Abstract
Depressive symptoms are frequently seen in patients with dementia and mild cognitive impairment (MCI). Evidence suggests that there may be a link between current depressive symptoms and Alzheimer disease (AD)-associated pathological changes, such as an increase in cortical amyloid-β (Aβ). However, limited in vivo studies have explored the relationship between current depressive symptoms and cortical Aβ in patients with MCI and AD. Our study, using a large sample of 455 patients with MCI and 153 patients with AD from the Alzheimer’s disease Neuroimaging Initiatives, investigated whether current depressive symptoms are related to cortical Aβ deposition. Depressive symptoms were assessed using the Geriatric Depression Scale and Neuropsychiatric Inventory-depression/dysphoria. Cortical Aβ was quantified using positron emission tomography with the Aβ probe 18F-florbetapir (AV-45). 18F-florbetapir standardized uptake value ratio (AV-45 SUVR) from the frontal, cingulate, parietal, and temporal regions was estimated. A global AV-45 SUVR, defined as the average of frontal, cingulate, precuneus, and parietal cortex, was also used. We observed that current depressive symptoms were not related to cortical Aβ, after controlling for potential confounds, including history of major depression. We also observed that there was no difference in cortical Aβ between matched participants with high and low depressive symptoms, as well as no difference between matched participants with the presence and absence of depressive symptoms. The association between depression and cortical Aβ deposition does not exist, but the relationship is highly influenced by stressful events in the past, such as previous depressive episodes, and complex interactions of different pathways underlying both depression and dementia.
Keywords: dementia, depression, beta-amyloid
Introduction
There is mounting evidence in support of a link among depression, mild cognitive impairment (MCI), and Alzheimer disease (AD).1–4 Depressive symptoms occur very commonly in patients with MCI,5 with previous findings reporting that the comorbidity rate is approximately 44%.6 Current literature suggests that depression in MCI is a strong risk factor for AD.7,8 A lifetime history of depression (LMD) confers an increased risk of developing AD, and a single episode of late-life depression increases the risk of developing AD by 4- to 5-fold.9 Studies have shown that patients with MCI having depressive symptoms are more likely to develop AD than those without depression.10,11 A recent study noted that currently patients with depression having MCI, who have elevated amyloid-β (Aβ) in the frontotemporal region, are more likely to convert to AD than nondepressed patients.12 These aforementioned findings suggest a complex relationship among depression, MCI, and AD.
Further, depression is strongly linked with the pathophysiology of neurodegenerative disorders.13,14 One study demonstrated that patients with AD having an LMD have higher hippocampal deposits of Aβ than those without an LMD.15 In addition, our group recently showed that patients with MCI having an LMD had higher Aβ accumulation mainly in the bilateral frontal region in comparison to those without an LMD.16 Therefore, the literature suggests that past depressive episodes are associated with elevated Aβ level in patients with MCI and AD.
Nevertheless, the relationship between current depressive symptoms and Aβ deposition in MCI remains to be characterized. Although several studies of peripheral Aβ levels in the elderly patients have not demonstrated an association between Aβ and current depressive symptoms,17–21 there are others suggesting a positive relationship.22–24 Moreover, there are no studies that have examined the relationship between symptoms of depression and cortical Aβ levels. It is also important to note that previous studies did not clearly distinguish LMD from the current depressive symptoms. At least 50% of patients who recover from their first depressive episode will have additional episodes, and approximately 80% of those with LMD will have recurrent episodes.25,26 These inconsistent findings suggest that the association between current depressive episode and Aβ level may be attributable to past depressive episodes rather than current depressive symptoms. Therefore, it is critically important to investigate the association between cortical Aβ accumulation and current depressive symptoms while controlling for the confounding effect of LMD to understand the role of AD pathology in patients with MCI and AD who have comorbid depression.
Presently, cortical Aβ deposition can be measured with positron emission tomography (PET) using radiotracers such as 18F-florbetapir (AV-45).27 A recent finding using PET-AV-45 demonstrated that in patients with depression, Aβ accumulation is increased in frontal, temporal, and parietal regions, in comparison to nondepressed patients.28 In addition, there is evidence of elevated Aβ accumulation in the cingulate region in patients with depression.29 In this study, investigating these 4 a priori regions—frontal, temporal, parietal, and cingulate— we examined cortical Aβ deposition in 455 patients with MCI and 153 patients with AD from Alzheimer’s Disease Neuroimaging Initiatives (ADNI)30 databases. The primary objective was to assess the relationship between cortical Aβ deposition in the 4 aforementioned regions of interests (ROIs) and current depressive symptoms, controlling for LMD in vivo. Findings from this study will further characterize the relationship between current depressive symptoms and cortical Aβ deposition in patients with MCI and AD. Negative results would suggest that current depressive symptoms in MCI and AD might be explained by multiple factors independent of Aβ pathology.
Method
Study Patients and Diagnosis
The entire data set was downloaded from ADNI-1, ADNI-2, and ADNI Grand Opportunity (ADNI-GO) databases on March 29, 2014.30 The ROI-based AV-45 data set that we used was updated as of May 2, 2014. Briefly, in ADNI-1, 800 participants, including controls, patients with amnestic MCI, and patients with mild AD, were recruited from 50 different sites in Canada and the United States.31 More details about participant recruitment procedure are described in the previous study.16
Eligibility criteria in ADNI-2 and ADNI-GO were identical to those in ADNI.32 For our current study, we only included patients with MCI who had both a T1-weighted magnetic resonance imaging (MRI) scan and an AV-45 PET scan completed approximately at the same time. The diagnostic inclusion criteria of MCI were early MCI, late MCI, normal-to-MCI, and dementia-to-MCI. The diagnostic inclusion criteria of AD were dementia, MCI-to-dementia, and normal-to-dementia.
