender and stroke symptoms: a review of the current literature. The Journal of Neuroscience Nursing. 2010;42(2):80–87. doi: 10.1097/jnn.0b013e3181ce5c70. [DOI] [PubMed] [Google Scholar]
- 2.Wang H.-R., Chen M., Wang F.-L., et al. Comparison of therapeutic effect of recombinant tissue plasminogen activator by treatment time after onset of acute ischemic stroke. Scientific Reports. 2015;5 doi: 10.1038/srep11743.11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan P. W., Zorowitz R., Bates B., et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36(9):e100–e143. doi: 10.1161/01.str.0000180861.54180.ff. [DOI] [PubMed] [Google Scholar]
- 4.Detante O., Jaillard A., Moisan A., et al. Biotherapies in stroke. Revue Neurologique. 2014;170(12):779–798. doi: 10.1016/j.neurol.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini L., Bennis Y., Guillet B., Velly L., Bruder N., Pisano P. La thérapie cellulaire de l’accident vasculaire cérébral ischémique: du mythe à la réalité. Revue Neurologique. 2013;169(4):291–306. doi: 10.1016/j.neurol.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Melendez M., Yawno T., Jenkin G., Miller S. L. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Frontiers in Neuroscience. 2013;7, article 194 doi: 10.3389/fnins.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moisan A., Favre I. M., Rome C., et al. Microvascular plasticity after experimental stroke: a molecular and MRI study. Cerebrovascular Diseases. 2014;38(5):344–353. doi: 10.1159/000368597. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y., Walczak P., Bulte J. W. M. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials. 2013;34(22):5521–5529. doi: 10.1016/j.biomaterials.2013.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detante O., Moisan A., Dimastromatteo J., et al. Intravenous administration of mTc 99-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplantation. 2009;18(12):1369–1379. doi: 10.3727/096368909X474230. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y., Wu J., Ju R., Chen Z., Xu Q. Comparison of intracerebral transplantation effects of different stem cells on rodent stroke models. Cell Biochemistry and Function. 2015;33(4):174–182. doi: 10.1002/cbf.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong J., Chan A., Morad L., Kornblum H. I., Fan G., Carmichael S. T. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabilitation and Neural Repair. 2010;24(7):636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmichael S. T. Cellular and molecular mechanisms of neural repair after stroke: making waves. Annals of Neurology. 2006;59(5):735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 13.Baeten K. M., Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Developmental Neurobiology. 2011;71(11):1018–1039. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliss T., Guzman R., Daadi M., Steinberg G. K. Cell transplantation therapy for stroke. Stroke. 2007;38(2):817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 15.Bakshi A., Keck C. A., Koshkin V. S., et al. Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Research. 2005;1065(1-2):8–19. doi: 10.1016/j.brainres.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 16.Jendelová P., Kubinová Š., Sandvig I., Erceg S., Sandvig A., Syková E. Current developments in cell- and biomaterial-based approaches for stroke repair. Expert Opinion on Biological Therapy. 2015;16(1):43–56. doi: 10.1517/14712598.2016.1094457. [DOI] [PubMed] [Google Scholar]
- 17.Kelly S., Bliss T. M., Shah A. K., et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblum S., Wang N., Smith T. N., et al. Timing of intra-arterial neural stem cell transplantation after hypoxia-ischemia influences cell engraftment, survival, and differentiation. Stroke. 2012;43(6):1624–1631. doi: 10.1161/STROKEAHA.111.637884. [DOI] [PubMed] [Google Scholar]
- 19.Tornero D., Wattananit S., Grønning Madsen M., et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136(12):3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 20.Crain B. J., Tran S. D., Mezey E. Transplanted human bone marrow cells generate new brain cells. Journal of the Neurological Sciences. 2005;233(1-2):121–123. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y.-P., Seçkin H., Zci Y., Du Z. W., Yan Y.-P., Başkaya M. K. Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism. 2009;29(4):780–791. doi: 10.1038/jcbfm.2009.1. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Li Y., Wang L., et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.STR.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 23.Lu D., Sanberg P. R., Mahmood A., et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplantation. 2002;11(3):275–281. [PubMed] [Google Scholar]
