Abstract
Purpose
The purpose of this study was to determine which morphokinetic variables are related to embryo gender in a cohort of consecutive live births obtained through single blastocyst transfer following mild ovarian stimulation.
Methods
Eighty-one live births (49 % of them females) from successfully treated, consecutive infertile patients (maternal age 36.9 ± 3.8 years, range 28–46) who underwent minimal ovarian stimulation, prolonged embryo culture in a time-lapse monitoring (TLM) incubator and elective single blastocyst transfers during 2012–2014. Early (PNf, t2–t9, cc2a, b, s2, s3) and late (tM, tSB, tfullB, texpB1, and texpB2) morphokinetic variables were scored according to published consensus criteria and were normalized to the time of pronuclear fading. For each variable, the ranges with the highest proportion of female embryos (optimal range) were determined by detailed examination of histograms.
Results
Female embryo gender was associated both with late cleavage (t8), morula (tM), and blastocyst stage morphokinetic variables. The strongest associations (adjusted ORs, 7.0–7.8) were found for late, expanded stage blastocyst parameters; tfullB, texpB1, and texpB2. The proportion of female embryos was 69–71 and 25–26 % inside and outside of the optimal ranges, respectively. This allowed to predict 74–78 % of them, increasing their proportion by 57 % compared to the average.
Conclusions
Although the sample size of our cohort was limited, our findings suggest that several expanded blastocyst stage morphokinetic parameters are associated with female embryo gender. If confirmed on a larger sample these could be potentially used to increase the proportion of female embryos among non-invasively selected blastocysts following single embryo transfer.
Keywords: Time-lapse monitoring, Blastocyst culture, Female gender, Single embryo transfer, In vitro fertilization
Introduction
In recent years, time-lapse monitoring (TLM) technology has emerged as a potentially promising way to improve the classical morphological selection of embryos [1]. To date, a number of studies were conducted to determine whether morphokinetic variables are correlated with important outcomes such as blastocyst formation, implantation potential, and even aneuploidy status [2–4]. These resulted in different predictive models that were shown to improve overall clinical outcomes in the setting of blinded, retrospective studies and randomized clinical trials [5, 6].
Meanwhile, other TLM studies focused on whether morphokinetic variables are affected by a number of external factors including patient- (obesity, smoking) and treatment-related characteristics (gonadotropin dose, stimulation protocol) as well as laboratory and culture conditions (fertilization method, oxygen concentration, culture media) [7–13]. Among these potential confounders, embryo gender was only investigated by a handful of studies so far. Whereas the first Turkish study did not find any gender-specific differences in embryo development kinetics among time-lapse-monitored embryos, a more recently published Spanish one did find a significant difference in a few TLM variables [14, 15]. This has led to the establishment of a hierarchical model that could define subsets with increasing proportion of female embryos from 42 up to 71 % [15]. As the results of these two studies are contradicting, further investigations in this area are still necessary because TLM technology provides an inherently more precise way of evaluating embryo kinetics than any previous investigation based on static morphological criteria [16–19].
Therefore, the aim of the present retrospective analysis, in a series of confirmed live births originating from a cohort of unselected infertile patients undergoing mild ovarian stimulation and single blastocyst transfer, was to evaluate the association between embryo gender and early (cleavage stage) and late (morula and blastocyst stage) morphokinetic variables and to identify those that could be used as potential predictors.
