Abstract
An increasing body of evidence indicates that astrocytes contribute to the governance and fine tuning of stem and progenitor cell production during brain development. The effect of astrocyte function in cell production in neurodevelopmental disorders is unknown. We used the Neural Colony Forming Cell assay to determine the effect of astrocyte conditioned media (ACM) on the generation of neurospheres originating from either progenitor cells or functional stem cells in the knock out (KO) Fragile X mouse model. ACM from both normal and Fmr1-KO mice generated higher percentages of smaller neurospheres indicative of restricted proliferation of the progenitor cell population in Fmr1-KO brains. Wild type (WT) neurospheres, but not KO neurospheres, showed enhanced responses to ACM from the Fmr1-KO mice. In particular, Fmr1-KO ACM increased the percentage of large neurospheres generated, representative of spheres produced from neural stem cells. We also used 2D DIGE to initiate identification of the astrocyte-secreted proteins with differential expression between Fmr1-KO and WT cortices and hippocampi. The results further support the critical role of astrocytes in governing neural cell production in brain development and point to significant alterations in neural cell proliferation due to astrocyte secreted factors from the Fragile X brain.
Highlights:
• We studied the proliferation of neural stem and progenitor cells in Fragile X.
• We examined the role of astrocyte-secreted factors in neural precursor cell biology.
• Astrocyte-secreted factors with differential expression in Fragile X identified.
Keywords: Fragile X syndrome, neural stem cells, neurospheres, astrocyte-secreted factors, Fmr1 knockout mice, 2D DIGE
Introduction
Fragile X Syndrome (FXS) is the most common single-gene cause of autism spectrum disorder and intellectual impairment (Wang et al., 2012), with a frequency of about 1 in 7,100 males and 1 in 11,100 females (Hunter et al., 2014). FXS is associated with impairments in learning and memory, hyperactivity, hypersensitivity to sensory stimuli, increased susceptibility to seizures, and autistic behaviors (Penagarikano et al., 2007). The majority of FXS cases result from the epigenetic transcriptional silencing of the Fragile X Mental Retardation 1 gene (FMR1), which subsequently prevents the expression of fragile X mental retardation protein (FMRP) (Pieretti et al., 1991; Verkerk et al., 1991; Coffee et al., 1999, 2002; Eiges et al., 2007). FMRP is responsible for the translational regulation of many mRNAs (Miyashiro et al., 2003; Darnell et al., 2011; Ascano et al., 2012) including those involved in synaptic plasticity (Bassell and Warren, 2008; Darnell et al., 2011), and has been implicated in regulating the proliferation and differentiation of embryonic and adult neural precursor cells (NPCs) (Castrén et al., 2005; Eadie et al., 2009; Tervonen et al., 2009; Luo et al., 2010; Guo et al., 2011). A loss of FMRP and the associated memory and learning impairments observed in FXS, may therefore be linked to aberrant regulation of NPCs in the hippocampus.
There is a strong correlation between learning and memory capacity and hippocampal neurogenesis. A hallmark of the dentate gyrus (DG) of the hippocampal formation is lifelong neurogenesis to which NPCs contribute (Altman and Das, 1965). The enhanced plasticity of immature newborn neurons is suggested to enable learning and memory (Deng et al., 2010; Snyder and Cameron, 2012). In fact, many neurological diseases marked by cognitive decline such as Alzheimer’s, Parkinson’s, depression, and epilepsy, all show alterations in hippocampal neurogenesis (Zhao et al., 2008). Similarly, conditional knockout of FMRP in adult NPCs results in increased NPC proliferation, and the consequent impairment of hippocampus dependent learning (Guo et al., 2011). Notably, the majority of DG granule cells are born postnatally in the rodent brain (Altman and Bayer, 1990), and most adult born neurons are generated from early-born NPCs that reside in the DG as it is forming (Mathews et al., 2010). It is unknown whether early postnatal dysregulation of NPCs has ramifications on neurogenesis throughout life, or whether later interventions can correct abnormalities. Regulation of neurogenesis via FMRP is likely to impact early-born NPCs in the developing DG since expression of FMRP peaks at postnatal day 7 (Lu et al., 2004), which may underlie abnormal hippocampus-dependent memory in 3-week-old Fragile X mice (Bilousova et al., 2009).
The role of FMRP and its downstream effects have been largely limited to studies of neural populations in isolation. However, emerging evidence suggests that the glial environment and the role of FMRP in these cells are just as critical for proper brain development. Astrocytes for example, previously believed to be only support cells, secrete factors that actively regulate neurogenesis, neural function and communication (Barres, 2008). When cortical astrocytes devoid of FMRP are co-cultured with wild type (WT) neurons, the growth of the neurons is affected and normal synaptic formations among them are limited (Jacobs and Doering, 2010a). Of particular interest, the astrocytes within the hippocampus have been shown to promote NPC proliferation and neuronal differentiation (Song et al., 2002), and we hypothesized that a lack of FMRP in astrocytes may contribute to altered neural precursor/stem cell production.
To test this hypothesis, we used the Neural Colony Forming Cell (NCFC) Assay to examine the proliferation of neural stem cells within WT or Fragile X mice (Fmr1-KO) hippocampal neurospheres cultured in the presence of astrocyte conditioned media (ACM). The NCFC assay is specifically designed to distinguish between functional stem cells that form neurospheres more than 2 mm in diameter from neurospheres produced by the general precursor cell population with limited proliferative capacity (Louis et al., 2008). We found that Fragile X progenitor-derived neurospheres showed restricted proliferation in the presence of both WT and Fmr1-KO ACM. On the other hand, the proliferation of WT neural progenitor-derived neurospheres selectively decreased with Fmr1-KO ACM, while that of WT stem cell-derived neurospheres was enhanced. Interestingly, one population of WT neural progenitor-derived neurospheres showed decreased proliferation in the presence of cortical Fmr1-KO ACM, but not hippocampal Fmr1-KO ACM, highlighting a regional difference in astrocyte-secreted factors. Additionally, we compared the protein expression profiles of secreted factors from cortical and hippocampal astrocytes between WT and Fmr1-KO brains. Of the multiple proteins with differential expression that we detected, we identified the following proteins using mass spectrometry: multidrug resistance protein 1B, microphthalmia-associated transcription factor (MITF), haptoglobin, fasciculation, and elongation protein zeta 2, antithrombin III, and serum albumin. Together, these results indicate that signals derived from Fmr1-KO astrocytes affect the proliferation of WT neurospheres, and that Fmr1-KO neurospheres have intrinsic deficits in responding to environmental cues as evident in their indiscriminate response to WT versus Fmr1-KO ACM.
Materials and Methods
Animals
All animal experiments were performed in accordance with the guidelines set by the Canadian Council on Animal Care and were approved by the McMaster University Animal Research Ethics Board. The transgenic mouse colony was established from breeding pairs of FVB.129P2(B6)-Fmr1tm1Cgr mice obtained from Dr. Carl Dobkin at the New York State Institute for Basic Research in Developmental Disabilities (Staten Island, NY, USA). The WT and Fmr1-knockout (KO) mice were housed and maintained at the McMaster University Central Animal Facility.