- 24.Shen L. H., Li Y., Chen J., et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38(7):2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 25.Shen L. H., Li Y., Chen J., et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. Journal of Cerebral Blood Flow and Metabolism. 2007;27(1):6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Cabrer P., Justicia C., Wiedermann D., Hoehn M. Stem cell mediation of functional recovery after stroke in the rat. PLoS ONE. 2010;5(9):11. doi: 10.1371/journal.pone.0012779.e12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englund U., Björklund A., Wictorin K., Lindvall O., Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):17089–17094. doi: 10.1073/pnas.252589099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishibashi S., Sakaguchi M., Kuroiwa T., et al. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. Journal of Neuroscience Research. 2004;78(2):215–223. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- 29.Daadi M. M., Lee S. H., Arac A., et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplantation. 2009;18(7):815–826. doi: 10.3727/096368909x470829. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa H., Tajiri N., Shinozuka K., et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;44(12):3473–3481. doi: 10.1161/strokeaha.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moisan A., Pannetier N., Grillon E., et al. Intracerebral injection of human mesenchymal stem cells impacts cerebral microvasculature after experimental stroke: MRI study. NMR in Biomedicine. 2012;25(12):1340–1348. doi: 10.1002/nbm.2806. [DOI] [PubMed] [Google Scholar]
- 32.Roitbak T., Li L., Cunningham L. A. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1&-regulated VEGF signaling. Journal of Cerebral Blood Flow and Metabolism. 2008;28(9):1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horie N., Pereira M. P., Niizuma K., et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosell A., Morancho A., Navarro-Sobrino M., et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0073244.e73244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taguchi A., Soma T., Tanaka H., et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. The Journal of Clinical Investigation. 2004;114(3):330–338. doi: 10.1172/jci200420622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlongan C. V., Lind J. G., Dillon-Carter O., et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Research. 2004;1010(1-2):108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 37.Shinozuka K., Dailey T., Tajiri N., Ishikawa H., Kaneko Y., Borlongan C. Stem cell transplantation for neuroprotection in stroke. Brain Sciences. 2013;3(1):239–261. doi: 10.3390/brainsci3010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Q., Li Y., Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136(1):123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 39.Chopp M., Li Y., Zhang Z. G. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40(3, supplement):S143–S145. doi: 10.1161/strokeaha.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Liu Z., Xin H., Chopp M. The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia. 2014;62(1):1–16. doi: 10.1002/glia.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., McIntosh K., Chen J., et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Experimental Neurology. 2006;198(2):313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 42.Shen L. H., Li Y., Chopp M. Astrocytic endogenous glial cell derived neurotrophic factor production is enhanced by bone marrow stromal cell transplantation in the ischemic boundary zone after stroke in adult rats. Glia. 2010;58(9):1074–1081. doi: 10.1002/glia.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Chen J., Zhang C. L., et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 44.Ohtaki H., Ylostalo J. H., Foraker J. E., et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheikh A. M., Nagai A., Wakabayashi K., et al. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: contribution of fractalkine and IL-5. Neurobiology of Disease. 2011;41(3):717–724. doi: 10.1016/j.nbd.