Abstract
Objective. To evaluate effects of tofacitinib or adalimumab on patient-reported outcomes (PROs) in patients with moderate to severe RA and inadequate responses to MTX.
Methods. In this 12-month, phase 3, randomized controlled trial (ORAL Standard), patients (n = 717) receiving background MTX were randomized to tofacitinib 5 or 10 mg twice daily (BID), adalimumab 40 mg once every 2 weeks or placebo. PROs included HAQ-Disability Index, Patient Global Assessment of Arthritis, Patient Assessment of Arthritis Pain, health-related quality of life (Short Form-36 [SF-36]), fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue) and sleep (Medical Outcomes Study-Sleep).
Results. At month 3, tofacitinib 10 mg BID treatment resulted in significant changes from baseline vs placebo across all PROs, sustained to month 12, with the highest number of patients reporting improvements ⩾minimum clinically important differences vs placebo (P < 0.05). Changes from baseline at month 3 with tofacitinib 5 mg BID and adalimumab were similar and statistically significant vs placebo across most PROs, excluding SF-36 Mental Component Score and Social Functioning, Role Emotional, and Mental Health domains, with significantly more patients reporting improvements ⩾minimum clinically important differences. Numbers Needed to Treat were lowest for tofacitinib 10 mg BID and similar between tofacitinib 5 mg BID and adalimumab.
Conclusion. Patients with moderate to severe RA and inadequate responses to MTX reported improvements across a broad range of PROs with tofacitinib 5 and 10 mg BID and adalimumab that were significantly superior to placebo.
Keywords: tofacitinib, Janus kinase, rheumatoid arthritis, phase 3, patient-reported outcomes
Rheumatology key messages
Tofacitinib 5 and 10 mg twice daily improved patient-reported outcomes vs placebo in RA patients.
Adalimumab 40 mg every other week improved patient-reported outcomes vs placebo in RA patients.
In RA patients, tofacitinib treatment improved patient-reported outcomes from month 3 to month 12.
Introduction
Pain, fatigue and poor physical functioning associated with RA affect health-related quality of life (HRQoL), resulting in a significant disease burden [1–4]. Additionally, the broad impact of RA frequently leads to sleep disturbances and difficulty performing everyday activities, including social and occupational roles [5, 6].
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. Tofacitinib 5 and 10 mg twice daily (BID) has been investigated in six phase 3 randomized controlled trials (RCTs) as monotherapy or in combination with non-biologic DMARDs, mostly MTX [7–12]. One of these phase 3 RCTs (ORAL Standard; A3921064; NCT00853385) included the tumor necrosis factor inhibitor (TNFi) adalimumab, administered in combination with background MTX, as an active control [7]. In addition to demonstrating clinically meaningful reductions in the signs and symptoms of RA, TNFis can significantly improve patient-reported outcomes (PROs) [13]. Therefore, it is of interest to examine whether tofacitinib 5 and 10 mg BID similarly affect PROs, in addition to the reported improvements in signs and symptoms of RA, physical function and structural preservation previously demonstrated in the clinical development program for tofacitinib [7–12]. Indeed, improvements in a number of PROs have been reported in tofacitinib phase 3 RCTs [14–18]. Here we report PROs from the tofacitinib ORAL Standard phase 3 RCT, which investigated the effects over 12 months of tofacitinib 5 and 10 mg BID, and adalimumab, in patients with moderate to severely active RA and inadequate responses to MTX.
Methods
Full details of the randomized, double-blind ORAL Standard trial design have been reported previously [7]. Patients ⩾18 years old with active RA (defined by the ACR 1987 Revised Criteria [19]) were randomized to receive oral tofacitinib 5 or 10 mg BID, adalimumab 40 mg subcutaneous injection self-administered once every 2 weeks, or placebo (advanced to either tofacitinib 5 or 10 mg BID). All patients had received 7.5–25 mg of MTX weekly with sufficient residual disease activity to meet entry criteria. Key exclusion criteria included: current treatment with other synthetic/biologic anti-rheumatic agents; prior treatment with adalimumab; lack of response to prior TNFi treatment; and current active or inadequately treated infection with Mycobacterium tuberculosis. After the publication of the ORAL Standard primary manuscript [7], one of its study sites (nine patients randomized) was found to be non-compliant to study procedures, and those patients have been removed from the analyses presented here.
At month 3, patients receiving placebo without ⩾20% improvements in swollen and tender joint counts (defined as non-responders) were advanced blindly to tofacitinib 5 or 10 mg BID (dose was determined at initial randomization). At month 6, all remaining placebo patients were advanced to tofacitinib 5 or 10 mg BID. Non-responders who were initially randomized to tofacitinib or adalimumab continued with the same regimens for the trial duration.
The final protocol, amendments and informed consent documentation of the phase 3 study were approved by the Institutional Review Board and the Independent Ethics Committee of the investigational centers. All patients provided written, informed consent according to the Declaration of Helsinki (updated 2008). This analysis was included in the ethical approval.
Assessment of PROs
Mean change from baseline in HAQ-Disability Index (HAQ-DI) score at month 3 was a co-primary endpoint. HAQ-DI, Patient Global Assessment of Arthritis (PtGA) and Patient Assessment of Arthritis Pain (Pain; both evaluated using a 100 mm visual analog scale) were included as part of the ACR response criteria. With the exception of HAQ-DI, mean changes from baseline across all other PROs were pre-defined secondary endpoints and included: Short Form-36 (SF-36, Version 2, Acute), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) and Medical Outcomes Study (MOS)-Sleep [20].
Minimum clinically important differences (MCIDs) have been defined in published studies using anchor-based methods [21] as follows: a 0.22-point decrease from baseline in HAQ-DI score [20]; 10 mm decreases from baseline in PtGA and Pain [22]; and increases from baseline of ⩾2.5 points in SF-36 Physical (PCS) and Mental Component Summary (MCS) scores; ⩾5 points in individual SF-36 domain scores [20]; and 4 points in FACIT-F score [20]. No MCID was available for MOS-Sleep [23].