Materials and methods
Study patients and follow-up
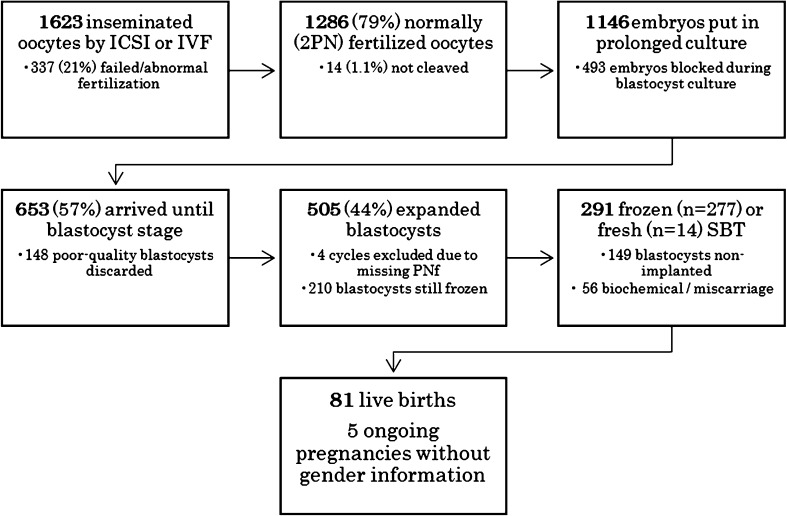
All consecutive infertile patients whose embryos were submitted to prolonged embryo culture reaching expanded blastocyst stage (n = 501) in a TLM incubator between October 2012 (the acquisition of a single EmbryoScope incubator) and December 2014 at our center, and then subsequently underwent single vitrified-warmed embryo transfers until August 2015 (n = 291) and delivered a healthy newborn until October 2015 (n = 81) were included in this retrospective analysis (Fig. 1). Institutional Review Board approval was not required for the present study because in our center all patients undergoing IVF treatment gave informed consent to the anonymous use of their data for retrospective reviews.
Fig. 1.
Flow chart of study events
Natural cycle IVF and minimal ovarian stimulation protocols
Clomiphene citrate (CC)- or letrozole-based minimal stimulation was used in the majority of cycles, whereas unstimulated natural cycle IVF represented a smaller proportion of cases. Details of the CC-based minimal stimulation and natural cycle IVF protocols were described previously [20, 21]. Patients were not selected according to their age and this treatment option was offered over a wide age range.
Oocyte retrieval and fertilization
Transvaginal ultrasound-guided oocyte retrieval was performed without anesthesia using a very thin 21–22-G needle (Kitazato Medical Co., Ltd., Tokyo, Japan). Mature (MII) oocytes were inseminated by conventional IVF or ICSI. Subsequently ICSI-injected oocytes were placed in pre-equilibrated slides (EmbryoSlide, Vitrolife, Gothenburg, Sweden), whereas IVF-inseminated oocytes were first cultured in a conventional, tri-gas, water jacket incubator (Astec, Japan) and transferred to a TLM incubator the next morning if fertilization was confirmed.
Prolonged embryo culture in a TLM incubator and elective vitrification
In our center elective blastocyst culture, vitrification and subsequent single vitrified-warmed blastocysts transfer (SVBT) are routinely practiced in most patients. Prolonged embryo culture was performed in a time-lapse incubator (EmbryoScope, Vitrolife, Gothenburg, Sweden) according to previously described methodology [3]. Briefly, normally fertilized 2PN zygotes were incubated at 37 °C in a 5 % O2 atm and cultured individually until day 3 in Quinn’s Advantage Cleavage Medium and subsequently until blastocyst stage in Quinn’s Advantage Blastocyst Medium. During cleavage stage, selection was performed by classical morphology criteria according to published consensus criteria [22]. Embryos that reached the blastocyst stage by day 5 or 6 were eligible for elective vitrification (Cryotop, Kitazato Medical Co., Ltd., Tokyo, Japan) as soon as they expanded to a size of at least 160 μm [23, 24]. Expanded blastocysts were graded into high- (ICM and TE score: AA, AB or BA) or low-quality (ICM and TE score: BB, BC, CB or CC) categories [25, 26]. Electively, vitrified blastocysts were transferred in the following 1 to 3 months after the oocyte retrieval in a spontaneous natural or hormonal replacement cycle [27].
