Abstract
Recent studies show right hemisphere has a unique contribution to emotion processing. The present study investigated EEG using non-linear measures during emotional processing in PD patients with respect to motor symptom asymmetry (i.e., most affected body side). We recorded 14-channel wireless EEGs from 20 PD patients and 10 healthy age-matched controls (HC) by eliciting emotions such as happiness, sadness, fear, anger, surprise and disgust. PD patients were divided into two groups, based on most affected body side and unilateral motor symptom severity: left side-affected (LPD, n = 10) or right side-affected PD patients (RPD, n = 10). Nonlinear analysis of these emotional EEGs were performed by using approximate entropy, correlation dimension, detrended fluctuation analysis, fractal dimension, higher order spectra, hurst exponent (HE), largest Lyapunov exponent and sample entropy. The extracted features were ranked using analysis of variance based on F value. The ranked features were then fed into classifiers namely fuzzy K-nearest neighbor and support vector machine to obtain optimal performance using minimum number of features. From the experimental results, we found that (a) classification performance across all frequency bands performed well in recognizing emotional states of LPD, RPD, and HC; (b) the emotion-specific features were mainly related to higher frequency bands; and (c) predominantly LPD patients (inferred right-hemisphere pathology) were more impaired in emotion processing compared to RPD, as showed by a poorer classification performance. The results suggest that asymmetric neuronal degeneration in PD patients may contribute to the impairment of emotional communication.
Keywords: EEG, Emotion, Hemispheric lateralization, Non-linear methods, Parkinson’s disease
Background
Parkinson’s disease (PD) is characterized by the progressive loss of dopamine neurons in the substantia nigra of the midbrain, and is associated with motor symptoms including tremor, bradykinesia and rigidity (Han et al. 2013). Apart from these motor symptoms, there has been increasing attention to the role played by emotional processes in PD patients. Indeed, a huge number of studies have been conducted in the recent years with the goal to understand if PD patients are still able to correctly identify, discriminate, and rate the emotional content of the stimuli (e.g., pictures, prerecorded speech samples, written sentences) (Gray and Tickle-Degnen 2010; Péron et al. 2012). Unfortunately, the experimental results so far are inconsistent and quite difficult to interpret. Some researchers reported that PD patients perform worse than healthy control (HC) participants in a number of recognition tasks, there is also evidence that the two groups do not differ in the same tasks (Gray and Tickle-Degnen 2010; Péron et al. 2012).
More recently, lateralization (left versus right hemisphere) of emotion processing with respect to most affected body side in PD patients has been debated. For instance, (Clark et al. 2008) reported no asymmetry effects on explicit emotion categorization while Ariatti et al. (2008) and Yip et al. (2003) reported problems in categorizing disgust prosody in patients with right-affected PD patients. In addition, Ventura et al. (2012) reported that left-affected PD patients (LPD) exhibit problems in the recognition of sadness emotion. Lately Garrido-Vásquez et al. (2013) reported altered emotional salience detection from prosody in patients primarily suffering from right-affected PD patients (RPD) using event related potential measures. Some studies showed that patients with left- or right affected motor symptoms side perform poorly in emotional prosodic identification tasks compared to HC (Pell and Baum 1997; VanLancker and Sidtis 1992). Thus, results regarding the influence of hemispheric asymmetric PD patients on emotion processing remain inconclusive. However, most of the research in this area have dealt with behavioral responses (i.e. participants were asked to match, to identify or to rate emotional stimuli), which are known to be impaired in PD (Wieser et al. 2006). It could be that this overall behavioral impairment causes impaired performances in evaluative emotion recognition and rating tasks. Furthermore, only commonly used statistical tools were employed to analyze the obtained behavioral responses.
Machine learning algorithms are becoming increasingly popular in psychology and psychophysiology research and they indeed might be useful as an additional tool to traditional statistical methods. The expression of an emotion occurs as a result of physiological changes in the central nervous system (CNS) and/or autonomic nervous system (ANS). For instance, the muscle tension in the face gives rise to facial actions (Picard et al. 2001). Researchers have showed significant differences between the emotional states in HC participants using different biosignals such as electroencephalogram (EEG), electrocardiogram (ECG), electromyogram (EMG), skin conductance (SC), skin temperature (ST), respiration rate (RR) and blood volume pulse (BVP) (Valenza et al. 2012; Verma and Tiwary 2014). These biosignals, being an activity of the CNS and/or ANS reflects the inherent state of the person which makes the suppression of emotions or social masking impossible. In particular, emotion research on EEG signals for HC participants show promising results (since the EEG signals are recorded from origin of emotion genesis) and hence can be enhanced to understand the emotion processing of PD patients.
Moreover, it is well known that underlying physiological mechanisms of biological systems are non-linear processes. As the human brain is composed of billions of complicatedly interconnected neurons whose responses are non-linear, it may be regarded as a complex, non-linear dynamic system. To analyse the output of such a system, non-linear signal processing techniques are applicable. There has been growing evidence that non-linear research studies have been devoted to analysis of EEG signals recorded from participants with pathological conditions such as Alzheimer’s, dementia, depression, disturbed cognition, epilepsy, schizophrenia and sleep disorders as well as the brain function related to emotional states (Bleton and Sejdic 2015; Liu et al. 2015; Poppy and Speckens 2015; Stam 2005). These studies shows that non-linear analyses are more in tune with nature of EEG signals and systems, thus, they are widely used in biological and medical applications.
In this study, we set out to investigate nonlinear analysis of EEG during emotion processing in PD patients with respect to motor symptom asymmetry (i.e., most affected body side). For this purpose, we extracted non-linear features such as approximate entropy (AE), correlation dimension (CD), detrended fluctuation analysis (DFA), fractal dimension (FD), higher order spectrum (HOS), hurst exponent (HE), largest Lyapunov exponent (LLE) and sample entropy (SE). These extracted features were ranked using analysis of variance (ANOVA) method. Two different classifiers namely fuzzy K-nearest neighbor (FKNN) and support vector machine (SVM) were used to examine the performance of extracted features based on ranking to classify six basic emotional states (happiness, sadness, fear, anger, surprise, and disgust) of LPD, RPD compared to HC participants.