HAQ-DI, PtGA, Pain and SF-36 were measured at all time points, including early discontinuation. FACIT-F and MOS-Sleep were assessed at baseline, months 1, 3, 6 and 12, or early discontinuation.
Statistical analysis
Endpoints were expressed as changes from baseline and analyzed using a longitudinal mixed-effect repeated-measures model. This model was based on the full analysis set (all patients who received ⩾1 dose of study drug and ⩾1 post-baseline assessment). Treatment, week and treatment-by-week interaction were included as fixed effects, along with patients as a random effect. Estimates of mean changes for each treatment as well as mean differences from placebo were derived from the model as least squares means (LSMs), along with corresponding standard errors. The study was not designed to formally test non-inferiority of tofacitinib compared with adalimumab.
The percentage of patients reporting improvements ⩾MCID at month 3 was compared between active and placebo treatment groups (except for MOS-Sleep) in a post hoc analysis by forming a Z-score using the normal approximation to binomial rates. Statistical significance was declared at P ⩽ 0.05 based on two-sided tests, with no correction for multiple secondary comparisons. The Numbers Needed to Treat (NNTs) were calculated as the inverse of the following: rate of patients in the placebo group failing to report improvements ≥ MCID minus rate of patients on active treatment failing to report improvements ⩾ MCID (i.e. the estimated number of patients that must be treated to prevent one additional negative outcome).
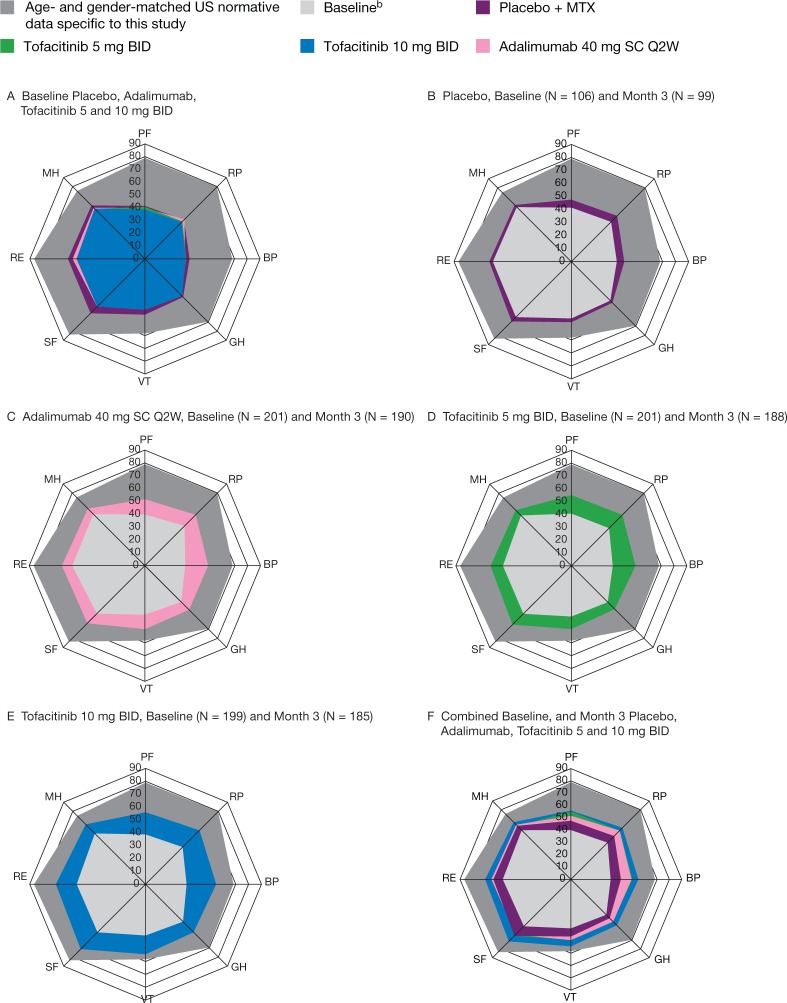
SF-36 data were compared with a published data set reporting means and standard deviations of SF-36 scores by age group and gender, as well as normed values, from a US population of healthy individuals [24]. The reported means and standard deviations were used to inverse-transform the norming of these patients, and the values were re-weighted using weights from the age ranges and genders observed in the present study. The results from the current study (e.g. at baseline) could then be compared with the values from healthy individuals as a benchmark. SF-36 domain data are displayed using Spydergram© plots, in which eight vertices (representing the eight SF-36 domains) radiate for the origin in a circular plot. Mean values are plotted on the vertices and connected by a line for each condition. The area is filled with colour for ease of review and comparison (e.g. with overlaid mean re-weighted values from healthy individuals). A larger area indicates higher HRQoL (on average) for patients.
Results
Patients
Randomized patients (n = 717, full analysis set) across treatment groups were predominantly female (range 75.0–85.3%) and white (67.3–74.0%), with mean disease duration ranging from 6.9 to 9.0 years [7]. Patient demographics and disease activity were similar across treatment groups [7]. In total, 556 (77.5%) patients completed the 12-month trial [7]; discontinuations were similar between tofacitinib and adalimumab treatment groups, including those due to adverse events [7].
Baseline values
At baseline, mean HAQ-DI scores ranged from 1.41 to 1.52, indicating impaired physical function; mean PtGA and Pain scores were 54.33–60.04 (Table 1). Large decrements in reported HRQoL were evident compared with an age- and gender-matched US normative population (Fig. 1) as a benchmark; SF-36 PCS and MCS scores were approximately 2 and 1 standard deviations lower than the normative value of 50, respectively [24]. Baseline domain scores were lowest in Physical Functioning, Role Physical and Bodily Pain domains: 36.0–38.9 points below normative data. Next lowest were Social Functioning and Role Emotional domains (29.7–31.3 points lower), followed by General Health (28.9 points lower).