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Vendrame M., Gemma C., de Mesquita D., et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells and Development. 2005;14(5):595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 47.Martino G., Pluchino S. The therapeutic potential of neural stem cells. Nature Reviews. Neuroscience. 2006;7(5):395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Hur T. Immunomodulation by neural stem cells. Journal of the Neurological Sciences. 2008;265(1-2):102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Lee S.-T., Chu K., Jung K.-H., et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(3):616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 50.Jin K., Mao X., Xie L., et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. Journal of Cerebral Blood Flow and Metabolism. 2010;30(3):534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osanai T., Kuroda S., Yasuda H., et al. Noninvasive transplantation of bone marrow stromal cells for ischemic stroke: preliminary study with a thermoreversible gelation polymer hydrogel. Neurosurgery. 2010;66(6):1140–1147. doi: 10.1227/01.neu.0000369610.76181.cf. [DOI] [PubMed] [Google Scholar]
- 52.Lin C.-M., Lin J.-W., Chen Y.-C., et al. Hyaluronic acid inhibits the glial scar formation after brain damage with tissue loss in rats. Surgical Neurology. 2009;72(supplement 2):S50–S54. doi: 10.1016/j.wneu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Schaar K. L., Brenneman M. M., Savitz S. I. Functional assessments in the rodent stroke model. Experimental and Translational Stroke Medicine. 2010;2, article 13 doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv W., Li W.-Y., Xu X.-Y., Jiang H., Bang O. Y. Bone marrow mesenchymal stem cells transplantation promotes the release of endogenous erythropoietin after ischemic stroke. Neural Regeneration Research. 2015;10(8):1265–1270. doi: 10.4103/1673-5374.162759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emerich D. F., Silva E., Ali O., et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplantation. 2010;19(9):1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- 56.Guan J., Zhu Z., Zhao R. C., et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34(24):5937–5946. doi: 10.1016/j.biomaterials.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 57.Yu H., Cao B., Feng M., et al. Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2010;293(5):911–917. doi: 10.1002/ar.20941. [DOI] [PubMed] [Google Scholar]
- 58.Browne S., Pandit A. Biomaterial-mediated modification of the local inflammatory environment. Frontiers in Bioengineering and Biotechnology. 2015;3, article 67 doi: 10.3389/fbioe.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kataoka H., Kono H., Patel Z., Rock K. L. Evaluation of the contribution of multiple DAMPs and DAMP receptors in cell death-induced sterile inflammatory responses. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0104741.e104741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rock K. L., Lai J.-J., Kono H. Innate and adaptive immune responses to cell death. Immunological Reviews. 2011;243(1):191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang B., Liao R. The paradoxical role of inflammation in cardiac repair and regeneration. Journal of Cardiovascular Translational Research. 2010;3(4):410–416. doi: 10.1007/s12265-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 62.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daley W. P., Peters S. B., Larsen M. Extracellular matrix dynamics in development and regenerative medicine. Journal of Cell Science. 2008;121(3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 64.Schachtrup C., Ryu J. K., Helmrick M. J., et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. The Journal of Neuroscience. 2010;30(17):5843–5854. doi: 10.1523/jneurosci.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pyka M., Wetzel C., Aguado A., Geissler M., Hatt H., Faissner A. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. European Journal of Neuroscience. 2011;33(12):2187–2202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- 66.Klausmeyer A., Conrad R., Faissner A., Wiese S. Influence of glial-derived matrix molecules, especially chondroitin sulfates, on neurite growth and survival of cultured mouse embryonic motoneurons. Journal of Neuroscience Research. 2011;89(2):127–141. doi: 10.1002/jnr.22531. [DOI] [PubMed] [Google Scholar]