Time-lapse annotations
Seven plane focal images were taken and recorded every 20 min shortly after insemination up until a period of approximately 160 h. Early (PNf, t2–t9) and late (full compaction at morula stage, start of blastulation, and full blastocyst) morphokinetic time points were scored in accordance with recently published consensus criteria [28]. To determine the degree of blastocyst expansion more objectively, two additional TLM variables (texpB1 and texpB1 and texpB2) were also introduced; the time point when the horizontal diameter of the expanded blastocyst reached 130 and 160 μm, respectively [29]. Annotations were done using EmbryoViewer by two experienced embryologists and re-checked by a third person. In order to control for the different insemination techniques used (38 and 62 % of the cohort was fertilized with conventional IVF or ICSI, respectively), static morphokinetic variables were standardized to a common time point of pronuclear fading [8, 29, 30].
Outcome measures and statistical analysis
The main outcome measure was the proportion of female newborns per live birth. Neonatal gender was confirmed by patient questionnaires approximately 1–2 months after delivery. Baseline cycle characteristics as well as standardized time-lapse variables were compared between female and male live birth groups. Metric variables were compared by the Mann-Whitney U test due to a non-normal distribution. Nominal variables were analyzed by the chi-squared test. p < 0.05 was considered statistically significant. Subsequently, at first, histograms for all morphokinetic variables were checked to determine the existence of an “optimal range” with the highest proportion of female newborns. The whole range of values for each TLM variable was displayed in histograms with (arbitrarily defined) bins of equal width (2.5 h). For each bin, the percentage of females in each of the corresponding columns was calculated and those with an above average rate were grouped together. In this way, those (six) TLM variables (t8, tM, tSB, tfullB, texpB1 and texpB2) where such an optimal range could be observed were converted into categorical ones (inside versus outside of the optimal female range) for further analysis (Fig. 2) [3]. A logistic multivariate analysis was also performed to adjust for maternal age (years) which was the only confounding factor found to be significantly different among baseline characteristics. Statistical calculations were performed with the “R” software package.
Fig. 2.
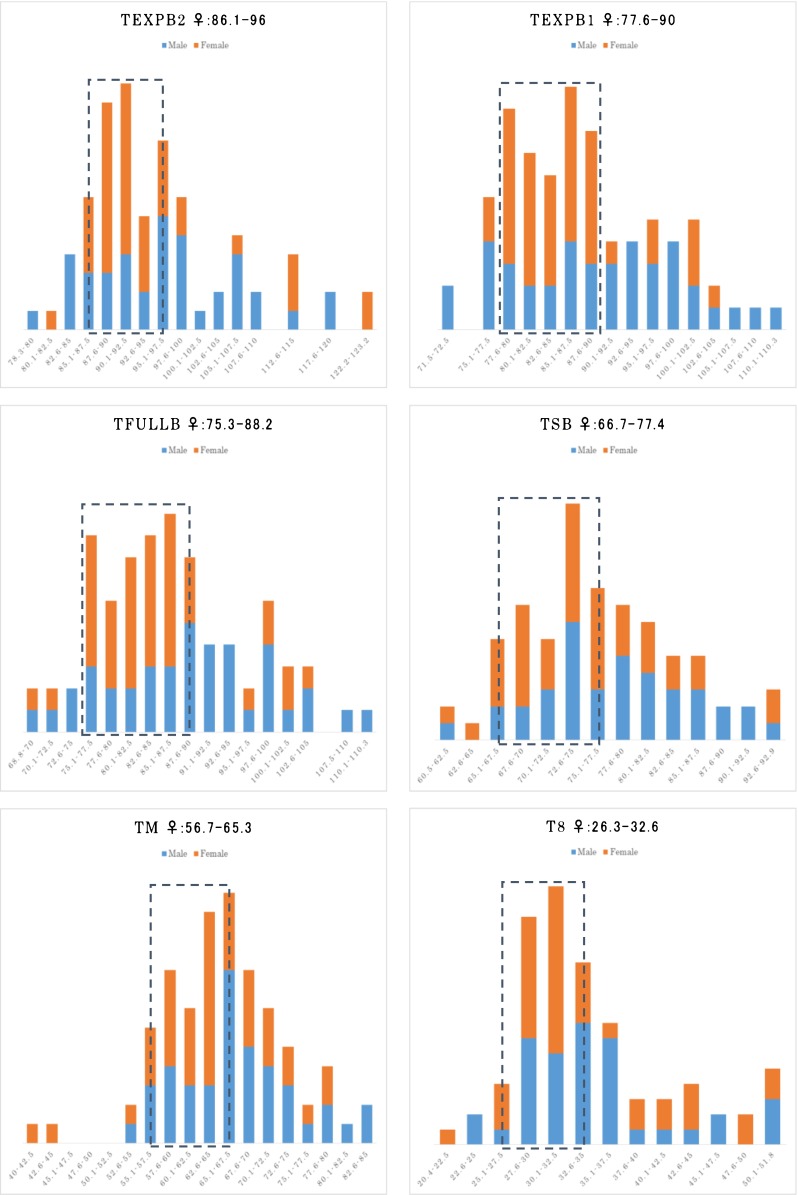