Table 1.
Baseline PRO measures (mean) and LSM changes from baseline at month 3
| PRO measure | Baseline, mean (SD) |
LSM (SE) change from baseline to month 3a |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Adalimumab 40 mg Q2W | Placebo | Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Adalimumab 40 mg Q2W | |
| + MTX | + MTX | + MTX | + MTX | + MTX | + MTX | + MTX | + MTX | |
| n = 104b | n = 198b | n = 197b | n = 199b | n = 96 | n = 185 | n = 183 | n = 188 | |
| HAQ-DI | 1.41 (0.68) | 1.50 (0.64) | 1.52 (0.63) | 1.50 (0.58) | −0.24 (0.05) | −0.54 (0.04)*** | −0.61 (0.04)*** | −0.50 (0.04)*** |
| PtGA | 54.33 (21.42) | 60.04 (21.42) | 56.46 (23.85) | 57.06 (22.26) | −7.27 (2.25) | −23.79 (1.67)*** | −26.38 (1.68)*** | −21.47 (1.66)*** |
| Pain | 55.03 (21.44) | 59.19 (21.09) | 58.85 (22.23) | 56.29 (21.97) | −9.50 (2.19) | −26.74 (1.63)*** | −27.82 (1.64)*** | −22.49 (1.62)*** |
| FACIT-F | 30.38 (10.30) | 28.15 (10.49) | 28.64 (10.82) | 27.92 (10.12) | 1.57 (0.79) | 5.85 (0.59)c,*** | 6.88 (0.59)*** | 5.04 (0.58)** |
| MOS-Sleep | 41.34 (19.65)d | 43.12 (19.96)e | 42.29 (19.42)e | 43.19 (19.52)f | −3.57 (1.43)g | −7.31 (1.07)h,* | −8.43 (1.06)i,* | −4.49 (1.05)j |
| SF-36 | ||||||||
| PCS | 33.07 (6.28) | 33.14 (7.74) | 32.70 (7.79) | 32.70 (6.80) | 3.17 (0.70) | 6.98 (0.52)c,*** | 7.81 (0.52)*** | 6.26 (0.52)j,** |
| MCS | 43.25 (10.67) | 39.78 (11.69) | 40.16 (11.16) | 40.61 (11.69) | 1.77 (0.88) | 3.16 (0.66)c | 6.09 (0.66)*** | 3.38 (0.65)j |
| Physical Functioning | 32.38 (9.44) | 32.06 (9.53) | 31.20 (9.47) | 31.70 (8.94) | 2.98 (0.83) | 6.24 (0.62)c,* | 7.02 (0.62)*** | 4.99 (0.61)* |
| Role Physical | 34.55 (7.99) | 33.91 (9.09) | 33.60 (9.14) | 34.79 (8.73) | 2.90 (0.84) | 6.01 (0.62)* | 7.15 (0.62)*** | 5.30 (0.62)* |
| Bodily Pain | 34.58 (6.60) | 33.46 (7.56) | 33.30 (7.41) | 33.08 (7.26) | 3.73 (0.84) | 8.18 (0.62)c,*** | 10.07 (0.62)*** | 7.96 (0.62)*** |
| General Health | 36.33 (8.44) | 35.30 (8.90) | 35.80 (8.96) | 35.23 (7.99) | 2.12 (0.69) | 4.44 (0.51)* | 6.38 (0.52)*** | 4.93 (0.51)j,** |
| Vitality | 42.61 (8.93) | 40.57 (9.24) | 40.74 (9.39) | 39.89 (9.57) | 2.21 (0.82) | 4.97 (0.61)* | 7.21 (0.61)*** | 5.35 (0.60)* |
| Social Functioning | 39.44 (11.26) | 36.25 (10.79) | 36.23 (11.63) | 36.24 (11.56) | 3.46 (0.91) | 5.39 (0.68) | 7.98 (0.68)*** | 4.44 (0.67) |
| Role Emotional | 37.30 (12.06) | 34.07 (12.52) | 34.17 (12.66) | 35.59 (12.10) | 2.10 (1.05) | 4.07 (0.78) | 6.90 (0.79)** | 4.04 (0.78) |
| Mental Health | 41.08 (10.61) | 39.00 (11.42) | 39.13 (11.22) | 39.67 (11.25) | 2.02 (0.87) | 3.36 (0.64) | 5.73 (0.65)** | 3.83 (0.64) |
aHAQ-DI at month 3, as previously reported, was a co-primary end point [7]. bNumber of patients with available baseline patient-reported outcome data. cn = 184. dn = 103. en = 196. fn = 198. gn = 95. hn = 181. in = 182. jn = 187. *P < 0.05, **P < 0.001 and ***P < 0.0001 vs placebo + MTX. BID: twice daily; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI: Health Assessment Questionnaire-Disability Index; LSM: least squares mean; MCS: Mental Component Summary; MOS: Medical Outcomes Study; MTX: methotrexate; Pain: Patient Assessment of Arthritis Pain; PCS: Physical Component Summary; PRO: patient-reported outcome; PtGA: Patient Global Assessment of Arthritis; SD: standard deviation; SE: standard error; SF-36: Short-Form 36; Q2W: once every 2 weeks.
Fig. 1.
Spydergram©a representing mean overall SF-36 domain scores at month 3 (full analysis set, no imputation)
All patients received background MTX. aStudy values were normed using population mean and standard deviations, see [24]. b(A–E) report treatment group baselines (A using separate colours); (F) reports weighted combined baseline across all treatment groups. Placebo, n = 106 at baseline and n = 99 at month 3; tofacitinib 5 mg BID, n = 201 at baseline and n = 188 at month 3; tofacitinib 10 mg BID, n = 199 at baseline and n = 185 at month 3; adalimumab, n = 201 at baseline and n = 190 at month 3. BID: twice daily; BP: Bodily Pain; GH: General Health; MH: Mental Health; MTX: methotrexate; PF: Physical Functioning; Q2W: once every 2 weeks; RE: Role Emotional; RP: Role Physical; SC: subcutaneous; SF: Social Functioning; SF-36: Short Form-36; VT: Vitality.