Materials used
Participants
Twenty right-handed individuals (10F, 10M) with Parkinson’s disease (including 10 LPD and 10 RPD motor symptoms) and 20 healthy right-handed age (range of 45 and 65 years), gender, education level-matched controls participated in this study (Table 1). Side of symptom onset was obtained from self-report, and current affected side was determined by the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn et al. 1987) and self-reports. The severity of Parkinsonian symptoms was I–III on the Hoehn and Yahr stage scale (Han et al. 2013; Hoehn and Yahr 1967). All PD patients were on medication (dopamine replacement therapy), and were recruited through the Neurology Unit outpatient service at the Department of Medicine of the Hospital University Kebangsaan Malaysia (HUKM) medical center in Kuala Lumpur, Malaysia. The HC participants were recruited through the hospital’s medical unit community and/or from patient’s relatives. All participants were native speakers of Malaysia and handedness was determined by self-report and confirmed by Edinburgh Handedness Inventory (EHS) (Oldfield 1971). This test consisted of 10 questions asking for the preferred hand for a series of activities (e.g., writing, throwing, and using scissors).
Table 1.
Summary of demographic and clinical characteristics of LPD, RPD, and HC participants
| Characteristics | LPD (n = 10) | RPD (n = 10) | HC (n = 20) | Test’s value | p value* |
|---|---|---|---|---|---|
| Age (45–65 years) | 57.60 ± 5.32 | 59.10 ± 3.75 | 58.10 ± 2.95 | F(2,37) = 0.365 | 0.697 |
| Gender | F = 5, M = 5 | F = 5, M = 5 | F = 11, M = 9 | x2 = 0.066 | 0.796 |
| Education (years) | 11.30 ± 4.27 | 11.20 ± 3.49 | 11.05 ± 3.34 | F(2,37) = 0.005 | 0.995 |
| MMSE (25–30) | 27.50 ± 1.35 | 26.40 ± 1.26 | 27.15 ± 1.63 | F(2,37) = 2.181 | 0.127 |
| H&Y (I/II/III) | 2.30 ± 0.67 | 2.50 ± 0.53 | NA | F(1,18) = 0.545 | 0.470 |
| Motor UPDRS | 16.70 ± 1.70 | 18.30 ± 4.60 | NA | F(1,18) = 1.066 | 0.470 |
| Disease severity (range 1–12 years) | 6.25 ± 3.26 | 6.45 ± 3.95 | NA | F(1,18) = 0.015 | 0.903 |
| BDI (0–18) | 6.30 ± 3.13 | 6.60 ± 3.89 | 5.45 ± 2.18 | F(2,37) = 0.016 | 0.984 |
| EHS (1–10) | 9.90 ± 0.32 | 9.60 ± 0.70 | 9.84 ± 0.72 | F(2,37) = 0.429 | 0.655 |
Mean ± standard deviation scores are reported. One-way ANOVA was used to test the group effect
LPD left-affected PD patients, RPD right-affected PD patients, HC healthy controls, F female, M male, MMSE mini-mental state exam, H & Y Hoehn & Yahr, UPDRS Unified Parkinson’s’ Disease Rating Scale, BDI Beck Depression Inventory, NA not applicable
* Group effect is significant at p < 0.05 level
Inclusion and exclusion criteria
Participants who had normal or corrected vision and normal hearing capabilities were included in the study. Participants with history of neurological disease (e.g. stroke, epilepsy, and significant head trauma), significant vision impairment, depression severity (Beck Depression Inventory [BDI] score ≥18; (Beck et al. 1961; Schröder et al. 2006), and global cognitive deterioration (Mini Mental Sate Examination [MMSE] score ≤24; (Folstein et al. 1975; Wieser et al. 2006) were excluded from this study.
Ethical statement
Ethical approval of the study was granted by the HUKM Faculty of Medicine Institutional Review Board (Ref. number: UKM1.5.3.5/244/FF-354-2012), and informed written consent was obtained from each participant/caretaker prior to testing. Each participant was paid 50 Malaysian Ringgit (US $15) for their participation.
Experiment design
In the present study, the emotional stimuli were taken from different sources, namely International Affective Picture System (IAPS) database, International Affective Digitized Sounds (IADS) database and video clips collected from the internet resources. The emotions namely sadness, fear, and disgust are elicited using IAPS and IADS databases. Whereas, the elicitation of happiness, surprise, and anger emotion is attained by using video clips (through a pilot study). The experiment protocol had two sessions of three trials each with a break of 10–15 min in between the sessions. The participants were allowed to relax during the break since the continuous assessment may be too exhausting. The multimodal stimuli relating to the six emotional states (happiness, sadness, fear, anger, surprise and disgust) are displayed in random order for various trials. Each combination of picture and sound is presented for 6 s. To maximize the participants’ emotional reactivity, each clip block consisted of six combinations of the same emotional category and lasted for 36 s. Moreover, each video clips varied 36–45 s, depending on the length of the clip. Neutral images, which can calm down the participants, are displayed for 10 s at the start of each trial. This will help the participants return to the normal or neutral state away from emotional excitation. Besides, a 15 s rating interval (Hamdi et al. 2012) was provided in between the clips in which participants completed a 5-point self-assessment questionnaire. Each session takes approximately 30 min. A more detailed description of the stimuli materials selection and experimental procedure used in this experiment can be found in (Yuvaraj et al. 2014a, b).
EEG recordings
Emotional EEG signals were recorded using the Emotive EPOC 14-channel wireless (2.4 GHz band) neuroheadset with a sampling rate of 128 Hz (Hadjidimitriou and Hadjileontiadis 2012). The electrodes were arranged at the scalp sites AF3, AF4, F7, F8, F3, F4, FC5, FC6, T7, T8, P7, P8, O1 and O2, according to the 10–20 system, referenced to the common mode sense (CMS-left mastoid)/driven right leg (DRL-right mastoid) ground.