- 67.Delcroix G. J.-R., Schiller P. C., Benoit J.-P., Montero-Menei C. N. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010;31(8):2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 68.Pakulska M. M., Ballios B. G., Shoichet M. S. Injectable hydrogels for central nervous system therapy. Biomedical Materials. 2012;7(2) doi: 10.1088/1748-6041/7/2/024101.024101 [DOI] [PubMed] [Google Scholar]
- 69.Robert L. Hyaluronan, a truly ‘youthful’ polysaccharide. Its medical applications. Pathologie Biologie. 2015;63(1):32–34. doi: 10.1016/j.patbio.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Aurand E. R., Lampe K. J., Bjugstad K. B. Defining and designing polymers and hydrogels for neural tissue engineering. Neuroscience Research. 2012;72(3):199–213. doi: 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. European Polymer Journal. 2013;49(4):780–792. doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- 72.Tsai C.-Y., Woung L.-C., Yen J.-C., et al. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydrate Polymers. 2016;135:308–315. doi: 10.1016/j.carbpol.2015.08.098. [DOI] [PubMed] [Google Scholar]
- 73.Nawrotek K., Tylman M., Rudnicka K., Balcerzak J., Kamiński K. Chitosan-based hydrogel implants enriched with calcium ions intended for peripheral nervous tissue regeneration. Carbohydrate Polymers. 2016;136:764–771. doi: 10.1016/j.carbpol.2015.09.105. [DOI] [PubMed] [Google Scholar]
- 74.Mo L., Yang Z., Zhang A., Li X. The repair of the injured adult rat hippocampus with NT-3-chitosan carriers. Biomaterials. 2010;31(8):2184–2192. doi: 10.1016/j.biomaterials.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 75.Lee S., Valmikinathan C. M., Byun J., et al. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials. 2015;63:158–167. doi: 10.1016/j.biomaterials.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding Y., Qiao Y., Wang M., et al. Enhanced neuroprotection of acetyl-11-keto-β-boswellic acid (AKBA)-loaded O-carboxymethyl chitosan nanoparticles through antioxidant and anti-inflammatory pathways. Molecular Neurobiology. 2015 doi: 10.1007/s12035-015-9333-9. [DOI] [PubMed] [Google Scholar]
- 77.Moshayedi P., Carmichael S. T. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter. 2013;3(1) doi: 10.4161/biom.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gentile P., Chiono V., Carmagnola I., Hatton P. V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. International Journal of Molecular Sciences. 2014;15(3):3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skop N. B., Calderon F., Cho C. H., Gandhi C. D., Levison S. W. Improvements in biomaterial matrices for neural precursor cell transplantation. Molecular and Cellular Therapies. 2014;2, article 19 doi: 10.1186/2052-8426-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong Y., Zeng Y.-S., Zeng C.-G., et al. Synaptic transmission of neural stem cells seeded in 3-dimensional PLGA scaffolds. Biomaterials. 2009;30(22):3711–3722. doi: 10.1016/j.biomaterials.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 81.Hazekawa M., Sakai Y., Yoshida M., Haraguchi T., Uchida T. Single injection of ONO-1301-loaded PLGA microspheres directly after ischaemia reduces ischaemic damage in rats subjected to middle cerebral artery occlusion. Journal of Pharmacy and Pharmacology. 2012;64(3):353–359. doi: 10.1111/j.2042-7158.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 82.Klose D., Laprais M., Leroux V., et al. Fenofibrate-loaded PLGA microparticles: effects on ischemic stroke. European Journal of Pharmaceutical Sciences. 2009;37(1):43–52. doi: 10.1016/j.ejps.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Wei Y. T., Zu Z. H., et al. Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharmaceutical Research. 2011;28(6):1406–1414. doi: 10.1007/s11095-011-0452-3. [DOI] [PubMed] [Google Scholar]
- 84.Bible E., Chau D. Y. S., Alexander M. R., Price J., Shakesheff K. M., Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30(16):2985–2994. doi: 10.1016/j.biomaterials.2009.02.012. [DOI] [Google Scholar]
- 85.Wong D. Y., Hollister S. J., Krebsbach P. H., Nosrat C. Poly(ε-Caprolactone) and poly (L-Lactic-Co-Glycolic Acid) degradable polymer sponges attenuate astrocyte response and lesion growth in acute traumatic brain injury. Tissue Engineering. 2007;13(10):2515–2523. doi: 10.1089/ten.2006.0440. [DOI] [PubMed] [Google Scholar]