Efficacy
HAQ-DI, PtGA and Pain
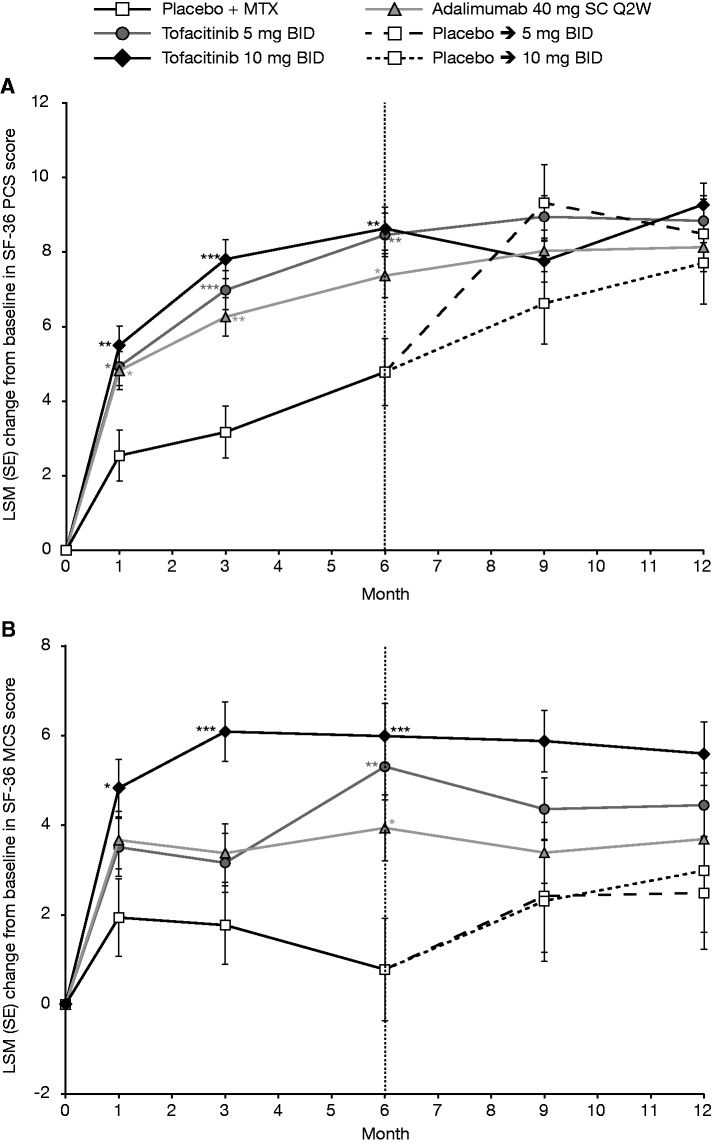
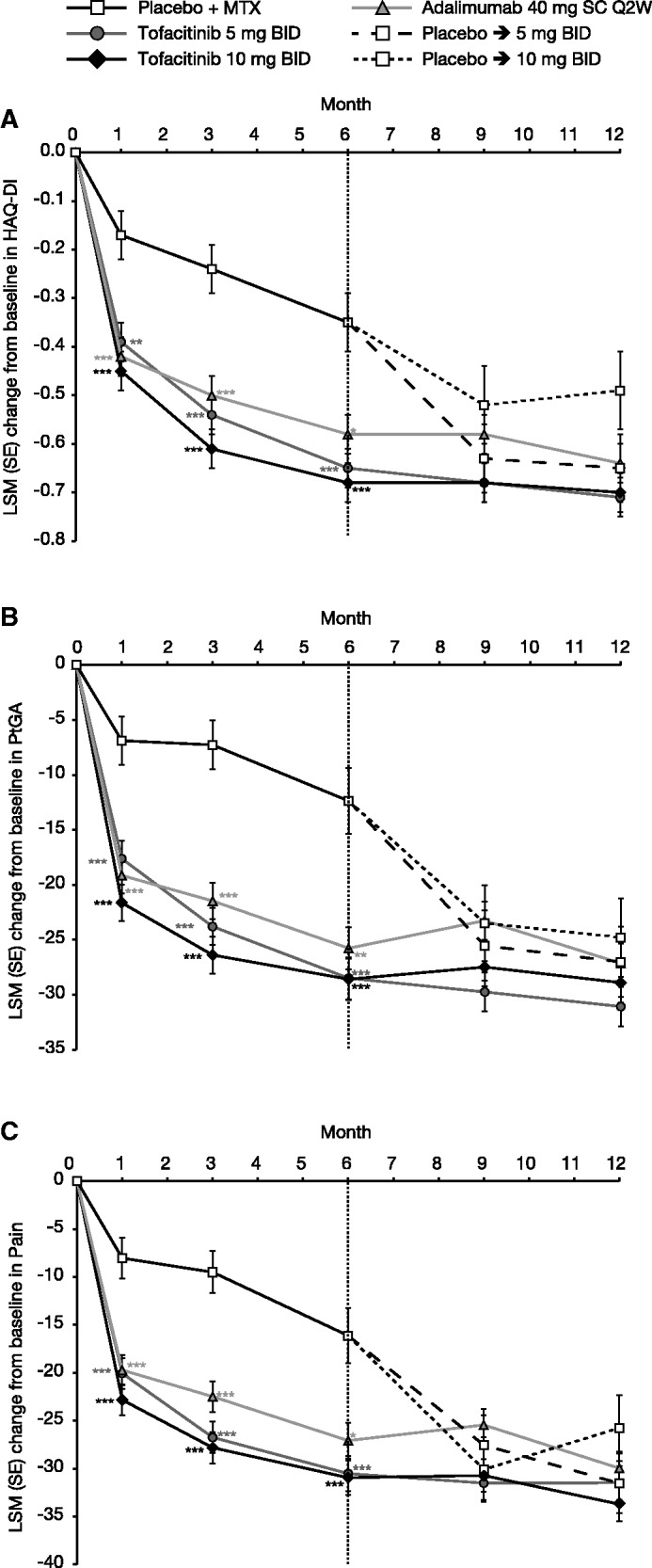
At month 3, treatment with both tofacitinib doses and adalimumab resulted in significant improvements vs placebo (P < 0.0001) in HAQ-DI, PtGA and Pain (Table 1). LSM changes from baseline at month 3 were sustained to month 12 in these three treatment groups (Fig. 2A–C).