Methodology
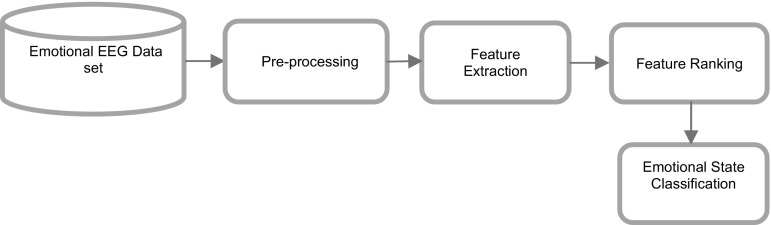
The emotional EEG data obtained from the experiments were analysed through several procedures, including preprocessing, feature extraction, feature ranking and emotional state classification, as shown in Fig. 1.
Fig. 1.
Block diagram of emotional state classification from EEG signals
Preprocessing
The time-series waveform of EEG data were pre-processed using thresholding method to remove movement artifacts (such as eye movement/blinking), in which data that are found to have amplitudes of more than 80 μV are discarded from the study (Gotlib et al. 1998; Poppy and Speckens 2015). Then, a 6th order bandpass IIR Butterworth filter was used to extract signals in the frequency range of 1–49 Hz. The standard frequency bands of interest were delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz) and gamma (30–49). Each channel of the artifact-free emotional EEG data was divided into 6 s length of epochs without overlapping using time windows (Yuvaraj et al. 2014b). Finally, the non-linear features were computed on each epoch of the emotional EEG data of LPD, RPD and HC participants and are subsequently explained.
Feature extraction
The non-linear methods of AE, CD, DFA, FD, HOS, HE, LLE and SE, described in this section, were used to extract the features from the pre-processed EEG signals. A total of 11 non-linear features were extracted from LPD, RPD and HC emotional EEG signals by using these methods.
Approximate entropy (AE)
This method is used in time series signal data analysis to quantify the regularity. The AE values are inversely proportional to the degree of regularity of a time series. In this work, the formula proposed by (Pincus and Goldberger 1994) is used to detect the variations in the emotional EEG signals that are not reflected in the amplitude or peaks. AE is given by (Subha et al. 2010)
| 1 |
where m specifies the embedding dimension, r is the radial distance, N is the total number of data samples and C(r) is the correlation integral. For this study, m is set to 2 and r is set to 0.2 times of the standard deviation of the data. These values are selected on the basis of previous studies indicating good statistical validity for AE using these values (Pincus and Goldberger 1994).
Correlation dimension (CD)
This method measures the dimensionality of signal in relation to its geometrical reconstruction in phase space. Herein, we have used the approach proposed by (Grassberger and Procassia 1983). Mathematically, CD can be described by (Subha et al. 2010)
| 2 |
where c(r) is the correlation integral, r is the radial distance around each reference point and m is the embedding dimension. In our analysis we have chosen an embedding dimension of 10 and radial distance of 0.2.
Detrended fluctuation analysis (DFA)
This method was proposed by (Peng et al. 1995) as a technique to quantify the fractal properties of the time series non-stationary signals. In this work, a fractal scaling component “α” was used to describe the nature of emotional EEG signals. This exponent gives an indication of the short and long term correlation behaviour of signals (Yuvaraj et al. 2014b).
Fractal dimension (FD)
This method is used to measure the EEG signal complexity and is a powerful tool for transient detection. Different algorithms have been used by researchers to determine the value of FD (Higuchi 1988; Katz 1998). In this work, Higuchi’s method was used.
Higher order spectrum (HOS)
HOS elicits both amplitude and phase information of a given signal. It offers good noise immunity and yields good results, even for weak and noisy signals. HOS consist of moment and cumulant spectra and can be used for both deterministic signals and random processes (Chua et al. 2010). In this work, we derived the features from the third-order statistics of the signal, namely, the bispectrum and it is given by Eq. 3. The bispectrum displays symmetry and is evaluated in the principal domain region, denoted by Ω (Chandran and Elgar 1993). The bispectrum mean magnitude (BS_Mag), bispectrum phase entropy (BS_PhEnt), normalized bispectral entropy (BS_Ent1) and normalized bispectral squared entropy (BS_Ent2) were computed in this paper (Chua et al. 2010).
| 3 |
where is the bispectrum in the bifrequency , is the discrete time Fourier transform of the given signal, and * denotes complex conjugate. To calculate the above bispectrum features, we used epochs of 768 samples (6 s) with Hanning window of 50 % overlap at a sampling rate of 128 Hz. Each epoch was taken from the record of 1024 NFFT points.
Hurst exponent (HE)
This method is used to measure the self-similarity and predictability of the EEG time series signals. This method also indicates whether a range of signal samples shows any sign of asymptomatic behavior when observed for a particular time span. The HE is defined as (Subha et al. 2010)
| 4 |
where T is the duration of the sample of data, (M/N) is the corresponding value of rescaled range, M is the difference between the maximum deviation from the mean and minimum deviation from the mean and N is the standard deviation.
Largest Lyapunov exponent (LLE)
The LLE estimates how sensitive a system is to initial conditions and it estimates the predictability of a signal. The LLE extraction algorithm detects a nearest neighbor for each point and observes how the distance between them changes over time. The LLE is calculated using least squares fit to ‘average’ line defined by (Rosenstein et al. 1993)
| 5 |
where represents the distance between the th phase-space point and its nearest neighbour at th time step and denotes the average overall phase-space points.
Sample entropy (SE)
This method is a modified version of AE used for the assessment of time series signal complexity and regularity measurement. The SE value will be low for repeating signal patterns. It provides an improvised assessment of time-series regularity and better tool to study the dynamics of emotional EEG signals as compared to AE. SE is given by the formula (Subha et al. 2010)
| 6 |
where B(0) = N, the length of the input series and k is the embedding dimension. In our work, k value was found to be 2.
Feature ranking
The feature extraction step generally results in a large number of features, and many of these features might not have significant information to effectively differentiate six emotional states. Therefore, the common practice is to apply feature ranking algorithms on the extracted features to retain only those informative features useful for classification and also reduces the complexity for the classifier without affecting its performance. In this work, ANOVA was used for this purpose (Acharya et al. 2015; Kobayashi et al. 2011). For each non-linear feature, the test generates two parameters, namely the p value and F value. The p value is used to identify the significance of the features: the lower the p value, the higher the significance. The F value is used to rank the features: the higher the F value, the better is the rank and the feature.