Neural Colony Forming Cell Assay
WT and Fmr1-KO postnatal day 1 (P1) mouse pups were decapitated and the brains processed for the NCFC Assay, using a modified protocol (Louis and Reynolds, 2010). Briefly, hippocampi were microdissected, minced into small pieces, and enzymatically digested for 20 min at 37°C (Pacey et al., 2006). The enzymes were inactivated by incubating the cell pellet for 10 min at 37°C in 4 mL of 1 mg/mL solution of trypsin inhibitor (Roche, Mississauga, ON, Canada) dissolved in NeuroCult complete NSC proliferation medium (StemCell Technologies, Vancouver, Canada). Cells were resuspended in 1 mL of NeuroCult complete NSC proliferation medium in the presence of 20 ng/mL epidermal growth factor (EGF, Sigma–Aldrich, St. Louis, USA), 10 ng/mL fibroblast growth factor-basic (FGF-2, Sigma–Aldrich), and 2 μg/mL heparin (Sigma–Aldrich). The cell suspension was passed through a 40-μm cell strainer and the cell density brought to a concentration of 650 cells/μL. For 2 replicates/culture, the following components were added in order: 1.7 mL NeuroCult NCFC Serum-Free Medium without cytokines, 330 μL NeuroCult NSC Proliferation Supplements (mouse), 6.6 μL EGF (of 10 μg/mL), 3.3 μL FGF-2 (of 10 μg/mL), 6.6 μL heparin (of 0.2%), 25 μL of cells at 650 cells/μL to a final density of 8,000 cells/35 mm culture dish, and 1.3 mL collagen solution. The neurosphere colonies were replenished with Complete NeuroCult Replenishment medium at 7 and 14 days in vitro (DIV) containing 0.5 μg/mL EGF, 0.25 μg/mL FGF, and 0.1 mg/mL heparin.
Astrocyte-Conditioned Medium
Monolayers of cortical and hippocampal astrocytes were established (Jacobs and Doering, 2010b; Jones et al., 2012). P2 cortices of WT and Fmr1-KO mice were dissected and minced in Hank’s Buffered Salt Solution (Life Technologies, Burlington, ON, Canada) supplemented with HEPES (Life Technologies) and enzymatically digested in 0.25% trypsin (Life Technologies) supplemented with 1 mg/mL DNase (Roche). Enzymatic digestion of cells was inactivated by the addition of glial medium, which is comprised of minimal essential media (MEM) with Earl salts and L-glutamine (Life Technologies) supplemented with 10% horse serum (Life Technologies) and 0.6% glucose, and the cell suspension was passed through a 70 μm strainer. Cells were resuspended in glial medium and plated in T-75 cm2 flasks. Hippocampal astrocyte cultures were established using a modified protocol previously published (Jones et al., 2012). P2 hippocampi of WT and Fmr1-KO mice were dissected and minced in Hank’s Buffered Salt Solution supplemented with HEPES and mechanically digested in glial media of the same composition as that used for cortical cultures. Cells were resuspended in glial media and plated in T-75 cm2 flasks previously coated with 0.1 mg/mL Poly-D-Lysine Hydrochloride (Sigma–Aldrich) in 0.1 M borate buffer, pH 8.5. When hippocampal and cortical astrocytes reached 50% confluence, glial medium was switched to serum-free medium containing a final concentration of 1.25 ng/mL FGF-2 and no EGF (Pacey et al., 2006).
Astrocyte-conditioned media (ACM) was collected and concentrated 10X in concentrator tubes (Sartorius Vivaspin 20 Concentrator 5000 MWCO) at 3000 xg at 4°C. ACM was added to the NCFC assay when applicable at 1X concentration, and the concentrations of NeuroCult NCFC Serum-Free Medium, NeuroCult NSC Proliferation Supplements (mouse), EGF (10 μg/mL), FGF-2 (10 μg/mL), and heparin (0.2%) were adjusted to 9/10X at plating when ACM was used. Neurosphere colonies were cultured in different combinations listed in Table 1.
Table 1.
Plating combinations of neurospheres and astrocyte conditioned media. Sample size (n) in parentheses.
| Neurosphere genotype | Media condition |
|---|---|
| WT | No ACM (n = 4) |
| WT cortical ACM (n = 4) | |
| WT hippocampal ACM (n = 3) | |
| Fmr1-KO cortical ACM (n = 5) | |
| Fmr1-KO hippocampal ACM (n = 5) | |
| Fmr1-KO | No ACM (n = 5) |
| WT cortical ACM (n = 5) | |
| WT hippocampal ACM (n = 5) | |
| Fmr1-KO cortical ACM (n = 5) | |
| Fmr1-KO hippocampal ACM (n = 5) | |
As a control, neurosphere proliferation was tested in the presence of 1X concentrated SFM containing 1.25 ng/mL FGF-2.
Neurosphere Measurements
Neurosphere colonies were measured after 21 DIV on a 2 mm × 2 mm grid culture dish, using the 2X objective on the EVOS XL Core Cell Imaging System (Life Technologies). Images were obtained using the 4X objective. Individual neurospheres were classified into 4 categories based on diameter: ≤0.5 mm, 0.5–1 mm, 1–2 mm, ≥ 2 mm as previously documented (Louis and Reynolds, 2010). Analysis included WT (n = 9) and Fmr1-KO (n = 9) cultures without the addition of ACM, and with the addition of WT and Fmr1-KO cortical and hippocampal ACM in groups as shown in Table 1.
Proteomic Analysis
Astrocyte conditioned media from WT and Fmr1-KO cortices and hippocampi were analyzed for protein expression using two-dimensional difference in gel electrophoresis (2D DIGE). ACM was 2D DIGE-analyzed by Applied Biomics (Hayward, CA, USA). Spots with the highest differential expression between WT and Fmr1-KO ACM as determined by 2D DIGE were selected for identification (4 spots from cortical ACM and 4 from hippocampal ACM, Supplementary Tables S1 and S2). Protein identification was based on peptide fingerprint mass mapping (using MS data) and peptide fragmentation mapping (using MS/MS data).
Statistical Analyses
Statistical analysis was performed using GraphPad Prism (version 5.0). Two-way analysis of variance followed by Bonferroni’s post hoc test was used in Figure 1, and all other pairwise comparisons were done using a two-tailed Student’s t-test (Figures 2 and 3). Probabilities of p < 0.05 were considered significant. Data are expressed as group means and error bars represent the standard error of the mean (SEM).