Histograms of relevant TLM variables (in hours) with optimal female (orange: female, blue: male) gender ranges (within dotted lines). texpB 2 time to reach expanded blastocyst size of 160 μm, texpB 1: time to reach expanded blastocyst size of 130 μm, tfullB time to expanded blastocyst, tSB time to initiation of blastulation, tM time to full compaction (morula), t8 time to 8 discrete cells; all TLM variables were standardized to pronuclear fading
Results
Baseline cycle characteristics according to embryo gender
During the study period, following prolonged embryo culture in a time-lapse incubator, a total of 291 single blastocyst transfers were performed in infertile patients who underwent natural IVF or minimal ovarian stimulation cycles resulting in 81 confirmed live births with gender information (maternal age 36.9 ± 3.8 years, range 28–46). Female newborns consisted of 49 % (40/81) of the entire cohort. Baseline characteristics according to gender are summarized in Table 1. There were no significant differences among baseline variables such as BMI, basal FSH, infertility type, parity, the history of previous IVF treatment at other centers, current cycle rank, stimulation type, the number of retrieved eggs, the proportion of high-quality blastocysts, and male partner age. However, maternal age was slightly higher in the group of women who delivered female newborns (37.8 ± 3.2 versus 36 ± 4.1, p = 0.03).
Table 1.
Baseline cycle characteristics in groups with female or male live birth
| Embryo transfer cycles | Female live births (n = 40) | Male live births (n = 41) | p |
|---|---|---|---|
| Maternal age, years | 37.8 ± 3.2 | 36 ± 4.1 | 0.03a |
| BMI, (kg/m2) | 20.2 ± 2.3 | 20.7 ± 2.2 | 0.27a |
| Basal FSH (IU/mL) | 7.7 ± 4.1 | 10.1 ± 5.5 | 0.09a |
| Primary infertility, n (%) | 29 (73) | 27 (66) | 0.52b |
| Nulliparous, n (%) | 37 (93) | 35 (85) | 0.31b |
| No IVF treatment (at other centers), n (%) | 16 (40) | 18 (44) | 0.72b |
| Current cycle rank | 0.57b | ||
| 1–2 cycle, n (%) | 4 (10) | 7 (17) | |
| 3–4 cycles, n (%) | 29 (73) | 29 (71) | |
| 5 or higher, n (%) | 7 (17) | 5 (12) | |
| Stimulation type | 0.26b | ||
| Natural cycle, n (%) | 14 (35) | 9 (22) | |
| Clomiphene, n (%) | 18 (45) | 18 (44) | |
| Letrozole, n (%) | 8 (20) | 14 (34) | |
| Retrieved eggs, n | 5.7 ± 3.6 | 6.1 ± 3.5 | 0.50a |
| High-quality blastocysts transferred, n (%) | 15 (38) | 15 (37) | 0.93b |
| Male partner age, years | 38.9 ± 4.3 | 38.3 ± 4.7 | 0.48a |
aMann-Whitney U test,bchi-squared test
Comparison of morphokinetic variables according to embryo gender
There were no significant differences observed between female versus male blastocysts in the cleavage and morula stage, static (t2 to t9 and tM), or interval parameters (cc2a, b, s2 and s3). However, at the blastocyst stage, there was a statistically significant delay for male-gender blastocysts for the tfullB variable (time to reach fully expanded blastocyst) (Table 2).