Fig. 2.
LSM (SE) change from baseline (full analysis set, longitudinal model)
*P ≤ 0.05, **P < 0.001 and ***P < 0.0001 vs placebo + MTX. (A) Physical Functioning (HAQ-DI); (B) Patient Global Assessment of Arthritis; and (C) Patient Assessment of Arthritis Pain. Each individual graph has two separate analyses plotted. For baseline to month 6, patients who advanced at month 3 had their month 6 value set to missing for the purpose of statistically testing vs placebo. For post-month 6, all available patient data are included. BID: twice daily; HAQ-DI: Health Assessment Questionnaire-Disability Index; LSM: least squares mean; MTX: methotrexate; Pain: Patient Assessment of Arthritis Pain; PtGA: Patient Global Assessment of Arthritis; Q2W: once every 2 weeks; SC: subcutaneous; SE: standard error.
At month 3, significantly greater proportions of patients reported improvements ⩾MCID in HAQ-DI, PtGA and Pain vs placebo with both tofacitinib doses and adalimumab; the highest with tofacitinib 5 mg BID by PtGA (70.27%; P < 0.0001 vs placebo), and with tofacitinib 10 mg BID by HAQ-DI (70.49%; P < 0.001 vs placebo) and Pain (72.68%; P < 0.0001 vs placebo) (Table 2). NNTs for these outcomes, which provide an estimate of the number of patients that must be treated to prevent one additional negative outcome, ranged from 2.9 to 5.5 for tofacitinib 5 mg BID, 3.0 to 4.6 for tofacitinib 10 mg BID and 3.5 to 6.3 for adalimumab (Table 2).
Table 2.
Percentage of patients reporting improvements ≥MCID at month 3 and NNT to achieve ≥ MCID response (full analysis set, no imputation)
| PRO measure | Placebo | Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Adalimumab 40 mg Q2W |
|---|---|---|---|---|
| (MCID) | + MTX | + MTX | + MTX | + MTX |
| n = 96 | n = 185 | n = 183 | n = 188 | |
| HAQ-DI | 48.96 | |||
| % patients reporting ≥MCID (−0.22) | 67.03* | 70.49** | 64.89* | |
| NNT | 5.5 | 4.6 | 6.3 | |
| PtGA | 35.42 | |||
| % patients reporting ≥MCID (−10) | 70.27*** | 67.76*** | 64.36*** | |
| NNT | 2.9 | 3.1 | 3.5 | |
| Pain | 39.58 | |||
| % patients reporting ≥MCID (−10) | 69.19*** | 72.68*** | 64.36*** | |
| NNT | 3.4 | 3.0 | 4.0 | |
| FACIT-F | 35.42 | |||
| % patients reporting ≥MCID (4) | 55.98**a | 59.56*** | 53.19* | |
| NNT | 4.9 | 4.1 | 5.6 | |
| SF-36 | ||||
| PCS score | 51.04 | |||
| % patients reporting ≥MCID (2.5) | 67.93*a | 73.22** | 66.31*b | |
| NNT | 5.9 | 4.5 | 6.5 | |
| MCS score | 42.71 | |||
| % patients reporting ≥MCID (2.5) | 50.00a | 62.84* | 51.87b | |
| NNT | 13.7 | 5.0 | 10.9 | |
| Physical Functioning | 30.21 | |||
| % patients reporting ≥MCID (5.0) | 48.37*a | 53.55*** | 44.15* | |
| NNT | 5.5 | 4.3 | 7.2 | |
| Role Physical | 25.00 | |||
| % patients reporting ≥MCID (5.0) | 47.03** | 53.55*** | 41.49* | |
| NNT | 4.5 | 3.5 | 6.1 | |
| Bodily Pain | 32.29 | |||
| % patients reporting ≥MCID (5.0) | 54.35**a | 60.11*** | 53.19** | |
| NNT | 4.5 | 3.6 | 4.8 | |
| General Health | 20.83 | |||
| % patients reporting ≥MCID (5.0) | 37.30* | 50.27*** | 39.04** | |
| NNT | 6.1 | 3.4 | 5.5 | |
| Vitality | 29.17 | |||
| % patients reporting ≥MCID (5.0) | 51.89** | 59.02*** | 54.26*** | |
| NNT | 4.4 | 3.4 | 4.0 | |
| Social Functioning | ||||
| % patients reporting ≥MCID (5.0) | 50.00 | 58.38 | 63.93* | 55.85 |
| NNT | 11.9 | 7.2 | 17.1 | |
| Role Emotional | 29.17 | |||
| % patients reporting ≥MCID (5.0) | 40.54 | 47.54* | 40.43 | |
| NNT | 8.8 | 5.4 | 8.9 | |
| Mental Health | 35.42 | |||
| % patients reporting ≥MCID (5.0) | 43.78 | 50.82* | 46.28 | |
| NNT | 12.0 | 6.5 | 9.2 |
an = 184. bn = 187. *P < 0.05, **P < 0.001 and ***P < 0.0001 vs placebo + MTX. BID: twice daily; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI: Health Assessment Questionnaire-Disability Index; MCID: minimum clinically important difference; MCS: Mental Component Summary; MTX: methotrexate; NNT: number needed to treat; Pain: Patient Assessment of Arthritis Pain; PCS: Physical Component Summary; PRO: patient-reported outcome; PtGA: Patient Global Assessment of Arthritis; SF-36: Short-Form 36; Q2W: once every 2 weeks.