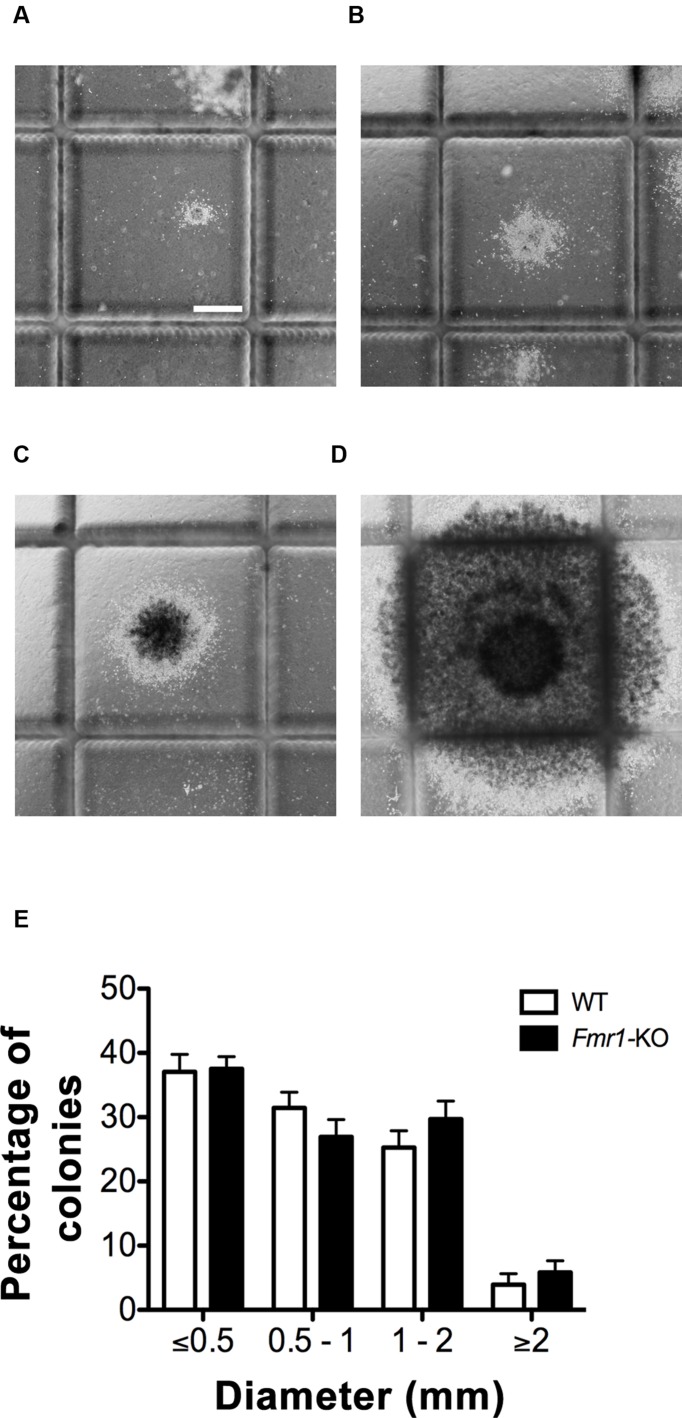
FIGURE 1.
Wild type (WT) and Fmr1-KO hippocampi generated four size categories of neurosphere colonies. (A) Neurospheres ≤0.5 mm in diameter. (B) Neurospheres 0.5–1 mm in diameter. (C) Neurospheres 1–2 mm. (D) Neurospheres ≥2 mm. (E) No difference in the percentage of neurosphere colonies present between WT and Fmr1-KO cultures in each of the four size categories. Scale bar = 500 μm (A–D). Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout.
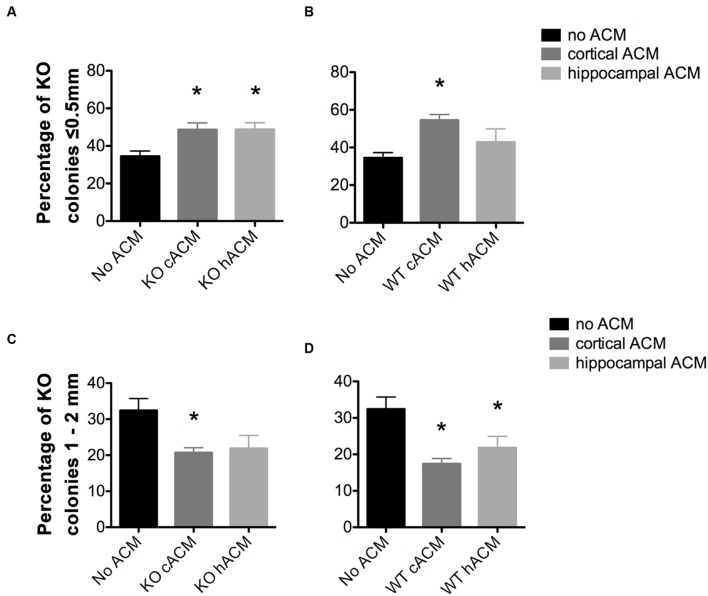
FIGURE 2.
Astrocyte conditioned media (ACM) decreased the size of Fmr1-KO progenitor-derived neurospheres. (A) Both cortical and hippocampal ACM from Fmr1-KO astrocytes increased the percentage of KO neurospheres ≤0.5 mm relative to KO neurospheres without ACM (p = 0.014; p = 0.014, respectively). (B) WT cortical ACM also increased the percentage of KO neurospheres (p = 0.001) ≤0.5 mm relative to KO neurospheres without ACM. (C) Cortical Fmr1-KO ACM decreased the percentage of KO neurospheres 1–2 mm relative to KO neurospheres without ACM (p = 0.011), and hippocampal KO ACM resulted in a near significant decrease (p = 0.063). (D) Cortical and hippocampal WT ACM decreased the percentage of neurospheres 1–2 mm (p = 0.0031, p = 0.049) relative to neurospheres without ACM. ∗ denotes a significant difference compared to neurospheres with no ACM. Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout; ACM, astrocyte conditioned media.
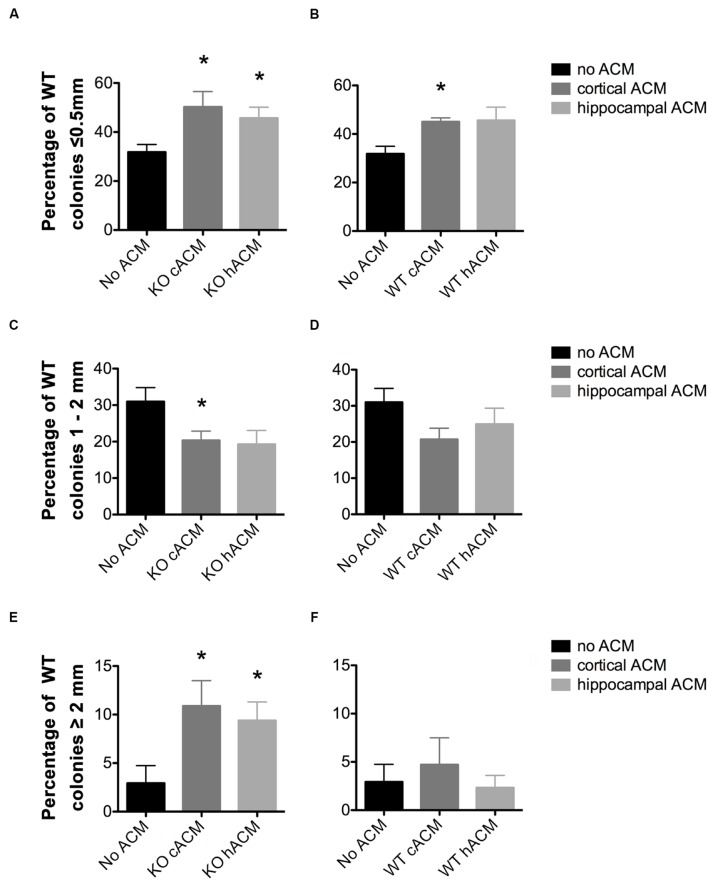
FIGURE 3.