Depressive Symptoms
Depressive symptoms were assessed using 2 different measures: Geriatric Depression Scale (GDS) Short-Form score and Neuropsychiatric Inventory depression/dysphoria (NPI-D) score. The GDS33 and NPI34 were obtained from ADNI-1, ADNI-2, and ADNI-GO. The GDS Short-Form is a 15-item self-assessment addressing depressive symptoms in elderly individuals.35 Its score ranges from 0 to 15, 15 referring to the most severe depressive symptoms, scores 5 or above are suggestive of depression, and scores 10 or above almost always indicate depression.36 The GDS is a valid and reliable way of measuring depressive symptoms in patients with MCI.37 For our study, the cutoff score used to distinguish between “low depressed” and “high depressed” was 5. The NPI-D was assessed using information from primary caretakers or informants of the participants. The severity score of NPI-D is classified into “mild,” “moderate,” or “severe” (score 1–3), and the frequency into “less than once a week,” “once a week,” “most days but not every day,” and “every day” (score 1–4).38 The score of NPI-D is the product of severity and frequency of the depressive symptoms and has a range from 0 to 12. The NPI-D also has high validity and good reliability in measuring depressive symptoms in elderly individuals.39 For our study, the definition of “absence of depressive symptoms” was having a score of 0 in both GDS and NPI-D scores and “presence of depressive symptom” as having a score 1 or above in both GDS and NPI-D scores. Participants who showed inconsistency in their scores were excluded. Medical history of cardiovascular diseases (CVDs), psychiatric disorders (PDs), neurological disorders (NDs), metabolic disorders (MDs), smoking, and alcohol and drug abuse was obtained by trained research assistants from patients with MCI and AD, their caretakers or informants during ADNI screening, and during follow-up visits.40 This was similarly done for ADNI-1, ADNI-GO, and ADNI-2. The presence of an LMD was determined using patients’ self-reported medical history from ADNI-1, ADNI-GO, and ADNI-2. Lifetime history of depression was defined using the procedure used in the previous study.16
The GDS assessment took place within a maximum of 6-month interval from the PET scan, and the NPI assessment took place within a maximum of 1-year interval from the scan.
Cortical Aβ Deposition
Data for AV-45 imaging were acquired from ADNI-2 and ADNI-GO. The detailed information about the technical scan procedure is described online.41 Each of the 2 ADNI initiatives reported ROI-based AV-45 standardized uptake value ratio (SUVR), which included bilateral frontal, anterior/posterior cingulate, lateral parietal and lateral temporal cortices, and global AV-45 SUVR, which is the average AV-45 of frontal, cingulate, precuneus, and parietal cortex regions, relative to the cerebellum. Region of interest-based AV-45 SUVR was estimated by dividing the average AV-45 mean from one of the ROIs into the AV-45 mean of a composite reference region (average of whole cerebellum, brainstems/pons, and eroded subcortical white matter was also used). The SUVR (ratio of SUV-specific or interest region to SUV reference region) was the outcome measure in order to reduce, as much as possible, any bias or error associated with differences in nonspecific binding between groups.42 More details about the PET analysis are described online.43
Scanning and Imaging Procedure
The ADNI study coordinators prepared AV-45, after referencing Avid Radiopharmaceuticals (Philadelphia, Pennsylvania, United States), Inc Clinical Supplies Guidance Document for the necessary steps prior to radiotracer injection.44,45 More details about AV-45 preparation and PET scanning are described in the previous study.16 Each participant also had an MRI scan session, including a T1-weighted scan. All patients’ PET AV-45 scans and the last clinical assessment of their diagnostic status were completed approximately at the same time.
Statistical Analysis
Each participant had assessments of depressive symptoms (GDS and NPI-D scores) and a PET AV-45 scan. Every participant had records of age, education years, gender, race, ethnicity, marital status, apolipoprotein (apoE4) genotype, Mini-Mental State Examination (MMSE) score, Clinical Dementia Rating score, NPI total score, Functional Activities Questionnaire (FAQ) score, Montreal Cognitive Assessment (MOCA) score, and medical history, which include history of LMD, CVD, ND, and PD, MD, history of drug abuse, history of alcohol abuse, history of smoking, current smoking status, years of smoking, and packs of cigarettes smoked during smoking years. Medical assessments were collected from ADNI-1, ADNI-2, and ADNI-GO database. Medical assessments and PET scans occurred within a maximum of a year interval between each other. Any participant with missing information of aforementioned records was excluded from the analyses.
First, partial Pearson correlations between Aβ deposition and depressive symptoms were performed for each group of patients with MCI and AD: (1) AV-45 SUVR and GDS score and (2) AV-45 SUVR and NPI-D score. Analyses were performed after controlling for aforementioned demographic and clinical factors.
Additionally, analyses of covariance (ANCOVAs) were performed for each group of patients with MCI and AD. We used 2 different approaches for ANCOVA. For the first approach, we separated participants based on low depressive symptom and high depressive symptom based on their GDS score. Then, participants with low depressive symptom were matched to those with high depressive symptom based on their demographic and clinical profiles. The 2 groups were matched for ApoE4 genotype, gender, race, ethnicity, marital status, MMSE score, and education years. For each participant with high depressive symptoms, 3 matched participants with low depressive symptoms were selectively found, and an ANCOVA was carried out controlling for any factor that was significantly different (P < .05) or at a trend level of difference between the 2 groups (P < .10). This process allowed 3 comparisons of AV-45 SUVR between the 2 groups.
For the second approach, we separated participants based on the presence and absence of depressive symptom based on their GDS and NPI-D scores. Participants without depressive symptoms were matched to those with depressive symptoms, based on their demographic and clinical profiles using the aforementioned variables. Then, we carried out an ANCOVA comparing the AV-45 SUVR between matched participants without depressive symptom and those with depressive symptoms, controlling for any factor significantly different or at a trend level of difference between the 2 groups. The statistical tests were performed using Statistical Package for the Social Sciences Version 21.0 (IBM, New York). The statistical significance was set at P < .05.
Results
Clinical and Demographic Profile
The clinical profile of patients with MCI (n = 455) and AD (n = 153) is shown in Supplementary Table 1a. The comparison of ROI-based cortical Aβ deposition between the 2 groups is shown in Supplementary Table 1b.