HRQoL by SF-36
As can be seen in Fig. 1, improvements from baseline to month 3 were generally observed across SF-36 domains with active treatment (Fig. 1B–E). Scores at month 3 were highest with tofacitinib 10 mg BID and lowest with placebo (Fig. 1F). At month 3, patients receiving tofacitinib 10 mg BID reported statistically significant LSM changes from baseline vs placebo in both PCS and MCS scores (both P < 0.0001) (Table 1), whereas those receiving tofacitinib 5 mg BID and adalimumab reported statistically significant LSM changes in PCS (P < 0.0001 and P < 0.001 vs placebo for tofacitinib 5 mg BID and adalimumab, respectively), but not MCS scores. Statistically significant LSM changes from baseline vs placebo in PCS scores were observed as early as month 1 across all active treatment groups, and improvements in PCS and MCS scores were sustained to month 12 (Fig. 3). With the exception of month 9, patients receiving tofacitinib 10 mg BID reported the largest LSM changes in PCS scores, followed by those receiving tofacitinib 5 mg BID. Patients receiving tofacitinib 10 mg BID reported improvements in MCS scores to month 3, which stabilised and numerically exceeded both the tofacitinib 5 mg BID and adalimumab treatment groups through month 12 (Fig. 3). Numerical improvements in MCS scores were observed with tofacitinib 5 mg BID and adalimumab by month 1; further improvements with tofacitinib 5 mg BID, and to a lesser extent adalimumab, were observed during months 3–6, with statistically significant LSM changes from baseline vs placebo observed at month 6 with tofacitinib 5 mg BID and adalimumab (Fig. 3). The highest percentages of patients reporting improvements ⩾MCID in PCS and MCS scores with the lowest NNTs (with lower values reflecting better efficacy) were reported in patients receiving tofacitinib 10 mg BID; NNTs and proportions reporting improvements ⩾MCID were similar among patients receiving tofacitinib 5 mg BID and adalimumab (Table 2).
Fig. 3.
LSM (SE) change from baseline in HRQoL (SF-36) (full analysis set, longitudinal model)
*P ≤ 0.05, **P < 0.001 and ***P < 0.0001 vs placebo + MTX. (A) Physical Component Summary score; (B) Mental Component Summary score. Each individual graph has two separate analyses plotted. For baseline to month 6, patients who advanced at month 3 had their month 6 value set to missing for the purpose of statistically testing vs placebo. For post-month 6, all available patient data are included. BID: twice daily; HRQoL: health-related quality of life; LSM: least squares mean; MCS: Mental Component Summary; MTX: methotrexate; PCS: Physical Component Summary; Q2W: once every 2 weeks; SC: subcutaneous; SE: standard error; SF-36: Short Form-36.
At month 3, reported improvements from baseline in all eight SF-36 domains were greatest with tofacitinib 10 mg BID vs placebo (P < 0.05; Table 1), and significantly more patients reported improvements ⩾MCID (Table 2). In the tofacitinib 5 mg BID and adalimumab treatment groups, LSM changes from baseline at month 3 were similar and statistically significant vs placebo across five domains, excluding Social Functioning, Role Emotional and Mental Health (Table 1), with significantly more patients reporting improvements ⩾MCID vs placebo (Table 2). NNTs ranged from 4.4 to 12.0 with tofacitinib 5 mg BID, 3.4 to 7.2 with tofacitinib 10 mg BID and 4.0 to 17.1 with adalimumab.
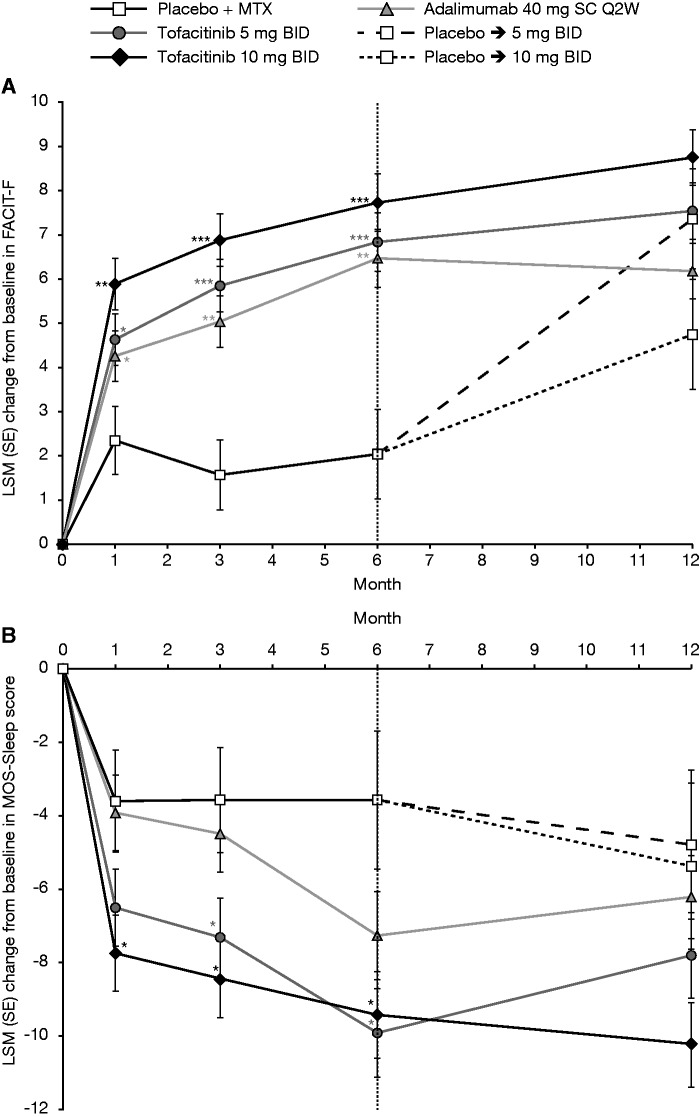
FACIT-F and MOS-Sleep
LSM changes from baseline at month 3 in FACIT-F and MOS-Sleep scores were statistically significant vs placebo with tofacitinib 5 and 10 mg BID; LSM changes in FACIT-F, but not MOS-Sleep scores, were significant vs placebo with adalimumab (Table 1). Improvements in fatigue and sleep scores were maintained through month 12 (Fig. 4). Fluctuations observed in the placebo to 5 mg BID and placebo to 10 mg BID groups between months 1–3 and months 3–6 reflected improvement and stabilization post-month 6, when all patients received active treatment (Fig. 4). More patients receiving tofacitinib 5 mg BID (55.98%; P < 0.001 vs placebo), 10 mg BID (59.56%; P < 0.0001 vs placebo) or adalimumab (53.19%; P < 0.05 vs placebo) reported improvements ⩾MCID by FACIT-F; NNTs were 4.9, 4.1 and 5.6, respectively (Table 2).