WT neurospheres respond more selectively to Fmr1-KO ACM. (A) Conditioned media from cortical and hippocampal KO astrocytes increased the percentage of neurospheres ≤0.5 mm relative to WT neurospheres without ACM (p = 0.046, p = 0.049, respectively). (B) WT Cortical ACM also increased the percentage of neurospheres ≤0.5 mm (p = 0.009) relative to WT neurospheres without ACM. WT hippocampal ACM caused a near significant increase in the percentage of WT neurospheres (p = 0.066). (C) Fmr1-KO cortical and hippocampal ACM decreased the percentage of WT neurospheres 1–2 mm (p = 0.049, p = 0.069, respectively). (D) No effect of WT ACM on WT neurospheres 1–2 mm in diameter. (E) Increased percentage of WT neurospheres ≥2 mm in diameter in the presence of cortical (p = 0.050) and hippocampal (p = 0.047) KO ACM relative to WT neurospheres without ACM. (F) No effect of WT ACM on WT neurospheres ≥2 mm in diameter. ∗ denotes significant difference from WT neurospheres with no ACM (p < 0.05). Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout; ACM, astrocyte conditioned media.
Results
WT and Fmr1-KO Cultures Generate Similar Sizes of Neurospheres
The NeuroCult® Neural Colony-Forming Cell (NCFC) Assay distinguishes stem from progenitor cell-derived neurospheres by generating clonally derived neurospheres in collagen that can be discriminated on the basis of size [(Louis et al., 2008); Figures 1A–D]. Neurosphere colonies larger than 2 mm in diameter contain cells with high proliferative potential and fulfill the functional criteria of stem cells (Louis et al., 2008). After 21 DIV, no differences were detected in the proportion of neurosphere colonies in each of the four size categories between WT and Fmr1-KO cultures (≤0.5 mm, 0.5–1 mm, 1–2 mm, ≥2 mm diameter, Figure 1E). However, the larger the neurosphere size category, the fewer the percentage of neurospheres in that category regardless of genotype (F3,64 = 68.16, p < 0.0001), as expected since fewer NPCs have an increased proliferative capacity (Reynolds and Rietze, 2005). Compared to neurospheres ≤0.5 mm in diameter, there were 86.9% fewer neurospheres with a diameter ≥2 mm. Together, these results indicated that while there were significantly more neurospheres with a lower proliferative potential, lack of FMRP did not affect the distribution of neurospheres in the 4 size categories after 21 DIV in our culture system, and thus the proportion of stem or progenitor cell-derived neurospheres was not different when comparing the two genotypes.
ACM Modulates the Size of Fmr1-KO Neurospheres
Our previous work has shown that signals from Fragile X astrocytes contribute to the immature neuronal morphology characteristic of Fragile X (Jacobs and Doering, 2010a; Jacobs et al., 2010), and so we examined whether Fragile X astrocyte-secreted factors could affect the proliferative capacity of neurospheres. We collected ACM from primary astrocyte cultures and added it to cultured NPCs in collagen as outlined (Table 1). When testing for variances between groups, we found a significant interaction between ACM conditions and Fmr1-KO neurosphere size (F12,80 = 2.69, p = 0.0042). ACM did not affect the total number of Fmr1-KO colonies generated (Supplementary Figure S1).
A larger proportion of Fmr1-KO neurospheres measured ≤0.5 mm in parallel to a smaller proportion of neurospheres that were 1–2 mm, in the presence of WT and Fmr1-KO ACM. Specifically, there was an increase in the percentage of small neurospheres (≤0.5 mm) in Fmr1-KO cultures (34.51 ± 2.76%) with the application of ACM from the Fmr1-KO cortex (48.62 ± 3.59%, p = 0.014; Figure 2A), Fmr1-KO hippocampus (48.77 ± 3.59%, p = 0.014, Figure 2A), and WT cortex (54.48 ± 3.01%, p = 0.001, Figure 2B). At the same time, application of ACM from the Fmr1-KO cortex (20.73 ± 1.35%, p = 0.011, Figure 2C), WT cortex (17.43 ± 1.42%, p = 0.0031, Figure 2D), and WT hippocampus (21.83 ± 3.13%, p = 0.049, Figure 2D) caused a decrease in the percentage of larger neurospheres (1–2 mm) relative to Fmr1-KO neurospheres without ACM (32.41 ± 3.30%). Interestingly, ACM did not affect the percentages of Fmr1-KO neurospheres measuring 0.5–1 mm or ≥2 mm (Supplementary Figure S2). These results demonstrated that astrocyte secreted factors decreased Fmr1-KO neurosphere size, suggesting that WT and Fmr1-KO ACM, from the cortex and the hippocampus, restricted the proliferation of progenitor-derived neurospheres.
WT Neurospheres Have an Enhanced Sensitivity to Fmr1-KO ACM
The addition of ACM significantly affected the generation of WT neurospheres of different sizes (F12,64 = 2.69, p = 0.0013). This is evident from the reduced total number of WT colonies generated in the presence of ACM from cortical Fmr1-KO astrocytes (Supplementary Figure S1). In addition, the percentage of WT neurospheres measuring ≤0.5 mm in diameter increased in the presence of both WT and Fmr1-KO ACM. ACM from the Fmr1-KO cortex (50.24 ± 6.28%, p = 0.046, Figure 3A), Fmr1-KO hippocampus (45.66 ± 1.59%, p = 0.049, Figure 3A), and WT cortex (45.04 ± 1.59%, p = 0.009; Figure 3B) increased the percentage of WT neurospheres ≤0.5 mm relative to WT neurospheres without ACM (31.83 ± 3.09%). Notably, ACM from WT astrocytes had no effect on WT neurospheres measuring 0.5–1 mm, 1–2 mm (Figure 3D), or ≥2 mm (Figure 3F).
On the other hand, ACM from the Fmr1-KO cortex (20.31 ± 2.58%, p = 0.049, Figure 3C) and Fmr1-KO hippocampus (19.26 ± 3.78%, p = 0.069, Figure 3C) reduced the percentage of WT neurospheres 1–2 mm diameter compared to WT neurospheres without ACM (30.97 ± 3.86%). Interestingly, only ACM from the Fmr1-KO cortex (18.58 ± 3.56%, p = 0.012, Supplementary Figure S2) decreased the percentage of WT neurospheres measuring 0.5–1 mm compared to those without ACM (34.27 ± 2.71%), potentially due to the genotype-region mismatch of Fmr1-KO cortical ACM and WT hippocampal neurospheres (data not shown).