Table 2.
Standardized TLM timings in female and male live birth groups
| TLM variables (in hours)** | Female live births (n = 40) | Male live births (n = 41) | p* |
|---|---|---|---|
| t2 | 2.6 ± 0.6 | 2.8 ± 0.6 | 0.19 |
| t3 | 13.3 ± 2.7 | 13.9 ± 1.5 | 0.71 |
| t4 | 14.6 ± 2.2 | 14.9 ± 3.1 | 0.62 |
| t5 | 26.9 ± 5.7 | 27.4 ± 5.4 | 0.59 |
| t6 | 28.9 ± 4.9 | 29.2 ± 4.5 | 0.76 |
| t7 | 30.7 ± 5.1 | 31 ± 4.3 | 0.70 |
| t8 | 34.3 ± 7.4 | 34.9 ± 7 | 0.38 |
| t9 | 48.2 ± 8.8 | 49.1 ± 9 | 0.67 |
| cc2a (t3-t2) | 10.7 ± 2.7 | 11.1 ± 1.5 | 0.88 |
| cc2b (t4-t2) | 12.1 ± 1.8 | 12.1 ± 3 | 0.27 |
| s2 (t4-t3) | 1.5 ± 2.3 | 1.1 ± 2.6 | 0.34 |
| s3 (t8-t5) | 7.9 ± 7.5 | 7.7 ± 6.7 | 0.74 |
| tM | 63.8 ± 7.8 | 67.3 ± 7.6 | 0.06 |
| tSB | 75.3 ± 7.3 | 78.1 ± 7.6 | 0.11 |
| tfullB | 84.4 ± 8.3 | 89 ± 10.2 | 0.022 |
| texpB1 | 86 ± 7.3 | 89.9 ± 10.3 | 0.07 |
| texpB2 | 94.8 ± 10 | 96.6 ± 9.8 | 0.18 |
*Mann-Whitney U test
**t2–t9 time to 2 to 9 discrete cells, cc2a/cc2b/s2/s3 duration of different embryo cell cycle events, tM time to full compaction (morula), tSB time to initiation of blastulation, tfB time to expanded blastocyst, texpB 1 time to reach expanded blastocyst size of 130 μm, texpB 2 time to reach expanded blastocyst size of 160 μm; all TLM variables were standardized to pronuclear fading
Association between embryo gender and morphokinetic variables
In a univariate analysis female embryo gender was associated with late cleavage stage (t8), morula stage (tM), early blastocyst stage (tSB), and also several late, expanded blastocyst stage (tfullB, texpB1 and texpB2) morphokinetic variables. For the first three, associations were less strong (odds ratios between 2.6 and 3.7), whereas the strongest association was found for expanded stage blastocyst parameters (odds ratios were between 6.4 and 7.2). Within the optimal range, the proportion of female newborns was between 69 and 71 % (conversely, 25–26 % outside of the optimal range) (Fig. 2). Being inside the optimal range predicted 74–78 % of all the female newborns, increasing their proportion by 51–57 % compared to the average. After adjusting for maternal age in a multivariate analysis all odds ratios increased slightly (Table 3).
Table 3.