Emotional state classification
Classifiers of FKNN and SVM were used to assess the association between EEG and emotional states of LPD, RPD and HC. The details are subsequently explained.
Fuzzy K-nearest neighbour (FKNN)
FKNN classifier assigns a class based on the predominant class among the k nearest neighbors. Euclidean distance was used as the metric in FKNN allocating fuzzy class membership before making decisions. The fuzzy strength parameter m is used to determine how heavily the distance is weighted when calculating each neighbor’s contribution to the membership value. Here, the m value is varied between 1 and 2 with steps of 0.01, and the classification performance is obtained using k-values between 1 and 10.
Support vector machine (SVM)
SVM classifier is a supervised learning method, which performs the classification by constructing hyperplane in an n-dimensional space where n is the number of input features. The hyperplane constructed separates input data classes. Using non-linear kernel function, the input data is transformed to a high-dimensional feature space so that the transformed data becomes more separable than the original input data. In this work, Radial Basis Function (RBF) kernel was used (Yuvaraj et al. 2014b). For RBF-SVM, we used the different combinations of the cost parameter (C) and kernel parameter (ϒ): and .
The tenfold cross validation method was used to evaluate the performance of classifiers. The original feature vectors were divided into ten equal sets. The first nine sets were used for training the classifier and the tenth set was used for testing the classifier. This process was then repeated ten times using different sets of test data. Classification performance was evaluated through the classification accuracy (CA) and is computed between six emotional states of LPD, RPD and HC as,
| 7 |
where refers to the six emotional states namely happiness, sadness, fear, anger, surprise and disgust of PD patients and HC (i.e., , and ), across delta, theta, alpha, beta, gamma EEG frequency bands and ALL (combination of five frequency bands). The overall performance of the classifier is evaluated by taking the average and standard deviation (SD) of the accuracies of the tenfold classification. Herein, ten participants (10) per group with six emotional states (6), six trials per emotion (6), and six epochs per channel (6) for each band resulted in a total of 2160 × 14 (electrodes) feature vectors, which were processed.
Results and discussion
Participants characteristics
ANOVA was conducted to ensure that the three participant groups (LPD, RPD and HC participants) were comparable with regard to demographic and clinical variables. As can be seen in Table 1, three groups did not differ in demographic variables such as age, gender (the ratios of male to female participants in each group [Chi square test]) and years of education. There were no significant differences between the groups for the MMSE, F (2, 37) = 2.181, p = 0.127, the BDI, F (2, 37) = 0.016, p = 0.984 and EHI scores, F (2, 37) = 0.429, p = 0.655. The scores on the measures of H & Y, F (1, 18) = 0.545, p = 0.470, disease severity, F (1, 18) = 0.015, p = 0.903 and motor performance, F (1, 18) = 1.066, p = 0.316, did not show any significant differences across PD groups. These results indicate that recruited PD groups and HC participants were matched in terms of demographic and clinical characteristics.
Experimental results and discussion
Tables 2, 3 and 4 show the ANOVA results of various features extracted by using the non-linear techniques described in previous section from LPD, RPD and HC across different emotion EEG frequency bands. The p values and F values indicate that all the extracted features were significant (p < 0.05) enough for classification. This ensures the probability of achieving better classification accuracy. In particular, emotional features of HC show very low p value (p < 0.00001) compared to LPD and RPD (p < 0.05) among the six emotional states. This may suggest that the EEG does not reflect the emotion processing accurately in PD patients, which could be interpreted as impairment in the brain’s processing ability of emotions. Furthermore, it is seen that the F value is low for the delta and theta frequency bands, and it is high for features computed from the alpha, beta, gamma and ALL frequency bands indicating that there is more difference between the six emotional states in those frequency bands for both HC and PD groups.
Table 2.
Results of feature ranking using ANOVA among the six emotional states in each frequency band of left-affected PD (LPD)
| Non-linear feature | Delta (1–4 Hz) | Theta (4–8 Hz) | Alpha (8–13 Hz) | Beta (13–30 Hz) | Gamma (30–49 Hz) | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | |
| AE | 0.007825 | 3.748 | 0.000883 | 4.632 | 5.93E−05 | 6.821 | 7.48E−07 | 7.174 | 5.46E−08 | 8.840 | 6.64E−08 | 9.948 |
| FD | 0.008913 | 3.771 | 0.000983 | 4.843 | 7.75E−04 | 6.218 | 3.69E−05 | 7.284 | 4.23E−04 | 8.294 | 2.96E−04 | 8.425 |
| LLE | 0.002819 | 3.038 | 0.000641 | 3.258 | 5.82E−04 | 6.413 | 1.68E−04 | 6.184 | 1.49E−04 | 7.163 | 1.53E−04 | 7.274 |
| BS_PhEnt | 0.001421 | 3.742 | 0.000801 | 3.854 | 0.000613 | 5.294 | 0.000345 | 5.294 | 0.000458 | 7.714 | 1.00E−04 | 7.519 |
| HE | 0.042179 | 3.489 | 0.000154 | 3.731 | 0.000927 | 5.618 | 0.000783 | 5.845 | 0.000936 | 6.843 | 0.000684 | 7.492 |
| BS_Ent1 | 0.024712 | 2.992 | 0.047832 | 3.945 | 0.042912 | 4.8374 | 0.000115 | 5.194 | 0.000891 | 5.942 | 0.000783 | 7.194 |
| BS_Ent2 | 0.018821 | 2.822 | 0.037081 | 3.620 | 0.005491 | 4.524 | 0.006929 | 4.713 | 0.000864 | 5.849 | 0.000241 | 6.130 |
| SE | 0.020032 | 2.643 | 0.036422 | 2.883 | 0.039481 | 4.741 | 0.005554 | 4.734 | 0.004726 | 5.524 | 0.006737 | 6.913 |
| BS_Mag | 0.018993 | 2.729 | 0.040567 | 2.472 | 0.006812 | 3.571 | 0.044824 | 4.004 | 0.006843 | 4.882 | 0.008423 | 5.825 |
| CD | 0.029961 | 2.783 | 0.027629 | 2.142 | 0.042184 | 3.732 | 0.016832 | 3.801 | 0.045573 | 4.852 | 0.007831 | 5.819 |
| DFA | 0.048002 | 2.732 | 0.014452 | 2.791 | 0.024891 | 3.117 | 0.047836 | 3.663 | 0.014842 | 4.743 | 0.006725 | 4.894 |
Table 3.