Fmr1-KO ACM Increases Stem Cell Production in WT Neurospheres
In sharp contrast, the percentage of WT neurospheres ≥2 mm, originating from functional stem cells, increased in the presence of ACM from the Fmr1-KO cortex (10.88 ± 2.62%, p = 0.050; Figure 3E) and the Fmr1-KO hippocampus (9.40 ± 1.92%, p = 0.047; Figure 3E) in comparison to cultures without ACM (2.93 ± 1.81%). The proportion of neurospheres ≥2 mm did not change in the presence of ACM from WT astrocytes from the cortex (4.71 ± 2.78%, p = 0.61; Figure 3F) or the hippocampus (2.31 ± 1.29%, p = 0.81 Figure 3F). Taken together, the results showed that Fmr1-KO ACM but not WT ACM significantly affected WT neurosphere size. This suggests that Fmr1-KO astrocytes provide aberrant signals in the control of NPC proliferation.
Differential Expression of Astrocyte-Secreted Proteins in Fragile X
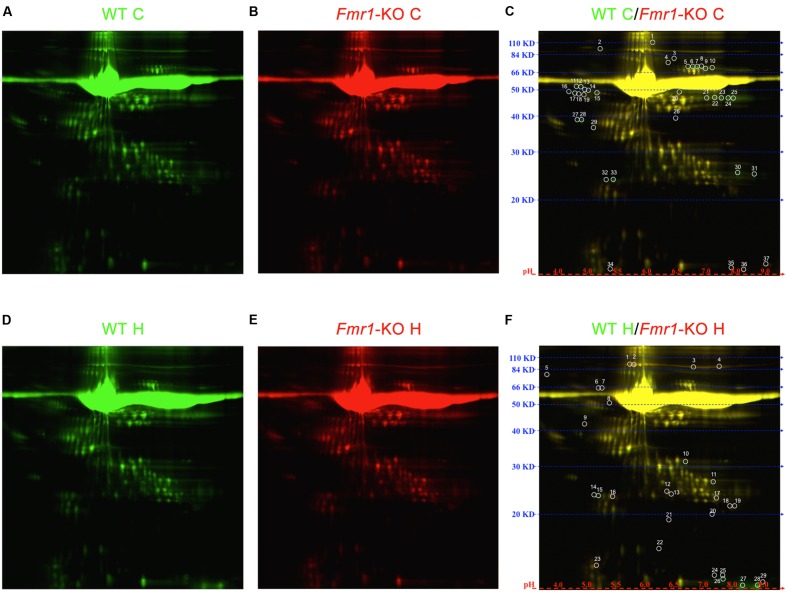
Proteomic analyses were performed on ACM to determine the identity of secreted proteins and compare between WT and Fmr1-KO astrocytes Data Sheet. 2D DIGE of spots that were at least expressed at ± 1.5 fold in Fmr1-KO compared to WT samples revealed 37 spots in cortical ACM (Figures 4A–C) and 29 spots in hippocampal ACM (Figures 4D–F). Eight of the spots were identified by mass spectrometry as: multidrug resistance protein 1B, MITF, haptoglobin, fasciculation, and elongation protein zeta 2 (FEZ2), antithrombin III, and serum albumin (Table 3). Three spots corresponded to serum albumin, which were not immediately adjacent on the gel possibly due to post-translational modifications or disintegration. Notably, the expression of the majority of astrocyte-secreted proteins from the Fmr1-KO cortex was downregulated relative to WT (34 out of 37 spots, Supplementary Table S1), whereas most secreted proteins from the Fmr1-KO hippocampus were upregulated compared to WT (18 out of 29 spots, Supplementary Table S2). Differences between cortical and hippocampal astrocyte-secreted factors again confirm the heterogeneity of astrocytes.
FIGURE 4.
2D DIGE of cortical and hippocampal ACM. Proteome map of ACM from (A) WT cortical samples or (B) Fmr1-KO cortical samples. (C) 37 spots showed at least 50% expression difference in ACM from Fmr1-KO cortices relative to their WT counterparts. Proteome map of ACM from (D) WT hippocampal samples or (E) Fmr1-KO hippocampal samples. (F) 29 spots showed at least 50% expression difference in ACM from Fmr1-KO hippocampi. Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout; ACM, astrocyte conditioned media; C, cortical; H, hippocampal; 2D DIGE, two-dimensional difference in gel electrophoresis.
Table 3.
Identity of proteins in ACM.
| Spot number | Gel | Protein | Fold difference (Fmr1-KO/WT) | Theoretical PI/MW | Protein score C.I. (%) | Total ion C.I. (%) |
|---|---|---|---|---|---|---|
| 2 | 1 (cortical ACM) | Multidrug resistance protein 1B | -4.9 | 8.5/140,905 | 0 | 97 |
| 16 | Microphthalmia-associated transcription factor (MITF) | -4.8 | 5.9/58,590 | 90 | 100 | |
| 28 | Haptoglobin | -10.6 | 5.9/38,728 | 100 | 100 | |
| 33 | Fasciculation and elongation protein zeta-2 (FEZ2) | -12.9 | 4.5/39,076 | 99 | 91 | |
| 8 | 2 (hippocampal ACM) | Antithrombin-III | 2.4 | 6.1/51,971 | 100 | 100 |
| 25 | Serum albumin | -7.0 | 5.8/68,648 | 100 | 100 | |
| 27 | Serum albumin | -25.7 | 5.8/68,648 | 100 | 100 | |
| 28 | Serum albumin | -45.3 | 5.8/68,648 | 100 | 100 | |
Discussion
In this study, we examined potential differences in the proportions of neural progenitor and neural stem cells between WT and Fmr1-KO NPCs in vitro using the NCFC Assay. Our results confirmed that the NCFC Assay generates four size categories of neurospheres, in turn reflecting different proliferative potentials in the NPC population (Louis et al., 2008). We also found that Fmr1-KO neural progenitor cells had a non-autonomous cell deficit in correctly responding to astrocyte secreted factors. This was evident in the indiscriminate response of Fmr1-KO neurospheres to ACM from WT or Fmr1-KO astrocytes, which contrasted with the specific response of WT neurospheres to Fmr1-KO, but not WT, ACM (Table 2). Interestingly, Fmr1-KO ACM increased the proliferation of WT functional neural stem cells, highlighting the abnormal signals that astrocytes send to NPCs in the absence of FMRP.
Table 2.