- 86.Hwang D. W., Jin Y., Lee D. H., et al. In vivo bioluminescence imaging for prolonged survival of transplanted human neural stem cells using 3D biocompatible scaffold in corticectomized rat model. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0105129.e105129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito M., Shichinohe H., Houkin K., Kuroda S. Application of cell sheet technology to bone marrow stromal cell transplantation for rat brain infarct. Journal of Tissue Engineering and Regenerative Medicine. 2014 doi: 10.1002/term.1920. [DOI] [PubMed] [Google Scholar]
- 88.Caicco M. J., Cooke M. J., Wang Y., Tuladhar A., Morshead C. M., Shoichet M. S. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. Journal of Controlled Release. 2013;166(3):197–202. doi: 10.1016/j.jconrel.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Tzouanas S. N., Ekenseair A. K., Kasper F. K., Mikos A. G. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. Journal of Biomedical Materials Research Part A. 2014;102(5):1222–1230. doi: 10.1002/jbm.a.35093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banerjee A., Arha M., Choudhary S., et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoare T. R., Kohane D. S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- 92.Nisbet D. R., Crompton K. E., Horne M. K., Finkelstein D. I., Forsythe J. S. Neural tissue engineering of the CNS using hydrogels. Journal of Biomedical Materials Research—Part B Applied Biomaterials. 2008;87(1):251–263. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- 93.Yeh J.-C., Hsu Y.-T., Su C.-M., Wang M.-C., Lee T.-H., Lou S.-L. Preparation and characterization of biocompatible and thermoresponsive micelles based on poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) grafted on polysuccinimide for drug delivery. Journal of Biomaterials Applications. 2014;29(3):442–453. doi: 10.1177/0885328214533736. [DOI] [PubMed] [Google Scholar]
- 94.Kim D. H., Seo Y. K., Thambi T., et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials. 2015;61:115–125. doi: 10.1016/j.biomaterials.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 95.Lam J., Lowry W. E., Carmichael S. T., Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Advanced Functional Materials. 2014;24(44):7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lampe K. J., Mooney R. G., Bjugstad K. B., Mahoney M. J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. Journal of Biomedical Materials Research—Part A. 2010;94(4):1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- 97.Massensini A. R., Ghuman H., Saldin L. T., et al. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomaterialia. 2015;27:116–130. doi: 10.1016/j.actbio.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kai D., Prabhakaran M. P., Stahl B., Eblenkamp M., Wintermantel E., Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology. 2012;23(9) doi: 10.1088/0957-4484/23/9/095705.095705 [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., Cooke M. J., Sachewsky N., Morshead C. M., Shoichet M. S. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. Journal of Controlled Release. 2013;172(1):1–11. doi: 10.1016/j.jconrel.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 100.Nakaguchi K., Jinnou H., Kaneko N., et al. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells International. 2012;2012:7. doi: 10.1155/2012/915160.915160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skop N. B., Calderon F., Levison S. W., Gandhi C. D., Cho C. H. Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomaterialia. 2013;9(6):6834–6843. doi: 10.1016/j.actbio.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 102.Struzyna L. A., Katiyar K., Cullen D. K. Living scaffolds for neuroregeneration. Current Opinion in Solid State and Materials Science. 2014;18(6):308–318. doi: 10.1016/j.cossms.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Struzyna L. A., Wolf J. A., Mietus C. J., et al. Rebuilding brain circuitry with living micro-tissue engineered neural networks. Tissue Engineering Part: A. 2015;21(21-22):2744–2756. doi: 10.1089/ten.tea.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., Rossi F., Papa S., et al. Non-invasive in vitro and in vivo monitoring of degradation of fluorescently labeled hyaluronan hydrogels for tissue engineering applications. Acta Biomaterialia. 2016;30:188–198. doi: 10.1016/j.actbio.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 105.Hahn S. K., Park J. K., Tomimatsu T., Shimoboji T. Synthesis and degradation test of hyaluronic acid hydrogels. International Journal of Biological Macromolecules. 2007;40(4):374–380. doi: 10.1016/j.ijbiomac.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 106.Jha A. K., Tharp K. M., Ye J., et al. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fournier E., Passirani C., Montero-Menei C. N., Benoit J. P. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24(19):3311–3331. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 108.Magnusson J. P., Saeed A. O., Fernández-Trillo F., Alexander C. Synthetic polymers for biopharmaceutical delivery. Polymer Chemistry. 2011;2(1):48–59. doi: 10.1039/c0py00210k. [DOI] [Google Scholar]