Clinical Assessments
Mild cognitive impairment
In patients with MCI (n = 455), among 361 (79.34%) of those with score of 1 or above, 28 (6.15%) of them were suggestive of depression (GDS score of 5 or above). There was no patient with MCI having GDS scores of 10 or above (Supplementary Table 1c). One hundred thirty-seven (30.11%) of the 455 patients had a NPI-D score of 1 or above (Supplementary Table 1d).
Alzheimer disease
In patients with AD (n = 153), among 124 (81.05%) of those with score of 1 or above, 17 (11.11%) of them were suggestive of depression (GDS score of 5 or above) and 3 (1.96%) of them had scores of 10 or above (Supplementary Table 1c). Fifty-seven of the 153 patients (37.25%) had a NPI-D score of 1 or above (Supplementary Table 1d).
Statistical Analyses
The summary of statistical analyses looking at cortical Aβ deposition and depressive symptoms within the 2 groups is shown in Table 1.
Table 1.
Summary of Analyses Between Cortical Amyloid-β Deposition and Depressive Symptoms.
| Region of Interest |
||||||
|---|---|---|---|---|---|---|
| n | Frontal | Cingulate | Parietal | Temporal | Global | |
| A. MCI | ||||||
| GDS | ||||||
| Tests used | ||||||
| Partial correlation | 455 |
r < −0.05, P = .34 |
r < −0.03, P = .50 |
r < −0.01, P = .86 |
r < −0.05, P = .61 |
r < −0.05, P = .54 |
| Partial correlation—excluded not depressed | 361 |
r < −0.05, P = .49 |
r = −0.01, P = .86 |
r < −0.05, P = .97 |
r < −0.05, P = .82 |
r< −0.01, P = .90 |
| ANCOVA: low versus high depressed | 28 each |
F = 3.01, P = .09, η2 = 0.06 |
F = 2.46, P = .12 η2 = 0.05 |
F = 1.01, P = .32, η2 = 0.02 |
F = 1.57, P = .22, η2 = 0.03 |
F = 1.24, P = .27, η2 = 0.02 |
| 28 each |
F = 4.10, P = .05, η2 = 0.07 |
F = 2.74 P = . 10, η2 = 0.05 |
F = 2.01, P = . 16, η2 = 0.04 |
F = 3.13, P = .08, η2= 0.06 |
F = 0.50, P = .49, η2 = 0.0l |
|
| 28 each |
F = 2.50, P = .12, η2 = 0.05 |
F = 2.31, P = .13, η2 = 0.04 |
F = 1.14, P = .29, η2 = 0.02 |
F = 1.75, P = .19, η2 = 0.03 |
F = 1.00, P = .32, η2 = 0.02 |
|
| NPI-D | ||||||
| Test used | ||||||
| Partial correlation | 455 |
r < −0.10, P = .13 |
r < −0.10, P = .24 |
r< −0.01, P = .25 |
r< −0.10, P = .18 |
r < −0.10, P = .16 |
| Partial correlation—excluded not depressed | 137 |
r < 0.05, P = 0.85 |
r < 0.05, P = .85 |
r < 0.05, P = .83 |
r < 0.05, P = .83 |
r < 0.05, P = .88 |
| GDS + NPI-D | ||||||
| Test used | ||||||
| ANCOVA: not depressed versus depressed | 80 each |
F < 0.01, P = 0.98, η2 < 0.01 |
F<0.01, P = .99, η2 < 0.01 |
F < 0.01, P = .94, η2 < 0.01 |
F < 0.01, P = .96, η2 < 0.01 |
F < 0.01, P = .97, η2 < 0.01 |
| B. AD | ||||||
| GDS | ||||||
| Tests used | ||||||
| Partial correlation | 153 |
r < −0.10, P = .53 |
r < −0.01, P = .92 |
r < −0.05, P = .60 |
r< −0.10, P = .43 |
r < −0.10, P = .36 |
| Partial correlation—excluded not depressed | 124 |
r = −0.14, P = .17 |
r < −0.10, P = .43 |
r = −0.14, P = .16 |
r = −0.13, P = .18 |
r = −0.17, P = .090 |
| ANCOVA: low versus high depressed | 17 each |
F = 1.43, P = .24, η2 = 0.04 |
F = 0.80, P = .38, η2 = 0.03 |
F = 0.97, P = .33, η2 = 0.03 |
F = 1.97, P = . 17, η2 = 0.06 |
F = 0.63, P = .43, η2 = 0.02 |
| 17 each |
F = 0.03, P = .96, η2 < 0.01 |
F = 0.11, P = .75, η2 < 0.01 |
F < 0.01, P = .98, η2 < 0.01 |
F = 0.08, P = .71, η2 < 0.01 |
F = 0.16, P = .69, η2 < 0.01 |
|
| 17 each |
F = 0.21, P = .65, η2 < 0.01 |
F = 0.08, P = .78, η2 < 0.01 |
F = 0.42, P = .52, η2 = 0.01 |
F = 0.24, P = .63, η2 < 0.01 |
F = 1.06, P = .31, η2= 0.03 |
|
| NPI-D | ||||||
| Test used | ||||||
| Partial correlation | 153 |
r < 0.10, P = .40 |
r < 0.10, P = .29 |
r < 0.10, P = .26 |
r = 0.11, P = .21 |
r = 0.10, P = .24 |
| Partial correlation—excluded not depressed | 57 |
r < 0.05, P = .85 |
r < 0.05, P = .90 |
r = 0.13, P = .45 |
r < 0.10, P = .63 |
r = 0.12, P = .49 |
| GDS + NPI-D | ||||||
| Test used | ||||||
| ANCOVA: not depressed versus depressed | 22 each |
F = 0.83, P = .37, η2 = 0.02 |
F = 0.68, P = .42, η2 = 0.02 |
F = 1.07, P = .31, η2 = 0.03 |
F = 1.01, P = .32, η2 = 0.03 |
F = 0.49, P = .49, η2 = 0.01 |
Abbreviations: AD, Alzheimer disease; ANCOVA, analysis of covariance; GDS, Geriatric Depression Scale score; MCI, mild cognitive impairment; NPI-D, Neuropsychiatric Inventory score depression/dysphoria domain; η2 = partial eta squared (effect size).