Summary of results.
| Neurosphere Size | WT ACM | KO ACM | |
|---|---|---|---|
| WT Neurospheres | ≤0.5 mm | Δ↑ | Δ↑ |
| 0.5–1 mm | no Δ | Δ↓ | |
| 1–2 mm | no Δ | Δ↓ | |
| ≥2 mm | noΔ | Δ↑ | |
| KO Neurospheres | ≤0.5 mm | Δ↑ | Δ↑ |
| 0.5–1 mm | no Δ | no Δ | |
| 1–2 mm | Δ↓ | Δ↓ | |
| ≥2 mm | noΔ | no Δ | |
NPCs comprise populations of neural stem and progenitor cells. Neural stem cells self-renew and are multipotent, giving rise to all neural lineages, namely neurons, astrocytes, and oligodendrocytes. Neural progenitor cells, on the other hand, have limited proliferative capacity and restricted potential in differentiating to distinct lineages. Previous research has reported enhanced NPC proliferation in the embryonic SVZ (Castrén et al., 2005), the embryonic VZ and SVZ combined (Tervonen et al., 2009), and the adult DG (Luo et al., 2010; Guo et al., 2011) in association with lack of FMRP. Given this, we expected to observe differences between WT and Fmr1-KO cultures in the proportions of neural progenitor- or stem cell-derived neurospheres generated. Our results alternatively show that there were in fact no inherent differences between the proliferative propensities of the neurospheres of each genotype, in line with Eadie et al. (2009), who reported no differences in the proliferation of NPCs in the DG of adult Fmr1-KO mice in vivo (Eadie et al., 2009). It is noteworthy to mention that our study is the first to examine hippocampal NPCs from the Fmr1-KO mouse brain on an FVB genetic background. While we detected no differences in neural progenitor and stem cell proliferation in our culture system between WT and Fmr1-KO cultures after 21 DIV, differences may exist at other time points especially since FMRP is developmentally regulated (Till et al., 2012). Till et al. (2012) indicate that Fmr1-KO mice show delays in cortical development at time points that coincide with the highest FMRP levels in normal brains. Notably, astrocytes of the developing hippocampus express FMRP (Pacey and Doering, 2007), suggesting that astrocytes contribute to the hippocampal impairments seen in Fragile X.
We identified a subset of astrocyte-secreted factors whose expression was up- or down-regulated by at least 50% in Fmr1-KO samples. These included multidrug resistance protein 1B, MITF, haptoglobin, and FEZ2 in cortical ACM, and antithrombin III and serum albumin in hippocampal ACM. These factors were all downregulated with the exception of antithrombin III (Supplementary Tables S1 and S2). Notably, the expression of multidrug resistance protein 1 has been reported in human NPCs where it promotes the proliferation of NPCs (Yamamoto et al., 2009). Thus, our finding of decreased multidrug resistance protein 1 expression in Fmr1-KO cortical ACM is in agreement with the decreased neural progenitor proliferation detected in the presence of Fmr1-KO cortical ACM. Unlike the multidrug resistance family of proteins, the function of the FEZ family of proteins is elusive. Previous research has found that FEZ2 interacts with 59 proteins in categories that include transcription, translation, apoptosis, signal transduction, neuronal cell development, and cytoskeleton and centrosome (Alborghetti et al., 2011). The function of FEZ2 remains poorly understood at this time. The expression of albumin and antithrombin III in the developing brain under normal conditions suggests that they play an important role in brain development (Dziegielewska et al., 1984; Deschepper et al., 1991). In fact, astrocytes of the developing brain have been shown to secrete antithrombin III in culture, similar to our findings (Deschepper et al., 1991). Albumin is also necessary for astrocytes to provide energy to the developing brain (Tabernero et al., 1999) and to synthesize oleic acid (Tabernero et al., 2001). In turn, oleic acid promotes neuronal differentiation (Tabernero et al., 2001). Given that astrocytes show developmental delays in FXS (Jacobs et al., 2010), we suggest that the expression levels of albumin and antithrombin III that we detected in Fmr1-KO hippocampal ACM may mirror the expression levels in ACM from the WT hippocampus at earlier time points. We are currently studying the role of the astrocyte secreted factors in Fragile X in order to better understand the extent of astrocyte involvement in Fragile X and associated neurodevelopmental disorders.
Astrocyte-secreted factors, such as thrombospondin-1 (Lu and Kipnis, 2010), fibroblast growth factor-2 (Kirby et al., 2013), and clusterin (Cordero-Llana et al., 2011) promote NPC proliferation, neurogenesis, or both. Indeed, astrocytes are an important component of the hippocampal neurogenic niche (Song et al., 2002). Our previous work has shown that signals from Fmr1-KO astrocytes can alter the development and growth of co-cultured WT neurons, an effect not seen with WT astrocytes (Jacobs and Doering, 2010a). Thus, we hypothesized that ACM from WT astrocytes would correct aberrant proportions of progenitor and stem cell-derived Fmr1-KO neurospheres, whereas Fmr1-KO ACM would alter the proportions of each of the WT neurosphere size categories. Our results demonstrated that ACM from Fmr1-KO astrocytes did alter the proliferation of WT neural progenitor and functional stem cells, restricting the proliferation of neural progenitors while increasing the proliferation of neural stem-derived neurospheres. It remains to be determined why Fmr1-KO ACM exerts opposite effects on neural progenitor compared to neural stem cells.
Intriguingly, the percentage of WT neurospheres measuring ≤0.5 mm increased in the presence of WT and Fmr1-KO ACM from the cortex and the hippocampus, similar to Fmr1-KO neurospheres of the same size category. These neurospheres have a limited ability for self-renewal (Louis et al., 2008). The generic response of WT neurospheres to ACM is interesting, as it was only detected in this size category, and mirrors the effect of ACM on Fmr1-KO neurospheres. This suggests a non-cell autonomous response of WT NPCs of limited potential as demonstrated by their generic response to astrocyte-secreted factors regardless of FMRP expression.
Lack of FMRP caused a defect in Fmr1-KO NPCs to respond correctly to environmental cues. Fmr1-KO neurospheres showed an indiscriminate response toward WT and Fmr1-KO ACM. We found that ACM from WT and Fmr1-KO astrocytes caused a global increase in the proportion of Fmr1-KO neurospheres ≤0.5 mm. In parallel, ACM decreased the proportion of Fmr1-KO neurospheres 1–2 mm in diameter relative to Fmr1-KO neurospheres of the same size without ACM. Collectively, this indicates that ACM restricted the proliferation of Fmr1-KO neural progenitors without affecting the proliferation of stem cells. The fact that the percentages of some Fmr1-KO neurosphere size categories (i.e., 0.5–1 mm and ≥2 mm) did not change in the presence of ACM suggests that not all NPC populations are targets of astrocyte-secreted factors in Fragile X.
In contrast to Fmr1-KO NPCs, only ACM from Fmr1-KO astrocytes decreased the percentage of WT neurospheres 1–2 mm, and increased that of neurospheres ≥2 mm in diameter. The selective response of WT neurospheres to Fmr1-KO ACM points to a non-cell autonomous defect in NPC proliferation in the absence of FMRP caused by abnormal Fmr1-KO astrocytic cues. This was particularly evident in the effect of ACM on neurospheres ≥2 mm in diameter, where the percentage of WT neurospheres was increased in the presence of Fmr1-KO ACM, while that of Fmr1-KO neurospheres was not. This population of neurospheres represents multipotent and proliferative functional stem cells (Louis et al., 2008). The apparent aberrant signaling of Fmr1-KO astrocytes may be due to compensatory measures for the lack of responsiveness of Fmr1-KO neurospheres to cellular or environmental cues.