Results of feature ranking using ANOVA among the six emotional states in each frequency band of right-affected PD patients (RPD)
| Non-linear feature | Delta (1–4 Hz) | Theta (4–8 Hz) | Alpha (8–13 Hz) | Beta (13–30 Hz) | Gamma (30–49 Hz) | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | |
| AE | 0.000456 | 4.391 | 2.38E−04 | 5.341 | 4.28E−07 | 8.803 | 4.73E−06 | 8.705 | 8.29E−05 | 9.218 | 6.78E−07 | 10.829 |
| FD | 0.000864 | 4.221 | 1.51E−04 | 5.820 | 3.27E−04 | 7.554 | 4.81E−04 | 8.032 | 2.88E−04 | 8.362 | 3.81E−05 | 10.524 |
| LLE | 0.000462 | 3.104 | 0.000741 | 4.706 | 2.47E−04 | 6.521 | 3.90E−04 | 7.336 | 0.000571 | 8.421 | 5.78E−04 | 9.402 |
| BS_PhEnt | 0.007831 | 3.030 | 0.000948 | 4.225 | 0.000134 | 6.731 | 0.000782 | 7.628 | 0.000285 | 7.952 | 0.000371 | 9.331 |
| HE | 0.003217 | 3.421 | 0.000164 | 3.752 | 0.000728 | 5.628 | 0.000903 | 6.732 | 0.000119 | 7.463 | 0.000993 | 8.373 |
| BS_Ent1 | 0.008537 | 3.582 | 0.006386 | 2.831 | 0.007821 | 4.445 | 0.000554 | 6.925 | 0.000792 | 6.832 | 0.000781 | 7.442 |
| BS_Ent2 | 0.029850 | 3.827 | 0.028463 | 2.510 | 0.005284 | 4.382 | 0.000382 | 5.394 | 0.003819 | 4.573 | 0.000718 | 7.926 |
| SE | 0.017276 | 3.237 | 0.037821 | 2.842 | 0.007743 | 3.872 | 0.005929 | 4.783 | 0.028493 | 4.062 | 0.000583 | 5.937 |
| BS_Mag | 0.035174 | 2.993 | 0.018934 | 2.392 | 0.047569 | 3.929 | 0.002894 | 4.378 | 0.018472 | 3.002 | 0.000886 | 4.792 |
| CD | 0.038296 | 2.917 | 0.041094 | 2.297 | 0.016837 | 3.638 | 0.048953 | 4.834 | 0.038194 | 3.572 | 0.037143 | 4.728 |
| DFA | 0.048632 | 2.932 | 0.037821 | 2.106 | 0.036380 | 3.037 | 0.016824 | 3.834 | 0.027481 | 2.691 | 0.048184 | 4.701 |
Table 4.
Results of feature ranking using ANOVA among the six emotional states in each frequency band of HC participants
| Non-linear feature | Delta (1–4 Hz) | Theta (4–8 Hz) | Alpha (8–13 Hz) | Beta (13–30 Hz) | Gamma (30–49 Hz) | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | p value | F value | |
| AE | 2.05E−07 | 7.483 | 2.02E−08 | 7.473 | 3.36E−11 | 10.280 | 4.21E−13 | 10.837 | 6.37E−10 | 16.293 | 3.04E−09 | 17.589 |
| FD | 1.78E−06 | 7.350 | 7.16E−08 | 7.485 | 6.28E−06 | 10.321 | 4.88E−10 | 10.481 | 4.17E−06 | 12.382 | 1.51E−06 | 13.284 |
| LLE | 1.35E−04 | 7.849 | 6.04E−04 | 7.294 | 4.77E−05 | 9.203 | 2.84E−08 | 9.014 | 2.94E−05 | 12.003 | 1.00E−05 | 11.805 |
| BS_PhEnt | 0.000852 | 6.390 | 0.000369 | 6.892 | 4.13E−04 | 8.382 | 0.000434 | 9.495 | 0.000142 | 10.285 | 0.000175 | 10.294 |
| HE | 0.000575 | 6.257 | 0.000615 | 6.348 | 0.000242 | 7.794 | 0.000580 | 9.155 | 0.000126 | 9.927 | 0.000155 | 9.273 |
| BS_Ent1 | 0.000628 | 5.392 | 0.001439 | 5.339 | 0.000742 | 7.063 | 0.000692 | 8.105 | 0.006906 | 8.923 | 0.000990 | 9.775 |
| BS_Ent2 | 0.000223 | 4.298 | 0.001503 | 5.321 | 0.002758 | 6.937 | 0.002687 | 7.025 | 0.003084 | 8.781 | 0.009346 | 8.724 |
| SE | 0.001693 | 3.284 | 0.001396 | 4.217 | 0.009676 | 6.786 | 0.026289 | 7.291 | 0.016095 | 7.581 | 0.00388 | 8.396 |
| BS_Mag | 0.001524 | 2.927 | 0.001504 | 2.284 | 0.003145 | 6.754 | 0.026515 | 6.801 | 0.031581 | 6.482 | 0.004893 | 7.682 |
| CD | 0.001861 | 2.721 | 0.021341 | 2.114 | 0.025202 | 6.385 | 0.017712 | 4.826 | 0.045938 | 5.361 | 0.041714 | 6.482 |
| DFA | 0.045203 | 2.933 | 0.020495 | 2.284 | 0.043371 | 4.932 | 0.030188 | 3.482 | 0.015526 | 4.381 | 0.036498 | 6.391 |
The features were ranked using their F value and fed one by one as input to FKNN and RBF-SVM classifiers for emotional state classification. The classifiers were evaluated for their performance by using tenfold cross validation to determine the highest performance accuracy with minimum number of features. Table 5 shows the average classification performance of LPD, RPD and HC across delta, theta, alpha, beta, gamma, and ALL frequency bands using RBF-SVM and FKNN classifier. It can be observed that the classification performance using ALL frequency bands outperformed any individual frequency band in all the groups. This demonstrates that the connection between emotions and EEG patterns does not occur in only one particular band, but it is evident in ALL frequency bands. The maximum average accuracy with the combination of ALL frequency band was 58.28 % ± 3.23 %, 70.86 % ± 1.82 % and 83.39 % ± 2.04 % for LPD, RPD and HC participants, respectively using RBF-SVM classifier. We obtained this performance using top 4 (AE, FD, LLE and BS_PhEnt), 6 (AE, FD, LLE, BS_PhEnt, HE and Bs_Ent1) and 3 (AE, FD and LLE) ranked features