Relationship Between GDS Score Versus AV-45 SUVR
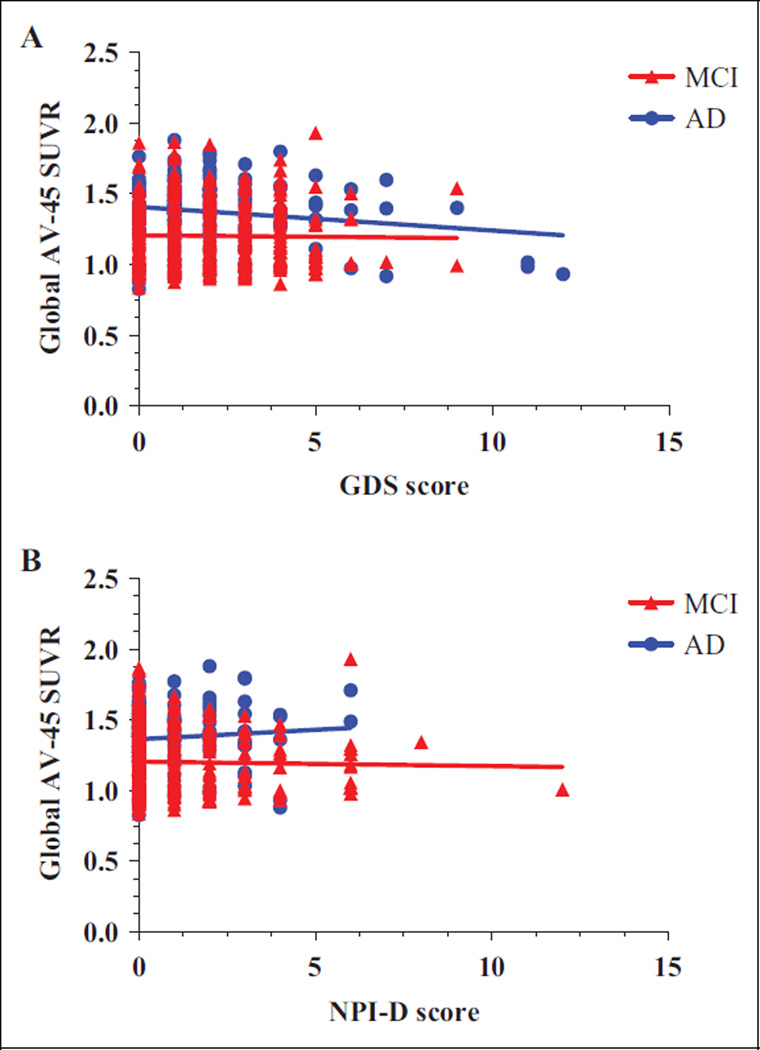
A partial Pearson correlation controlling for aforementioned covariates showed that no correlation existed between GDS score and AV-45 SUVR in frontal, cingulate, parietal, and temporal regions. There was no correlation between GDS score and global AV-45 SUVR both in patients with MCI and AD. A scatter graph illustrating the relationship between GDS score and global AV-45 SUVR is shown in Figure 1A. After excluding participants with GDS score of 0, no correlation was found between GDS score and frontal, cingulate, parietal, temporal, and global AV-45 SUVR in both groups.
Figure 1.
Scatter graphs of global cortical amyloid-β deposition versus GDS (A) and NPI-D (B) score in MCI and AD. AV-45 SUVR indicates florbetpair standardized uptake value ratio; GDS, Geriatric Depression Scale; Global, average SUVR of frontal, cingulate, precuneus, and parietal cortex regions, relative to the cerebellum; MCI, mild cognitive impairment; NPI-D, Neuropsychiatric Inventory score depression/dysphoria domain.
Relationship Between NPI-D Score Versus AV-45 SUVR
A partial Pearson correlation controlling for aforementioned covariates identified no correlation between NPI-D score and AV-45 SUVR in frontal, cingulate, parietal, and temporal regions. There was no correlation between NPI-D score and global AV-45 SUVR both in patients with MCI and AD. A scatter graph illustrating the relationship between NPI-D score and global AV-45 SUVR is shown in Figure 1B. After excluding participants with NPI-D score of 0, no correlation was found between NPI-D score and frontal, cingulate, parietal, temporal, and global AV-45 SUVR.
Severity of Depressive Symptom Versus Aβ Deposition
Mild Cognitive Impairment
In the first pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with MCI having low depressive symptoms (GDS score <5) and high depressive symptoms (GDS score >5), except for NPI, Clinical Dementia Rating Scale Sum of Boxes (CDRSB), and FAQ score (Supplementary Table 2a). The ANCOVA between the first pair of matched participants (n = 28 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between the 2 groups (Table 2A), after controlling for NPI, CDRSB, and FAQ score. In the second pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with MCI having low depressive symptoms and high depressive symptoms (Supplementary Table 2b), except for CDRSB score and history of PD. The ANCOVA between the second pair of matched participants (n = 28 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between groups (Table 2B), after controlling for CDRSB score and history of PD. In the third pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with MCI having low depressive symptoms and high depressive symptoms (Supplementary Table 2c), except for MOCA, CDRSB, and NPI score. The ANCOVA between the third pair of matched participants (n = 28 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between groups (Table 2C), after controlling for MOCA, CDRSB, and NPI score.
Table 2.