The addition of Fmr1-KO cortical ACM to WT neurospheres caused the most robust changes, affecting the total number of neurospheres generated (Supplementary Figure S1) as well as the percentages of neurospheres in all size categories relative to WT neurospheres without ACM (Supplementary Figure S2), which highlights the heterogeneity of astrocytes (Matyash and Kettenmann, 2010). This is likely due to the genotypic-spatial mismatch between hippocampal WT neurospheres and cortical Fmr1-KO ACM. Indeed, of all combinations tested, ACM from Fmr1-KO cortical astrocytes decreased the percentage of WT colonies measuring 0.5–1 mm. Thus, the proliferation of NPCs that make up colonies 0.5–1 mm is not solely changed by astrocyte-secreted factors from Fragile X brains. Rather, the enhanced effect of Fmr1-KO cortical ACM on WT hippocampal neurospheres resulted in our finding, which seems to only apply to neurospheres 0.5–1 mm. In agreement with the specificity of astrocytes, hippocampal astrocytes are shown to promote NPC proliferation and neurogenesis, whereas spinal cord astrocytes do not (Song et al., 2002). Similarly, ACM from the hippocampus increases the number of neurons differentiated from human NPCs, whereas cortical ACM does not (Cordero-Llana et al., 2011).
One of the functions of hippocampal neurogenesis is learning and memory (Deng et al., 2010). We thus hypothesized that impaired learning, characteristic of FXS, is attributed to aberrant NPC proliferation and neurogenesis. Fmr1-KO mice have demonstrated deficits in hippocampus-dependent learning such as exaggerated inhibitory avoidance extinction (Dölen et al., 2007) and trace conditioning tasks (Hayashi et al., 2007). Interestingly, conditional ablation of FMRP from adult NPCs results in learning deficits on the trace conditioning and delayed non-matching-to-place radial arm maze tasks, both of which require an intact hippocampus, and conditional restoration of FMRP results in improved performance on these tasks (Guo et al., 2011). Therefore, functional restoration of hippocampal neurogenesis may alleviate learning and memory impairments in Fragile X.
Summary
In this study, we examined the proliferation of hippocampal neural progenitor and stem cells from the early postnatal Fmr1-KO mouse brain. We found that Fmr1-KO progenitor-derived neurospheres showed decreased proliferation in response to WT and Fmr1-KO ACM, thereby demonstrating non-cell autonomous defects in responding correctly to astrocyte-secreted factors. In contrast, WT neurospheres showed a specific response to Fmr1-KO ACM, where Fmr1-KO ACM restricted the proliferation of neural progenitors, and enhanced the proliferation of stem cells. We also identified some of the astrocyte secreted factors with ± 1.5 fold expression difference in Fmr1-KO brains. To our knowledge, this is the first time that astrocyte-secreted factors are assayed and a number of them identified with mass spectrometry in relation to Fragile X. In turn, this work opens up new possibilities in investigating the functions of these factors and uncovering the role of astrocytes in Fragile X and neurodevelopmental disorders.
Author Contributions
MS conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. LD conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Hongjin Huang for 2D DIGE and mass spectrometry experiments, Dr. Jane Foster for valuable statistical input, and Dr. Angela Scott for critical reading of the manuscript.
Footnotes
Funding. This work was supported by grants to LD from NSERC, and Brain Canada/Azrieli Neurodevelopmental Research Program, and a Vanier Canada Graduate Scholarship to MS.
Supplementary Material
FIGURE S1 | Total number of colonies generated by the addition of ACM to WT and Fmr1-KO neurospheres. Abbreviations: WT, wild type; KO, Fmr1 knockout; ACM, astrocyte conditioned media; c, cortical; h, hippocampal.
FIGURE S2 | Summary of the effect of astrocyte conditioned media (ACM) on neurosphere size. (A) WT neurospheres, and (B) Fmr1-KO neurospheres. ∗ denotes significant difference from neurospheres with no ACM (p < 0.05). Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout; ACM, astrocyte conditioned media.
TABLE S1 | Spots from cortical ACM with at least 1.5 fold difference relative to WT.
TABLE S2 | Spots from hippocampal ACM with at least 1.5 fold difference relative to WT.
References
- Alborghetti M. R., Furlan A. S., Kobarg J. (2011). FEZ2 has acquired additional protein interaction partners relative to FEZ1: functional and evolutionary implications. PLoS ONE 6:e17426 10.1371/journal.pone.0017426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. (1990). Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 301 365–381. 10.1002/cne.903010304 [DOI] [PubMed] [Google Scholar]
- Altman J., Das G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124 319–335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Ascano M., Mukherjee N., Bandaru P., Miller J. B., Nusbaum J. D., Corcoran D. L., et al. (2012). FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492 382–386. 10.1038/nature11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A. (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60 430–440. 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Bassell G. J., Warren S. T. (2008). Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60 201–214. 10.1016/j.neuron.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T. V., Dansie L., Ngo M., Aye J., Charles J. R., Ethell D. W., et al. (2009). Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46 94–102. 10.1136/jmg.2008.061796 [DOI] [PubMed] [Google Scholar]
- Castrén M., Tervonen T., Kärkkäinen V., Heinonen S., Castrén E., Larsson K., et al. (2005). Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl. Acad. Sci. U.S.A. 102 17834–17839. 10.1073/pnas.0508995102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B., Zhang F., Ceman S., Warren S. T., Reines D. (2002). Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile x syndrome. Am. J. Hum. Genet. 71 923–932. 10.1086/342931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B., Zhang F., Warren S. T., Reines D. (1999). Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 22 98–101. 10.1038/8807 [DOI] [PubMed] [Google Scholar]
- Cordero-Llana O., Scott S. A., Maslen S. L., Anderson J. M., Boyle J., Chowhdury R.-R., et al. (2011). Clusterin secreted by astrocytes enhances neuronal differentiation from human neural precursor cells. Cell Death. Differ. 18 907–913. 10.1038/cdd.2010.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Van Driesche S. J., Zhang C., Hung K. Y. S., Mele A., Fraser C. E., et al. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146 247–261. 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Aimone J. B., Gage F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11 339–350. 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschepper C. F., Bigornia V., Berens M. E., Lapointe M. C. (1991). Production of thrombin and antithrombin III by brain and astroglial cell cultures. Brain Res. Mol. Brain Res. 11 355–358. 10.1016/0169-328X(91)90045-Y [DOI] [PubMed] [Google Scholar]
- Dölen G., Osterweil E., Rao B. S. S., Smith G. B., Auerbach B. D., Chattarji S., et al. (2007). Correction of fragile X syndrome in mice. Neuron 56 955–962. 10.1016/j.neuron.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., New H., Reynolds M. L., Saunders N. R. (1984). Synthesis of plasma proteins by rat fetal brain and choroid plexus. Int. J. Dev. Neurosci. 2 215–222. 10.1016/0736-5748(84)90015-7 [DOI] [PubMed] [Google Scholar]