for LPD, RPD and HC, respectively. The result suggests that EEG signals, being an activity of central nervous system, can reflect the underlying inherent emotional state changes of PD patients. The main significance of the proposed emotional classification system in the clinical environment is that it is completely non-invasive and automated. This can be used to identify emotion recognition impairments in PD patients with respect to most affected side and also to direct the patients for medical treatment, such as computerized cognitive rehabilitation. As seen in Table 5, it can be found that the classification performance of alpha, beta, and gamma bands (i.e., high frequency bands) is better than those of delta and theta bands (i.e., low frequency bands) in LPD, RPD and HC. This finding is in line with our previous emotion studies that high frequency bands play a more important role in emotion activities than low frequency bands (Yuvaraj et al. 2014b). Neuroanatomically, high frequency bands play a crucial role in integrating distributed neural processes into highly ordered cognitive functions and are important in a wide range of cognitive, perceptual, attention and emotion processes (Luo et al. 2007; Sammler et al. 2007). In addition, high frequency bands have been associated with emotional processing in the amygdala (one of the most important brain region for emotion) (Oya et al. 2002). Hence the distribution of high frequency band activation recorded from the scalp surface may be significant in the results for discovering links between emotional experiences and EEG recordings.
Table 5.
Average classification accuracy ± SD across different EEG frequency bands (features ranked using ANOVA)
| Classifier | Group | No. of features | EEG frequency band (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Beta | Gamma | ALL | |||
| FKNN m = 1.12, k = 5 |
LPD | 5 | 36.19 ± 3.46 | 42.76 ± 2.88 | 48.59 ± 1.39 | 50.83 ± 2.63 | 51.65 ± 1.39 | 54.28 ± 1.60 |
| RPD | 7 | 44.82 ± 2.77 | 54.29 ± 1.62 | 55.52 ± 2.97 | 60.35 ± 1.68 | 60.35 ± 2.00 | 63.19 ± 1.57 | |
| HC | 5 | 55.42 ± 1.75 | 58.39 ± 2.33 | 60.54 ± 1.24 | 64.28 ± 2.82 | 65.24 ± 1.75 | 71.42 ± 2.58 | |
| RBF-SVM | LPD | 4 | 40.49 ± 1.65 | 50.39 ± 3.05 | 52.30 ± 2.61 | 54.85 ± 1.95 | 55.20 ± 1.05 | 58.28 ± 3.23 |
| RPD | 6 | 52.29 ± 1.83 | 55.49 ± 2.91 | 64.99 ± 1.53 | 61.63 ± 2.28 | 61.28 ± 1.99 | 70.86 ± 1.82 | |
| HC | 3 | 60.49 ± 2.75 | 66.29 ± 1.73 | 67.38 ± 2.81 | 72.38 ± 1.73 | 74.84 ± 2.83 | 83.39 ± 2.04 | |
From Table 5, it can also be noted that the average classification performance of LPD and RPD patients is less compared to HC participants in all the frequency bands. This lower accuracy indicates that EEG features of PD patients may not reflect the emotional states effectively, which may due to the impairment in their brain’s processing ability of emotions. These results are in line with studies done by other researchers indicating that there is a decrease in brain’s complexity during emotion processing due to the dysfunction in the neural circuits of PD patients (Adolphs et al. 1996; Lawrence et al. 2007). Furthermore, the results are also compatible with the more general hypothesis that a loss of complexity appears when the biological systems become functionality impaired (Jeong et al. 1998). Notably, PD patients with greater symptom severity on the left side of the body (inferred right–hemisphere pathology) were specifically impaired in emotion recognition, as showed by decreased classification accuracy compared to RPD. This finding indicate that stronger right than left-hemispheric degeneration in PD may lead to impairments in emotion recognition. This results are in line with Garrido-Vasquez (Garrido-Vásquez et al. 2013; Ventura et al. 2012), who reported that LPD had more difficulty with emotion processing since there is evidence that the processing of emotional information is right-hemisphere dominant (Yuvaraj et al. 2013). Furthermore, the right hemisphere is thought to be involved in social awareness and the in the recognition of salient social cues (Ventura et al. 2012). Finally, considering the different classifiers, the average classification performance of SVM with RBF kernel outperforms FKNN classifier. Since the SVM classifier projects input data onto a higher dimensional feature space via a RBF kernel function, in which classification can be made more easily than in the original feature space.