Comparison of Cortical Amyloid-β Deposition Between Patients With Low Depression and High Depression Having MCI.
| Low Depressed MCI (n= 28) |
High Depressed MCI (n= 28) |
||||
|---|---|---|---|---|---|
| Region of Interests | Mean | SD | Mean | SD | P |
| A. First pair | |||||
| Frontal | 1.38 | 0.28 | 1.27 | 0.25 | .09 |
| Cingulate | 1.46 | 0.28 | 1.36 | 0.26 | .12 |
| Parietal | 1.37 | 0.27 | 1.32 | 0.28 | .32 |
| Temporal | 1.28 | 0.24 | 1.21 | 0.22 | .22 |
| Global | 1.18 | 0.20 | 1.16 | 0.24 | .27 |
| B. Second pair | |||||
| Frontal | 1.44 | 0.33 | 1.27 | 0.25 | .05 |
| Cingulate | 1.52 | 0.33 | 1.36 | 0.26 | .10 |
| Parietal | 1.46 | 0.37 | 1.32 | 0.28 | .16 |
| Temporal | 1.36 | 0.33 | 1.21 | 0.22 | .08 |
| Global | 1.19 | 0.21 | 1.16 | 0.24 | .49 |
| C. Third pair | |||||
| Frontal | 1.34 | 0.31 | 1.27 | 0.25 | .12 |
| Cingulate | 1.44 | 0.34 | 1.36 | 0.26 | .13 |
| Parietal | 1.36 | 0.29 | 1.32 | 0.28 | .29 |
| Temporal | 1.27 | 0.27 | 1.21 | 0.22 | .19 |
| Global | 1.18 | 0.23 | 1.16 | 0.24 | .32 |
Abbreviations: AV-45, 18F-florbetapir; Global, average AV-45 SUVR of frontal, cingulate, precuneus, and parietal cortex regions, relative to the cerebellum; MCI, mild cognitive impairment; SD, standard deviation; SUVR, standardized uptake value ratio.
Alzheimer disease
In the first pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with AD having low depressive symptoms (GDS score <5) and high depressive symptoms (GDS score >5), except for NPI score (Supplementary Table 3a). The ANCOVA between the first pair of matched patients with MCI having low depressive symptoms and high depressive symptoms (n = 17 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between the 2 groups (Table 3A), after controlling for NPI score. In the second pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with AD having low depressive symptoms and high depressive symptoms (Supplementary Table 3b), except for CDRSB score. The ANCOVA between the second pair of matched participants (n = 17 each) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between groups (Table 3B), after controlling for CDRSB score. In the third pair of matched samples, there was no difference in demographic and clinical profiles between matched patients with AD having low depressive symptoms and high depressive symptoms (Supplementary Table 3c), except for the history of MD. The ANCOVA between the third pair of matched participants (n = 17 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between groups (Table 3C), after controlling for the history of MD.
Table 3.
Comparison of Cortical Amyloid-β Deposition Between Patients With Low Depression and High Depression Having AD.
| Low Depressed AD (n= 17) |
High Depressed AD (n= 17) |
||||
|---|---|---|---|---|---|
| Region of Interests | Mean | SD | Mean | SD | P |
| A. First pair | |||||
| Frontal | 1.55 | 0.22 | 1.45 | 0.35 | .24 |
| Cingulate | 1.61 | 0.21 | 1.55 | 0.35 | .38 |
| Parietal | 1.53 | 0.23 | 1.45 | 0.35 | .33 |
| Temporal | 1.46 | 0.21 | 1.35 | 0.31 | .17 |
| Global | 1.34 | 0.18 | 1.28 | 0.24 | .43 |
| B. Second pair | |||||
| Frontal | 1.46 | 0.31 | 1.45 | 0.35 | .96 |
| Cingulate | 1.52 | 0.27 | 1.55 | 0.35 | .75 |
| Parietal | 1.46 | 0.30 | 1.45 | 0.35 | .98 |
| Temporal | 1.38 | 0.27 | 1.35 | 0.31 | .71 |
| Global | 1.31 | 0.26 | 1.28 | 0.24 | .69 |
| C. Third pair | |||||
| Frontal | 1.50 | 0.30 | 1.45 | 0.35 | .65 |
| Cingulate | 1.58 | 0.30 | 1.55 | 0.35 | .78 |
| Parietal | 1.51 | 0.25 | 1.45 | 0.35 | .52 |
| Temporal | 1.40 | 0.29 | 1.35 | 0.31 | .63 |
| Global | 1.35 | 0.21 | 1.28 | 0.24 | .31 |
Abbreviations: AD, Alzheimer disease; AV-45, 18F-florbetapir; Global, average AV-45 SUVR of frontal, cingulate, precuneus, and parietal cortex regions, relative to the cerebellum; SD, standard deviation; SUVR, standardized uptake value ratio.
Presence of Depressive Symptom Versus Aβ Deposition
Mild cognitive impairment
There was no difference in demographic and clinical profiles between matched patients with MCI having depressive symptoms (presence) and those without depressive symptoms (absence), except for history of LMD, PD, smoking, NPI total score, CDRSB, and FAQ score (Supplementary Table 4a). The ANCOVA between a pair of matched patients having MCI without depressive symptoms and with depressive symptoms (n = 80 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between groups (Table 4A), after controlling for history of LMD, PD, smoking, NPI total score, CDRSB, and FAQ score.
Table 4.
Comparison of Cortical Amyloid-β Deposition Between Patients Without Depression and With Depression Having MCI and AD.
| Not Depressed MCI (n = 80) |
Depressed MCI (n = 80) |
||||
|---|---|---|---|---|---|
| Region of Interests | Mean | SD | Mean | SD | P |
| A. MCI | |||||
| Frontal | 1.39 | 0.28 | 1.35 | 0.29 | .98 |
| Cingulate | 1.48 | 0.29 | 1.43 | 0.30 | .99 |
| Parietal | 1.39 | 0.28 | 1.35 | 0.27 | .94 |
| Temporal | 1.30 | 0.26 | 1.26 | 0.23 | .96 |
| Global | 1.19 | 0.21 | 1.19 | 0.22 | .97 |
| Not Depressed AD (n= 22) |
Depressed AD (n= 22) |
||||
| Region of Interests | Mean | SD | Mean | SD | P |
| B. AD | |||||
| Frontal | 1.51 | 0.28 | 1.58 | 0.33 | .37 |
| Cingulate | 1.59 | 0.28 | 1.64 | 0.31 | .42 |
| Parietal | 1.51 | 0.28 | 1.57 | 0.32 | .31 |
| Temporal | 1.44 | 0.28 | 1.50 | 0.30 | .32 |
| Global | 1.33 | 0.22 | 1.39 | 0.27 | .49 |
Abbreviations: AD, Alzheimer disease; MCI, mild cognitive impairment; SD, standard deviation.