- Eadie B. D., Zhang W. N., Boehme F., Gil-Mohapel J., Kainer L., Simpson J. M., et al. (2009). Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol. Dis. 36 361–373. 10.1016/j.nbd.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Eiges R., Urbach A., Malcov M., Frumkin T., Schwartz T., Amit A., et al. (2007). Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Stem Cell 1 568–577. 10.1016/j.stem.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Guo W., Allan A. M., Zong R., Zhang L., Johnson E. B., Schaller E. G., et al. (2011). Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 17 559–565. 10.1038/nm.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. L., Rao B. S. S., Seo J.-S., Choi H.-S., Dolan B. M., Choi S.-Y., et al. (2007). Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 104 11489–11494. 10.1073/pnas.0705003104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J., Rivero-Arias O., Angelov A., Kim E., Fotheringham I., Leal J. (2014). Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A 164A, 1648–1658. 10.1002/ajmg.a.36511 [DOI] [PubMed] [Google Scholar]
- Jacobs S., Doering L. C. (2010a). Astrocytes prevent abnormal neuronal development in the fragile x mouse. J. Neurosci. 30 4508–4514. 10.1523/JNEUROSCI.5027-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Doering L. C. (2010b). “Primary dissociated astrocyte and neuron coculture,” in Protocols for Neural Cell Culture, 4th Edn, ed. Dooring L. C. (New York: Humana Press; ), 269–283. [Google Scholar]
- Jacobs S., Nathwani M., Doering L. C. (2010). Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci. 11:132 10.1186/1471-2202-11-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. V., Cook D., Murai K. K. (2012). A neuron-astrocyte co-culture system to investigate astrocyte-secreted factors in mouse neuronal development. Methods Mol. Biol. 814 341–352. 10.1007/978-1-61779-452-0_22 [DOI] [PubMed] [Google Scholar]
- Kirby E. D., Muroy S. E., Sun W. G., Covarrubias D., Leong M. J., Barchas L. A., et al. (2013). Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife 2:e00362 10.7554/eLife.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S. A., Reynolds B. A. (2010). Neurosphere and Neural Colony-Forming Cell Assays. Protoc. Neural Cell Cult. 10 1–28. [Google Scholar]
- Louis S. A., Rietze R. L., Deleyrolle L., Wagey R. E., Thomas T. E., Eaves A. C., et al. (2008). Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells 26 988–996. 10.1634/stemcells.2007-0867 [DOI] [PubMed] [Google Scholar]
- Lu R., Wang H., Liang Z., Ku L., O’Donnell W. T., Li W., et al. (2004). The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl. Acad. Sci. U.S.A. 101 15201–15206. 10.1073/pnas.0404995101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Kipnis J. (2010). Thrombospondin 1–a key astrocyte-derived neurogenic factor. FASEB J. 24 1925–1934. 10.1096/fj.09-150573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Shan G., Guo W., Smrt R. D., Johnson E. B., Li X., et al. (2010). Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 6:e1000898 10.1371/journal.pgen.1000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews E. A., Morgenstern N. A., Piatti V. C., Zhao C., Jessberger S., Schinder A. F., et al. (2010). A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J. Comp. Neurol. 518 4479–4490. 10.1002/cne.22489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V., Kettenmann H. (2010). Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63 2–10. 10.1016/j.brainresrev.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Miyashiro K. Y., Beckel-Mitchener A., Purk T. P., Becker K. G., Barret T., Liu L., et al. (2003). RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37 417–431. 10.1016/S0896-6273(03)00034-5 [DOI] [PubMed] [Google Scholar]
- Pacey L., Stead S., Gleave J., Tomczyk K., Doering L. (2006). Neural stem cell culture: neurosphere generation, microscopical analysis and cryopreservation. Protoc. Exch. 10.1038/nprot.2006.215 [DOI] [Google Scholar]
- Pacey L. K. K., Doering L. C. (2007). Developmental expression of FMRP in the astrocyte lineage: implications for fragile X syndrome. Glia 55 1601–1609. 10.1002/glia.20573 [DOI] [PubMed] [Google Scholar]
- Penagarikano O., Mulle J. G., Warren S. T. (2007). The Pathophysiology of Fragile X Syndrome. Annu. Rev. Genom. Hum. Genet. 8 109–129. 10.1146/annurev.genom.8.080706.092249 [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., et al. (1991). Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66 817–822. 10.1016/0092-8674(91)90125-I [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Rietze R. L. (2005). Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods 2 333–336. 10.1038/nmeth758 [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Cameron H. A. (2012). Could adult hippocampal neurogenesis be relevant for human behavior? Behav. Brain Res. 227 384–390. 10.1016/j.bbr.2011.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Stevens C., Gage F. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417 39–44. 10.1038/417039a [DOI] [PubMed] [Google Scholar]
- Tabernero A., Lavado E. M., Granda B., Velasco A., Medina J. M. (2001). Neuronal differentiation is triggered by oleic acid synthesized and released by astrocytes. J. Neurochem. 79 606–616. 10.1046/j.1471-4159.2001.00598.x [DOI] [PubMed] [Google Scholar]
- Tabernero A., Medina A., Sánchez-Abarca L. I., Lavado E., Medina J. M. (1999). The effect of albumin on astrocyte energy metabolism is not brought about through the control of cytosolic Ca2+ concentrations but by free-fatty acid sequestration. Glia 25 1–9. [DOI] [PubMed] [Google Scholar]
- Tervonen T. A., Louhivuori V., Sun X., Hokkanen M.-E., Kratochwil C. F., Zebryk P., et al. (2009). Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome. Neurobiol. Dis. 33 250–259. 10.1016/j.nbd.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Till S. M., Wijetunge L. S., Seidel V. G., Harlow E., Wright A. K., Bagni C., et al. (2012). Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21 2143–2156. 10.1093/hmg/dds030 [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., et al. (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905 10.1016/0092-8674(91)90397-H [DOI] [PubMed] [Google Scholar]
- Wang T., Bray S. M., Warren S. T. (2012). New perspectives on the biology of fragile X syndrome. Curr. Opin. Genet. Dev. 22 256–263. 10.1016/j.gde.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Shofuda T., Islam M. O., Nakamura Y., Yamasaki M., Okano H., et al. (2009). ABCB1 is predominantly expressed in human fetal neural stem/progenitor cells at an early development stage. J. Neurosci. Res. 87 2615–2623. 10.1002/jnr.22094 [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132 645–660. 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 | Total number of colonies generated by the addition of ACM to WT and Fmr1-KO neurospheres. Abbreviations: WT, wild type; KO, Fmr1 knockout; ACM, astrocyte conditioned media; c, cortical; h, hippocampal.
FIGURE S2 | Summary of the effect of astrocyte conditioned media (ACM) on neurosphere size. (A) WT neurospheres, and (B) Fmr1-KO neurospheres. ∗ denotes significant difference from neurospheres with no ACM (p < 0.05). Abbreviations: WT, wild type; Fmr1-KO, Fmr1 knockout; ACM, astrocyte conditioned media.
TABLE S1 | Spots from cortical ACM with at least 1.5 fold difference relative to WT.
TABLE S2 | Spots from hippocampal ACM with at least 1.5 fold difference relative to WT.