- 109.Peppas N. A., Hilt J. Z., Khademhosseini A., Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):1345–1360. doi: 10.1002/adma.200501612. [DOI] [Google Scholar]
- 110.Place E. S., George J. H., Williams C. K., Stevens M. M. Synthetic polymer scaffolds for tissue engineering. Chemical Society Reviews. 2009;38(4):1139–1151. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- 111.Duconseille E., Woerly S., Kelche C., Will B., Cassel J.-C. Polymeric hydrogels placed into a fimbria-fornix lesion cavity promote fiber (re)growth: a morphological study in the rat. Restorative Neurology and Neuroscience. 1998;13(3-4):193–203. [PubMed] [Google Scholar]
- 112.Nguyen M. K., Lee D. S. Injectable biodegradable hydrogels. Macromolecular Bioscience. 2010;10(6):563–579. doi: 10.1002/mabi.200900402. [DOI] [PubMed] [Google Scholar]
- 113.Prestwich G. D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. Journal of Controlled Release. 2011;155(2):193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shu X. Z., Ahmad S., Liu Y., Prestwich G. D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. Journal of Biomedical Materials Research Part A. 2006;79(4):902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 115.Anderson J. M., Rodriguez A., Chang D. T. Foreign body reaction to biomaterials. Seminars in Immunology. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Je J.-Y., Kim S.-K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. Journal of Agricultural and Food Chemistry. 2006;54(18):6629–6633. doi: 10.1021/jf061310p. [DOI] [PubMed] [Google Scholar]
- 117.Esser P. R., Wölfle U., Dürr C., et al. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041340.e41340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahrens E. T., Bulte J. W. M. Tracking immune cells in vivo using magnetic resonance imaging. Nature Reviews Immunology. 2013;13(10):755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyamoto M., Kuroda S., Zhao S., et al. Bone marrow stromal cell transplantation enhances recovery of local glucose metabolism after cerebral infarction in rats: a serial 18F-FDG PET study. Journal of Nuclear Medicine. 2013;54(1):145–150. doi: 10.2967/jnumed.112.109017. [DOI] [PubMed] [Google Scholar]
- 120.Coquery N., Francois O., Lemasson B., et al. Microvascular MRI and unsupervised clustering yields histology-resembling images in two rat models of glioma. Journal of Cerebral Blood Flow & Metabolism. 2014;34(8):1354–1362. doi: 10.1038/jcbfm.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bible E., Dell'Acqua F., Solanky B., et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by 19F- and diffusion-MRI. Biomaterials. 2012;33(10):2858–2871. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin Y., Kim I.-Y., Kim I.-D., et al. Biodegradable gelatin microspheres enhance the neuroprotective potency of osteopontin via quick and sustained release in the post-ischemic brain. Acta Biomaterialia. 2014;10(7):3126–3135. doi: 10.1016/j.actbio.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 123.Bible E., Qutachi O., Chau D. Y. S., Alexander M. R., Shakesheff K. M., Modo M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials. 2012;33(30):7435–7446. doi: 10.1016/j.biomaterials.2012.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin Y.-C., Kim S.-W., Cheng F., et al. The effect of biodegradable gelatin microspheres on the neuroprotective effects of high mobility group box 1 A box in the postischemic brain. Biomaterials. 2011;32(3):899–908. doi: 10.1016/j.biomaterials.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 125.Cooke M. J., Wang Y., Morshead C. M., Shoichet M. S. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 2011;32(24):5688–5697. doi: 10.1016/j.biomaterials.2011.04.032. [DOI] [PubMed] [Google Scholar]