Alzheimer disease
There was no difference in demographic and clinical profiles between matched patients having AD without depressive symptoms and with depressive symptoms (Supplementary Table 4b), except for NPI total score and history of PD. An ANCOVA between a pair of matched patients having AD without depressive symptoms and with depressive symptoms (n = 22 in each group) showed that there was no difference in frontal, cingulate, parietal, temporal, and global AV-45 SUVR between the 2 groups (Table 4B), after controlling for NPI total score and history of PD.
Discussion
In this study, we demonstrated in a large sample (455 patients with MCI and 153 patients with AD) that current depressive symptoms were not linked to cortical Aβ deposition in patients with MCI and AD. Symptoms of depression were assessed with the GDS and NPI-D, 2 instruments widely used in the elderly, and AV-45 SUVR was used as a surrogate measure of cortical Aβ deposition in 4 ROIs: frontal, cingulate, parietal, and temporal regions. There was no association between current depressive symptoms and cortical Aβ levels, after controlling for multiple confounding factors, such as age and gender. Current literature highlights that there is a link between depression and atrophies in several cortical regions,46,47 such as hippocampus and orbitofrontal cortex. In order to account for potential atrophies in patients with depressive symptoms, controlling for total brain volume in the Pearson partial correlation did not influence the relationship between current depressive symptoms and cortical Aβ levels.
The Aβ protein hypothesis of AD states that increased production of Aβ plaques is the first step in the molecular cascade, ultimately leading to the impaired functions that manifest in dementia.48 This hypothesis proposes that amyloid accumulation in the brain occurs before the clinical symptoms and functional impairment of dementia. Brain atrophy is linked to cortical Aβ level only in the pre-MCI stage but not in MCI and AD stages,49 suggesting that cortical Aβ deposition and neuronal loss start prior to clinical evidence of cognitive decline. In addition, a recent study proposed that the rate of cortical Aβ deposition slows down over the course of life, whereas the process of neurodegeneration accelerates.50 A recent prospective study further reinforced that the process of cortical Aβ accumulation is extremely slow and protracted, showing approximately 3% of increased deposition per year.51 Therefore, current depressive symptoms in patients with established MCI or AD are less likely to be associated with the cortical Aβ level. Findings from a recent study by our group suggest that in patients with MCI, an LMD is linked to an increase in cortical Aβ deposition in the frontal region.16 Our previous and current findings strongly suggest that LMD is a stronger predictor of cortical Aβ accumulation than current depressive state in patients with MCI or AD. Overall, we can speculate that although LMD is strongly associated with cortical Aβ accumulation, current depressive symptoms might have an underlying pathology independent of Aβ neuropathological changes.
Our results are in accordance with a number of previous studies that did not find a relationship between depression and Aβ deposition. In a neuropathological study of older adults, increased Aβ accumulation was not associated with current depression.52 In a recent investigation, NPI-D score was unrelated to cortical Aβ deposition in patients with AD (n = 28).53 Similarly, another group concluded that depression was not associated with more severe AD-type pathology in patients with SCI and MCI.18 Moreover, another study observed no correlation between current Hamilton Depression Scale score and Aβ42 level in patients with late-life depression.54
The mechanism underlying why depressive symptoms are common in patients with MCI and AD is still elusive. Nevertheless, our current finding reinforces the recent hypothesis that states elevated risk of AD in old patients with depressive symptoms may not be due to abnormalities in Aβ deposition but rather due to reduced brain reserve secondary to pathological changes in patients.21 According to this hypothesis, Aβ accumulation makes brains vulnerable to other neurotoxic events that may result in emergence of symptoms of dementia. These events include reduced neurotrophic factors,55 elevated oxidative stress markers, elevated cortisol, higher white matter intensity volume, and neuroinflammation.21 Therefore, our negative result is in agreement with both the Aβ hypothesis of AD, proposed by Diniz et al, and a finding that suggests that MCI in late-life depression may be caused by non-AD pathology, such as vascular changes in the brain.56 Overall, based on previous literature and our current finding, we conclude that the association between depression and cortical Aβ deposition does not exist, but the relationship is highly influenced by the presence of LMD, heterogeneity of participants, and complex interactions of different pathways underlying both depression and dementia.
There are several limitations to this study. First, there was inconsistency between NPI-D and GDS measures of depression. Based on participants’ NPI-D score, more than half of the study participants included in ADNI did not have depressive symptoms, whereas according to GDS score, the majority of patients with MCI and AD had symptoms of depression. This inconsistency may be attributable to methodological differences between the scales. The GDS is a self-report measure, and NPI-D is based on information provided by participants’ caregivers or informants. The NPI-D is a more objective measure of participants’ depressive symptoms and is less affected by subject bias or cognitive decline. The GDS and NPI-D scores were modestly correlated in MCI and AD groups (n = 455, r = 0.25, P < .01 and n = 153, r = 0.19, P = .03, respectively). In order to increase the sensitivity of measuring depressive symptoms, we combined the 2 scales, especially as depression contains both subjective and objective clinical features. Second, few patients had severe depressive symptoms, which is attributable to the ADNI protocol’s preferential inclusion of patients with GDS less than 6. The majority of patients had mild to moderate or no symptoms of depression. The inclusion of patients with severe depression could have provided a wider range of symptoms and more conclusive analyses. Third, given that cortical Aβ deposition reaches a plateau in the early illness phase of AD,51 our study was limited in that we restricted our cohort to patients who had their PET scan and clinical assessment within a close temporal proximity. By including more patients, we could have increased the statistical power of our study. Fourth, we did not investigate the relationship between cortical Aβ accumulation and antide-pressant usage. There is one study showing that antidepressants may reduce cerebrospinal fluid (CSF) Aβ production in healthy individuals and transgenic AD mice.57 Future studies need to investigate how antidepressant usage influences cortical Aβ deposition in patients with MCI and AD. Lastly, the selection bias created by matching participants may influence group differences. To address this, we utilized 3 matched groups to allow comparisons between the 3 groups. Fifth, one of the inclusion criteria of participants in ADNI is their baseline GDS score less than six58; excluding patients who are depressed based on their GDS scores may be another limitation, which may reduce the sample sizes of participants with depression and mask the relationship between depressive symptoms and cortical Aβ levels. However, in our study, we did not restrict our inclusion criteria only to patients at baseline but also included patients with PET-AV-45 scan and clinical assessments at any time point (ie, 2 years after the baseline). This method allowed us to increase the patient samples and statistical power.