Fig. 4.
LSM (SE) change from baseline (full analysis set, longitudinal model)
*P ≤ 0.05, **P < 0.001 and ***P < 0.0001 vs placebo + MTX. (A) Fatigue (FACIT-F); (B) sleep (MOS-Sleep). Each individual graph has two separate analyses plotted. For baseline to month 6, patients who advanced at month 3 had their month 6 value set to missing for the purpose of statistically testing vs placebo. For post-month 6, all available patient data are included. BID: twice daily; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; LSM: least squares mean; MOS: Medical Outcomes Study; MTX: methotrexate; Q2W: once every 2 weeks; SC: subcutaneous; SE: standard error.
Discussion
The ORAL Standard trial was the first phase 3 study of tofacitinib to include an active control, thereby allowing an estimation of the relative efficacy and safety of tofacitinib vs the TNFi adalimumab, each administered with MTX, in patients with inadequate responses to MTX. However, it must be noted that the study was not designed to formally test the non-inferiority of tofacitinib vs adalimumab.
In this RCT, treatment with tofacitinib 5 and 10 mg BID or adalimumab all resulted in statistically significant and clinically meaningful improvements vs placebo in PROs that approached maximal at month 3, with further smaller improvements until month 6. This pattern of improvement was generally consistent with the primary efficacy data [7].
Previous studies [13, 25, 26] have highlighted the importance of physical functioning, pain and fatigue to patients with active RA [27]. In the current trial, improvements in HAQ-DI, PtGA and Pain, observed as early as month 1 and sustained to month 12, were consistent with changes reported in HRQoL and fatigue. Significantly greater proportions of patients reported improvements ⩾MCID in these outcomes with both tofacitinib doses and adalimumab, the highest being with tofacitinib 10 mg BID. The low NNTs, which provide an estimate of the number of patients that must be treated to prevent one additional negative outcome, observed with both tofacitinib doses (⩽5) for HAQ-DI, PtGA and Pain reflect the value of treatment to patients.
SF-36 summary and domain scores indicated that there was substantial disease burden and diminished HRQoL at baseline in this trial population (Fig. 1). Significant improvements in SF-36 PCS and MCS (tofacitinib 10 mg BID only) scores were observed with tofacitinib and adalimumab at month 3; improvements across five SF-36 domains were also observed with adalimumab and tofacitinib 5 mg BID and across all eight SF-36 domains with tofacitinib 10 mg BID. These results indicate that tofacitinib not only improves physical function, global assessments of disease activity, pain and fatigue, but also other aspects of HRQoL, particularly well-being and emotional health of patients with RA. Statistically significant and clinically meaningful improvements vs placebo in the SF-36 Vitality domain were observed across all active treatment groups, consistent with changes reported in FACIT-F scores, including the percentage of patients reporting improvements ⩾MCID.
RA can have a major negative psychological impact on patients; for example, it is associated with depression, which occurs in >13% of patients [27–29]. In this study, numerical improvements in Mental Health and Role Emotional domains of SF-36 were observed with tofacitinib 5 mg BID and adalimumab, and greater improvements in these domains were observed with tofacitinib 10 mg BID, which were statistically significant vs placebo. These results are consistent with observed improvements in these parameters with TNFis (e.g. adalimumab) [13, 30].
TNFis are proven to be efficacious treatments in patients with inadequate responses to MTX [31–33]. Improvements in PROs observed with adalimumab are consistent with previously published data in these patient populations, where significant improvements in HAQ-DI, PtGA, Pain and FACIT-F have been reported for up to 24 weeks of treatment [13, 34], and in HAQ-DI and SF-36 domains for up to 1 and 2 years of treatment [13, 30].
Sleep disturbance in RA is linked to pain, mood and disease activity. In a comparison between patients with RA and those with non-inflammatory disorders, up to 42% of sleep disturbance could be attributed to RA [5]. Patients receiving tofacitinib 5 or 10 mg BID or adalimumab reported numerically better improvements vs placebo at month 3 in FACIT-F and MOS-Sleep endpoints, which were sustained to month 12.
In conclusion, in the ORAL Standard phase 3 RCT, patients with moderate to severely active RA and inadequate responses to MTX reported clinically meaningful improvements across a broad range of PROs with tofacitinib 5 or 10 mg BID as well as with adalimumab. Maximal treatment effects were generally evident at month 3. The greatest improvements were observed with tofacitinib 10 mg BID, which were significantly superior to placebo across PROs at month 3. Significant improvements were also observed with tofacitinib 5 mg BID and with adalimumab across the majority of endpoints, with generally similar effect sizes observed between tofacitinib 5 mg BID and adalimumab.