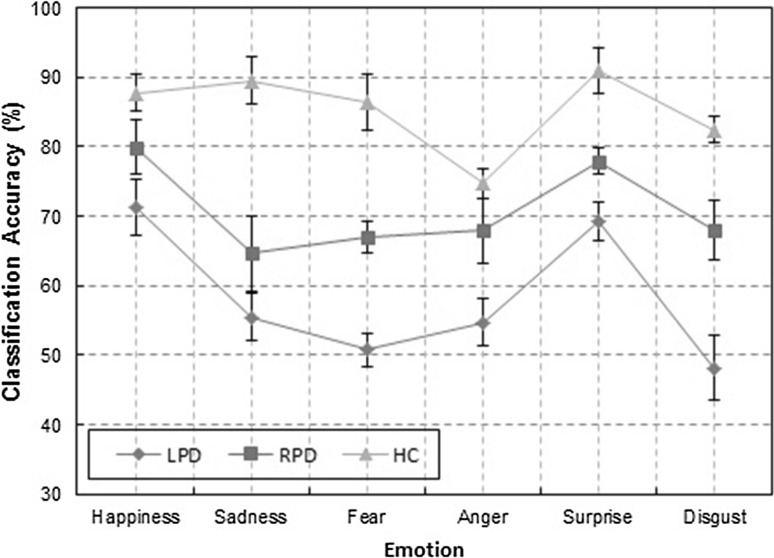
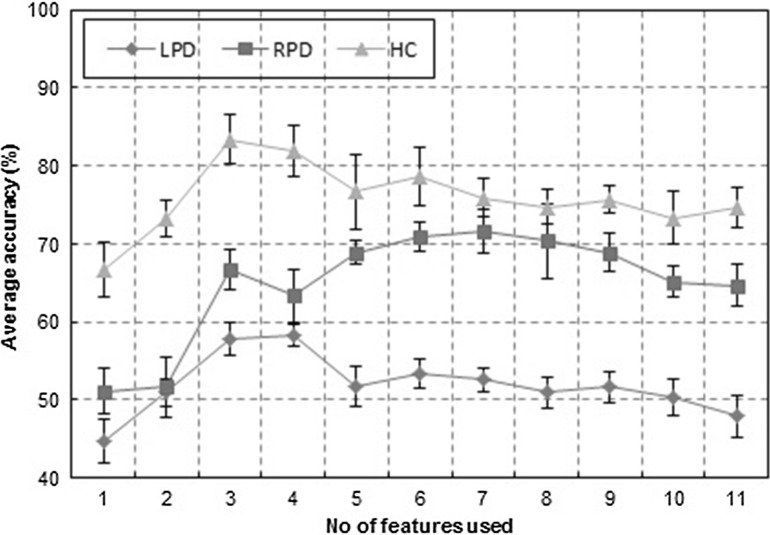
Figure 2 shows the plot of accuracy of individual emotion. It can be observed that the difficulties in PD patients appear to be more severe in recognizing negative emotions (anger, disgust, fear, and sadness) as compared to that of positive emotions (happiness and surprise), as revealed by lower classification accuracy. This evidence points to neuropathological changes in PD patients in many brain areas which are assumed to play key roles in negative emotion processing (Kober et al. 2008). These include limbic structures such as the amygdala, and the ventral striatum, which is centrally located within the basal ganglia’s limbic loop. Figure 3 shows the plot of average accuracy versus number of features for RBF-SVM classifier. It shows that beyond certain ranked features, there is a drop in the accuracy level.
Fig. 2.
Classification accuracy ± SD of Individual emotion for RBF-SVM classifier
Fig. 3.
Plot of average accuracy ± SD versus number of features for RBF-SVM classifier
On the limitation side, the use of small number of PD samples affects the reliability of the system. In order to generalize the proposed algorithm, further studies should use larger number of samples to examine the relationship between brain activity and emotions with respect to affected body side in PD. Furthermore, all PD patients were under different regimens of medication, and the two patient groups differed with respect to the distribution of motor subtypes. Therefore, the interpretation of our findings should be rather cautious and warrants further investigation.
Conclusion
This study demonstrates the utility of EEG signal in identifying true inherent emotional state of PD patients with respect to motor symptom asymmetry. Different nonlinear features were extracted across each EEG frequency band of LPD, RPD, and HC participants. After using feature ranking technique to select only the significant features, two classifiers namely FKNN and SVM were built and validated using the selected features. PD patients primarily suffering from right-hemisphere dysfunction (LPD) were more impaired in emotional communication compared to RPD. Our results may be useful for to develop a device that can automatically detect the emotional states would be helpful to medical practitioners to regulate the emotions for better clinical outcomes in PD patients.
Acknowledgments
This research was financially supported by Fundamental Research Grant Scheme (FRGS), Ministry of Higher Education, Malaysia. Grant No: 9003-00507.
References
- Acharya UR, Fujita H, Sudarshan VK, Bhat S, Koh JEW. Application of entropies for automated diagnosis of epilepsy using EEG signals: a review. Knowl Based Syst. 2015;88:85–96. doi: 10.1016/j.knosys.2015.08.004. [DOI] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Damasio AR. Corticle systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariatti A, Benuzzi F, Nichelli P. Recognition of emotions from visual and prosodic cues in Parkinson’s disease. Neurol Sci. 2008;29:219–227. doi: 10.1007/s10072-008-0971-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bleton H, Sejdic E. A cerebral blood flow evaluation during cognitive tasks following a cervical spinal cord injury: a case study using transcranial Doppler recordings. Cogn Neurodyn. 2015;9:615–626. doi: 10.1007/s11571-015-9355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, Elgar SL. Pattern recognition using invariants defined from higher order spectra-one dimensional inputs. IEEE Trans Signal Process. 1993;41:205–212. doi: 10.1109/TSP.1993.193139. [DOI] [PubMed] [Google Scholar]
- Chua KC, Chandran V, Acharya UR, Lim CM. Application of higher order statistics/spectra in biomedical signals-A review. J Med Eng Phys. 2010;32:679–689. doi: 10.1016/j.medengphy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2008;46:2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Committee M. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, Clane DB, editors. Recent developments in Parkinson’s Disease Macmillan health care information. New Jersey: Florham Park; 1987. pp. 53–163. [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-Mental State Examination: a practical method for grading the cognitive state of patients. Psychol Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garrido-Vásquez P, Pell MD, Paulmann S, Strecker K, Schwarz J, Kotz SA. An ERP study of vocal emotion processing in asymmetric Parkinson’s disease. Soc Cogn Affect Neurosci. 2013;8:918–927. doi: 10.1093/scan/nss094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Raganathan C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emot. 1998;12:449–478. doi: 10.1080/026999398379673. [DOI] [Google Scholar]
- Grassberger P, Procassia I. Measuring the strangeness of strange attractors. Phys D. 1983;9:189–208. doi: 10.1016/0167-2789(83)90298-1. [DOI] [Google Scholar]
- Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in parkinson’s disease. Neuropsychology. 2010;24:176–191. doi: 10.1037/a0018104. [DOI] [PubMed] [Google Scholar]
- Hadjidimitriou SK, Hadjileontiadis LJ. Toward an EEG-based recognition of music liking using time-frequency analysis. IEEE Trans Biomed Eng. 2012;59:3498–3510. doi: 10.1109/TBME.2012.2217495. [DOI] [PubMed] [Google Scholar]
- Hamdi H, Richard P, Suteau A, Allain P (2012) Emotion assessment for affective computing based on physiological responses. In: IEEE proceedings of world congress on computational intelligence, pp 10–15