Sixth, the causality between the level of cortical Aβ and depressive symptoms may be bidirectional. There is evidence of infusion of Aβ peptides inducing depressive-like behaviors in mice.59,60 Although these were rodent studies, it is very important to investigate whether there is a bidirectional relationship between depressive symptoms and Aβ levels in humans. We did not look into the potential role of Aβ promoting depression, and future studies should investigate this relationship.
Seventh, our finding shows no correlation between current depressive symptoms and cortical Aβ levels in 4 ROIs that showed increased Aβ deposition in populations with depression. In addition to ROI analyses, future studies should take a voxel-based approach to confirm whether any other regions show elevated cortical Aβ levels that may be related to current depressive symptoms. Eighth, MCI has heterogeneous etiologies; thus, patients with MCI have different causes explaining their illness state. In order to eliminate this confounding effect of heterogeneity of MCI, we classified patients with MCI into either “MCI due to AD” (n = 281) or “MCI not due to AD” (n = 91) based on their biomarker criteria, including positive cortical Aβ and high CSF phosphorylated tau, as suggested by previous studies.51,61 In each group, there was no relationship between current depressive symptoms, assessed by GDS and NPI-D score, and cortical Aβ deposition in frontal, parietal, cingulate and temporal regions (Supplementary Table 5a and b). Thus, current depressive symptoms were not linked to cortical Aβ, regardless of different etiologies of MCI. Lastly, future prospective studies investigating the changes in Aβ levels over time in patients with depression versus nondepressed patients should be carried out to verify no difference in the rate of amyloidogenesis between the 2 groups. This finding will further reinforce that cortical Aβ deposition is not influenced by late-life depressive symptoms.
In conclusion, using a large sample size, we did not find a relationship between current depressive symptoms and cortical Aβ accumulation in patients with MCI and AD. This finding suggests that the mechanisms associated with current depressive symptoms in AD-MCI may be different from AD pathology.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc; Biogen Idec Inc; Bristol-Myers Squibb Company; Eisai Inc; Elan Pharmaceuticals, Inc; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Medpace, Inc; Merck & Co, Inc; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc; Piramal Imaging; Servier; Synarc Inc; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada.
Appendix A
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies, and nonprofit organizations, as a $60 million, 5-year public private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer disease (AD).
Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California, San Francisco. The ADNI is the result of efforts of many coinvestigators from a broad range of academic institutions and private corporations, and participants have been recruited from over 50 sites across the United States and Canada. The initial goal of ADNI was to recruit 800 participants, but ADNI has been followed by ADNI Grand Opportunity (ADNI-GO) and ADNI-2.
To date, these 3 protocols have recruited over 1500 adults, ages 55 to 90 years, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow-up duration of each group is specified in the protocols for ADNI-1, ADNI-2, and ADNI-GO. Participants originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
Footnotes
Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California. Some data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
The online supplement tables are available at http://jgpn.sagepub.com/supplemental
References
- 1.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF, van Duijn CM, Chandra V, et al. Psychiatric history and related exposures as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(suppl 2):S43–S47. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- 3.Kokmen E, Beard CM, Chandra V, Offord KP, Schoenberg BS, Ballard DJ. Clinical risk factors for Alzheimer’s disease: a population-based case-control study. Neurology. 1991;41(9):1393–1397. doi: 10.1212/wnl.41.9.1393. [DOI] [PubMed] [Google Scholar]
- 4.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 6.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239–1250. doi: 10.3233/JAD-140405. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150(11):1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- 10.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 12.Brendel M, Pogarell O, Xiong G, et al. Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur J Nucl Med Mol Imaging. 2015;42(5):716–724. doi: 10.1007/s00259-014-2975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51(8):1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 14.Chi S, Yu JT, Tan MS, Tan L. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J Alzheimers Dis. 2014;42(3):739–755. doi: 10.3233/JAD-140324. [DOI] [PubMed] [Google Scholar]
- 15.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Chung JK, Plitman E, Nakajima S, et al. Lifetime history of depression predicts increased amyloid-beta accumulation in patients with mild cognitive impairment. J Alzheimers Dis. 2015;45(3):907–919. doi: 10.3233/JAD-142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramberger MG, Jelic V, Kareholt I, et al. Cerebrospinal fluid Alzheimer markers in depressed elderly subjects with and without Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2012;2(1):48–56. doi: 10.1159/000334644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auning E, Selnes P, Grambaite R, et al. Neurobiological correlates of depressive symptoms in people with subjective and mild cognitive impairment. Acta Psychiatr Scand. 2014;131(2):139–147. doi: 10.1111/acps.12352. [DOI] [PubMed] [Google Scholar]
- 19.Skogseth R, Mulugeta E, Jones E, et al. Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):559–563. doi: 10.1159/000137671. [DOI] [PubMed] [Google Scholar]
- 20.Gudmundsson P, Skoog I, Waern M, et al. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry. 2007;15(10):832–838. doi: 10.1097/JGP.0b013e3180547091. [DOI] [PubMed] [Google Scholar]
- 21.Diniz BS, Sibille E, Ding Y, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry. 2015;20(5):594–601. doi: 10.1038/mp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon YS, Kang SH, No HJ, et al. The correlation of plasma Abeta42 levels, depressive symptoms, and cognitive function in the Korean elderly. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1603–1606. doi: 10.1016/j.pnpbp.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Tsuruga K, Sugawara N, Yasui-Furukori N, et al. A positive correlation between serum amyloid beta levels and depressive symptoms among community-dwelling elderly individuals in Japan. Neuropsychiatr Dis Treat. 2014;10:1621–1627. doi: 10.2147/NDT.S67205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Direk N, Schrijvers EM, de Bruijn RF, et al. Plasma amyloid beta, depression, and dementia in community-dwelling elderly. J Psychiatr Res. 2013;47(4):479–485. doi: 10.1016/j.jpsychires.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Penel N, Yazdanpanah Y, Chauvet MP, et al. Prevention of surgical site infection after breast cancer surgery by targeted prophylaxis antibiotic in patients at high risk of surgical site infection. J Surg Oncol. 2007;96(2):124–129. doi: 10.1002/jso.20796. [DOI] [PubMed] [Google Scholar]
- 26.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149(8):999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 27.Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Dis. 2008;22(3):261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu KY, Hsiao IT, Chen CS, et al. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur J Nuclear Med Mol Imaging. 2014;41(4):714–722. doi: 10.1007/s00259-013-2627-0. [DOI] [PubMed] [Google Scholar]
- 29.Tosun D, Schuff N, Mathis CA, Jagust W, Weiner MW. Alzheimer’s Disease NeuroImaging I. Spatial patterns of brain amyloid-beta burden and atrophy rate associations in mild cognitive impairment. Brain. 2011;134(pt 4):1077–1088. doi: 10.1093/brain/awr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alzheimer’s Disease Neuroimaging I. ADNI Website. 2014 http://adni.loni.usc.edu/
- 31.Alzheimer’s Disease Neuroimaging I. ADNI General Procedures Manual. 2010 https://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf.