Acknowledgements
The study was designed by the Pfizer authors, Pfizer Inc, USA, in collaboration with the academic authors. All aspects of this study were funded by Pfizer Inc. The authors would like to thank the patients who were involved in this study, and the A3921064 investigators and study team. Editorial support under the direction of the authors was provided by Martin Goulding, PhD, and Anne Marie Reid, PhD, of Complete Medical Communications, and funded by Pfizer Inc. All authors vouched for the veracity and completeness of the data and data analyses, approved the final version of the manuscript and made the decision to submit for publication.
Funding: This work was supported by Pfizer Inc.
Disclosure statement: V.S. serves as a consultant to and attends advisory boards for AbbVie, Alder, Amgen Corporation, AstraZeneca, Biotest, BMS, Celltrion, CORRONA, Crescendo, Eupraxia, Genentech/Roche, GSK, Hospira, Janssen, Lilly, MerckSerono, Novartis, Pfizer Inc, Regeneron, Sandoz, Sanofi, UCB and Vertex. R.F.vV. has received research support and grants from AbbVie, Amgen, BMS, GSK, Pfizer Inc, Roche and UCB, and has been a consultant for and received honoraria from AbbVie, Biotest, BMS, Celgene, Crescendo, GSK, Janssen, Lilly, Merck, Novartis, Pfizer Inc, Roche, UCB and Vertex. E.B.L. has acted as a consultant to Pfizer Inc. R.F. has received consulting fees and clinical research grant support from Pfizer Inc. S.H.Z., D.G., T.K., B.W. and G.W. are shareholders and employees of Pfizer Inc.
References
- 1.Kosinski M, Kujawski SC, Martin R. et al. Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002;8:231–40. [PubMed] [Google Scholar]
- 2.Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs 2010;70:121–45. [DOI] [PubMed] [Google Scholar]
- 3.Matcham F, Scott IC, Rayner L. et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:123–30. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson T, Morris M, Calnan M, Richards P, Hewlett S. What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res 2010;62:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol 2006;33:1942–51. [PubMed] [Google Scholar]
- 6.Sokka T, Kautiainen H, Pincus T. et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther 2010; 12:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann R, Kremer J, Cush J. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 9.Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 10.van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 11.Kremer J, Li ZG, Hall S. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 12.Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 13.Strand V, Rentz AM, Cifaldi MA. et al. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol 2012;39:63–72. [DOI] [PubMed] [Google Scholar]
- 14.Strand V, Burmester GR, Zerbini CA. et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a Phase 3 trial. Arthritis Care Res 2015;67:475–83. [DOI] [PubMed] [Google Scholar]
- 15.Strand V, Kremer J, Li ZG. et al. Tofacitinib (CP-690,550) in combination with traditional disease-modifying anti-rheumatic drugs: patient-reported outcomes from a phase 3 study in patients with active rheumatoid arthritis and an inadequate response to disease-modifying anti-rheumatic drugs. Arthritis Rheum 2011;63:S1032–3. [Google Scholar]
- 16.Strand V, van der Heijde D, Zerbini CAF. et al. ORAL SCAN: effects of the oral JAK inhibitor tofacitinib in combination with methotrexate on patient reported outcomes in a 24-month phase 3 trial of active rheumatoid arthritis. Arthritis Rheum 2013;65:S996. Abstract No. 2334 (abstract). [Google Scholar]
- 17.Alten R, Strand V, Fleischmann R. et al. Effects of tofacitinib monotherapy versus methotrexate on patient-reported outcomes in the 2-year Phase 3 ORAL Start trial in methotrexate-naïve patients with rheumatoid arthritis. Ann Rheum Dis 2014;73(Suppl 2):118–9. Abstract No. OP0152 (abstract). [Google Scholar]
- 18.Strand V, Kanik K, Connell CA. et al. The effects of the oral JAK inhibitor CP-690,550 on patient reported outcomes in a phase 3 study of active rheumatoid arthritis. Ann Rheum Dis 2011;70(Suppl 3):88–9 (abstract). [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 20.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis 2008;67:260–5. [DOI] [PubMed] [Google Scholar]
- 21.Strand V, Boers M, Idzerda L. et al. It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38:1720–7. [DOI] [PubMed] [Google Scholar]
- 22.Strand V, Scott DL, Emery P. et al. Physical function and health related quality of life: analysis of 2-year data from randomized, controlled studies of leflunomide, sulfasalazine, or methotrexate in patients with active rheumatoid arthritis. J Rheumatol 2005;32:590–601. [PubMed] [Google Scholar]
- 23.Hays RD, Stewart AL. Sleep measures In: Stewart AL, Ware JE, Jr, eds. Measuring functioning and well-being. Durham, NC: Duke University Press, 1992, 235–9. [Google Scholar]
- 24.Ware JE, Kosinski M, Dewey JE. How to score version two of the SF-36 health survey. Lincoln, RI: QualityMetric Incorporated, 2000. [Google Scholar]
- 25.Strand V, Mease P, Burmester GR. et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther 2009;11:R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strand V, Smolen JS, van Vollenhoven RF. et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr A, Hewlett S, Hughes R. et al. Rheumatology outcomes: the patient’s perspective. J Rheumatol 2003;30:880–3. [PubMed] [Google Scholar]
- 28.Uguz F, Akman C, Kucuksarac S, Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry Clin Neurosci 2009;63:50–5. [DOI] [PubMed] [Google Scholar]
- 29.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 2013;52:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keystone EC, Kavanaugh AF, Sharp JT. et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. [DOI] [PubMed] [Google Scholar]
- 31.Bathon JM, Cohen SB. The 2008 American College of Rheumatology recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis: where the rubber meets the road. Arthritis Rheum 2008;59:757–9. [DOI] [PubMed] [Google Scholar]
- 32.Saag KG, Teng GG, Patkar NM. et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 33.Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinblatt ME, Keystone EC, Furst DE. et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. [DOI] [PubMed] [Google Scholar]