- Han CX, Wang J, Yi GS, Che YQ. Investigation of EEG abnormalities in the early stage of Parkinson’s disease. Cogn Neurodyn. 2013;7:351–359. doi: 10.1007/s11571-013-9247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T. Approach to an irregular time series on the basis of the fractal theory. Phys D. 1988;31:277–283. doi: 10.1016/0167-2789(88)90081-4. [DOI] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jeong J, Kim SY, Han SH. Non-linear dynamical analysis of the EEG in Alzheimer’s disease with optimal embedding dimension. Electroencephalogr Clin Neurophysiol. 1998;106:220–228. doi: 10.1016/S0013-4694(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Katz MJ. Fractals and the analysis of waveforms. Comput Biol Med. 1998;18:145–156. doi: 10.1016/0010-4825(88)90041-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Mark BL, Turin W. Probabaility, random processes and statistical analysis: applications to communications, Signal processing queueing theory and mathematical finance. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wagera TD. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Impaired recogition of facial expression of anger in Parkinson’s disease patients acutely withdrawn from dopamine replacement theraphy. Neuropsychologia. 2007;45:65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Liu X et al (2015) Multiple characteristics analysis of Alzheimer’s electroencephalogram by power spectral density and Lempel–Ziv complexity. Cogn Neurodyn 1–13 [DOI] [PMC free article] [PubMed]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J NeuroSci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell MD, Baum SR. Unilateral brain damage, prosodic comprehension deficits, and the acoustic cues to prosody. Brain Lang. 1997;57:195–214. doi: 10.1006/brln.1997.1736. [DOI] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series Chaos: interdisciplinary. J Nonlinear Sci. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- Péron J, Dondaine T, Jeune FL, Grandjean D, Vérin M. Emotional processing in Parkinson’s disease: a systematic review. Mov Disord. 2012;27:186–199. doi: 10.1002/mds.24025. [DOI] [PubMed] [Google Scholar]
- Picard RW, Vyzas E, Healey J. Toward machine emotional intelligence: analysis of affective physiological state. IEEE Trans Pattern Anal Mach Intell. 2001;23:1175–1191. doi: 10.1109/34.954607. [DOI] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266(4 Pt 2):H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Poppy PLS, Speckens AE. Multi-dimensional modulations of α and γ cortical dynamics following mindfulness-based cognitive therapy in major depressive disorder. Cogn Neurodyn. 2015;9:13–29. doi: 10.1007/s11571-014-9308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein MT, Collins JJ, Luca CJD. A practical method for calculating largest Lyapunov exponents from small data sets. Phys D Nonlinear Phenom. 1993;65:117–134. doi: 10.1016/0167-2789(93)90009-P. [DOI] [Google Scholar]
- Sammler D, Grigutsch M, Fritz T, Koelsch S. Music and emotion: electrophysiological correlates of the processing of pleasant and unpleasant music. Psychophysiology. 2007;44:293–304. doi: 10.1111/j.1469-8986.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- Schröder C, et al. Perception of emotional speech in Parkinson’s disease. Mov Disord. 2006;21:1774–1778. doi: 10.1002/mds.21038. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116:2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Subha DP, Joseph PK, Acharya UR, Lim CM. EEG signal analysis: a survey. J Med Syst. 2010;34:195–212. doi: 10.1007/s10916-008-9231-z. [DOI] [PubMed] [Google Scholar]
- Valenza G, Lanata A, Scilingo EP. The role of nonlinear dynamics in affective valence and arousal recognition. IEEE Trans Affect Comput. 2012;3:237–249. doi: 10.1109/T-AFFC.2011.30. [DOI] [Google Scholar]
- VanLancker D, Sidtis JJ. The identification of affective, prosodic stimuli by left and right-hemisphere damaged subjects: all errors are not created equal. J Speech Hear Res. 1992;35:963–970. doi: 10.1044/jshr.3505.963. [DOI] [PubMed] [Google Scholar]
- Ventura MI, Baynes K, Sigvardt KA, Unruh AM, Acklin S, Kirsch HE, Disbrow EA. Hemispheric asymmetries and prosodic emotion recognition deficits in Parkinson’s disease. Neuropsychologia. 2012;50:1936–1945. doi: 10.1016/j.neuropsychologia.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Verma GK, Tiwary US. Multimodal fusion framework: a multiresolution appraoch for emtotion classification and recognition from physiological signals. Neuroimage. 2014;102:162–172. doi: 10.1016/j.neuroimage.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Muhlberger A, Alpers G, Macht M, Ellgring H, Pauli P. Emotion processing in parkinson’s disease: dissociation between early neuronal processing and explicit ratings. Clin Neurophysiol. 2006;117:94–102. doi: 10.1016/j.clinph.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Yip JT, Lee TM, Ho SL, Tsang KL, Li LS. Emotion recognition in patients with idiopathic Parkinson’s disease. Mov Disord. 2003;18:1115–1122. doi: 10.1002/mds.10497. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M, Norlinah MI, Sundaraj K, Khairiyah M. Review of emotion recognition in stroke patients. Dement Geriatr Cogn Disord. 2013;36:179–196. doi: 10.1159/000353440. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, et al. Inter-hemispheric EEG coherence analysis in Parkinson’s disease: assessing brain activity during emotion processing. J Neural Transm. 2014;122:237–252. doi: 10.1007/s00702-014-1249-4. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M, Norlinah MI, Sundaraj K, Omar MI, Khairiyah M, Palaniappan R. Optimal set of EEG features for emotional state classification and trajectory visualization in Parkinsosn’s disease. Int J Psychophysiol. 2014;94:482–495. doi: 10.1016/j.ijpsycho.2014.07.014. [DOI] [PubMed] [Google Scholar]