- 32.Alzheimer’s Disease Neuroimaging I. ADNI GO Procedures Manual. 2008 http://adni.loni.usc.edu/wp-content/uploads/2008/07/ADNI GO ProceduresManual06102011.pdf.
- 33.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 34.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Kurlowicz L, Greenberg SA. The Geriatric Depression Scale (GDS) New York, NY: The Hartford Institute for Geriatric Nursing, New York University, College of Nursing; 2007. [Google Scholar]
- 37.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Connor DJ, Sabbagh MN, Cummings JL. Comment on administration and scoring of the Neuropsychiatric Inventory in clinical trials. Alzheimers Dement. 2008;4(6):390–394. doi: 10.1016/j.jalz.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 40.Alzheimer’s Disease Neuroimaging I. ADNI Procedure Manual-Alzheimer’s Disease. 2012 http://www.adniinfo.org/Scientists/Pdfs/adniproceduresmanual12.pdf.
- 41.Alzheimer’s Disease Neuroimaging I. ADNI_AV45_Methods_JagustLab_04.29.14.pdf. 2014 http://adni.bitbucket.org/ucberkeleyav45.html.
- 42.Perani D, Schillaci O, Padovani A, Nobili FM, et al. A survey of FDG- and amyloid-PET imaging in dementia and GRADE analysis. BioMed Res Int. 2015;7:67. doi: 10.1155/2014/785039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alzheimer’s Disease Neuroimaging I. ADNI Study Documents. 2014 http://adni.loni.usc.edu/methods/documents/
- 44.Alzheimer’s Disease Neuroimaging I. ADNI-GO PET Technical Procedures Manual AV-45 & FDG. 2011 http://adni.loni.usc.edu/wp-content/uploads/2010/05/ADNIGOPETTechManual01142011.pdf.
- 45.Alzheimer’s Disease Neuroimaging I. ADNI 2 PET Technical Procedures Manual AV-45. 2011 http://adni.loni.usc.edu/wpcontent/uploads/2010/05/ADNI2PETTechManual0142011.pdf.
- 46.Egger K, Schocke M, Weiss E, et al. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164(3):237–244. doi: 10.1016/j.pscychresns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98(22):12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 49.Chetelat G, Villemagne VL, Bourgeat P, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- 50.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 52.Tsopelas C, Stewart R, Savva GM, et al. Neuropathological correlates of late-life depression in older people. Br J Psychiatry. 2011;198(2):109–114. doi: 10.1192/bjp.bp.110.078816. [DOI] [PubMed] [Google Scholar]
- 53.Mori T, Shimada H, Shinotoh H, et al. Apathy correlates with prefrontal amyloid beta deposition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85(4):449–455. doi: 10.1136/jnnp-2013-306110. [DOI] [PubMed] [Google Scholar]
- 54.Reis T, Brandao CO, Freire Coutinho ES, Engelhardt E, Laks J. Cerebrospinal fluid biomarkers in Alzheimer’s disease and geriatric depression: preliminary findings from Brazil. CNS Neurosci Ther. 2012;18(7):524–529. doi: 10.1111/j.1755-5949.2012.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teixeira AL, Barbosa IG, Diniz BS, Kummer A. Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomarkers Med. 2010;4(6):871–887. doi: 10.2217/bmm.10.111. [DOI] [PubMed] [Google Scholar]
- 56.Hughes TM, Kuller LH, Lopez OL, et al. Markers of cholesterol metabolism in the brain show stronger associations with cerebro-vascular disease than Alzheimer’s disease. J Alzheimers Dis. 2012;30(1):53–61. doi: 10.3233/JAD-2012-111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheline YI, West T, Yarasheski K, et al. An antidepressant decreases CSF Abeta production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6(236):236re4. doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledo JH, Azevedo EP, Clarke JR, et al. Amyloid-beta oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry. 2013;18(10):1053–1054. doi: 10.1038/mp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colaianna M, Tucci P, Zotti M, et al. Soluble beta amyloid(1–42): a critical player in producing behavioural and biochemical changes evoking depressive-related state? Br J Pharmacol. 2010;159(8):1704–1715. doi: 10.1111/j.1476-5381.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nettiksimmons J, Harvey D, Brewer J, et al. Subtypes based on cerebrospinal fluid and magnetic resonance imaging markers in normal elderly predict cognitive decline. Neurobiol Aging. 2010;31(8):1419–1428. doi: 10.1016/j.neurobiolaging.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.