Association between female gender and categorical morphokinetic variables (inside versus outside of the optimal female range)
| Categorical TLM variables | Unadjusted ORs | Adjusted ORs* | Adjusted p* |
|---|---|---|---|
| t8 | 2.9 (1.2–7.4) | 2.8 (1.1–7.6) | 0.03 |
| tM | 3.7 (1.5–9.7) | 3.7 (1.5–10.1) | 0.007 |
| tSB | 2.6 (1–6.5) | 3.0 (1.2–8.1) | 0.02 |
| tfullB | 7.2 (2.7–20.2) | 7.3 (2.7–21.4) | 0.00012 |
| texpB1 | 6.4 (2.5–17.9) | 7.0 (2.6–20.9) | 0.0002 |
| texpB2 | 6.8 (2.6–18.9) | 7.8 (2.9–23.6) | 0.00014 |
*adjusted for female age
Discussion
Our retrospective study involving a moderate number of newborns originating from elective single embryo transfers of time-lapse-monitored blastocysts, has found that embryo gender was strongly associated with late, expanded blastocyst stage morphokinetic variables. The strongest variables predicted approximately three quarters of the resulting female newborns, which, compared to the average, increased their proportion by more than 50 %.
Previous human studies on the relation of embryo morphology/kinetics and gender were inconclusive, whereas some found a faster development for male blastocysts; others were unable to show such as difference [17, 19]. Some authors have also expressed concerns that embryo selection using prolonged embryo culture could lead to a male-biased sex ratio. This was supported by a meta-analysis involving four earlier studies [31] but was refuted by subsequent large cohort studies [16, 18]. A common pitfall for all these previous investigations was that embryo evaluation was based on static criteria instead of TLM technology which currently allows a more precise and detailed comparison of kinetic parameters. To date, only two recently published, retrospective studies investigated the influence of embryo gender on the time-lapse parameters of a developing embryo [14, 15].
Serdarogullari et al. presented the findings of a 2-year study, involving a total of 138 analyzed, KID (known implantation data) embryos (78 female versus 60 male as confirmed after birth) originating from a mixture of day 2–3 and day 5 embryo transfers [14]. As a main finding, cell kinetics were not significantly different for cleavage or blastocyst stage morphokinetic parameters; therefore, the authors concluded that selecting the embryos by time-lapse criteria does not create a gender bias. However, the comparison of TLM variables was done by simple statistical test only (comparing means and standard deviations) and not by examining quartiles or detailed histograms which could explain the discordant findings of subsequent studies.
A subsequent, larger Spanish study, from one of the groups that pioneered TLM research, involved a time-lapse monitored dataset of 327 analyzed embryos from a single-center obtained over a 1-year period [15]. In contrast with the previous study, all included <40-year-old patients were undergoing PGD; thus, embryo gender was ascertained by day 3 array CGH chromosomal analysis. Single or double embryo transfer was performed on day 5. When simple statistical tests were used, there were no differences between morphokinetic variables according to embryo gender, but with a more sophisticated approach (converting continuous variables into categorical ones by examining four quartiles), significantly different proportions of female embryos were observed for two parameters, s2 and tM. For s2 (t4–t3), being inside the defined “sex range” increased the probability of being female by 32 % (from 44 to 58 %), whereas for tM, the increase was 35 % (from 46 to 62 %). Combining these two variables resulted in a four-category hierarchical predictive model where the proportion of female embryos gradually increased from 42 to 71 % (from category D to A). However, it is important to note that the two middle categories (B and C), which together consisted 39 % of the entire cohort, were not predictive at all, consisting of 53 % of females which was very similar to the average proportion of 49 %.
The authors concluded that embryo development as observed by time-lapse monitoring was clearly affected by embryo gender implying that embryo selection methods based on kinetics could also potentially affect the sex ratio of resulting newborns. The authors also suggested that their model could be used as a non-invasive alternative for sex selection in those countries where PGD is not allowed although the model’s practical applicability could be severely limited by the previously mentioned shortcomings (for example, the fact that only two of the categories contain a notably different proportion of female embryos compared to the expected average rate).
Apart from confirming the general findings of the previous Spanish study (tM was also associated with female gender in our study), our investigation suggests that advanced stage morphokinetic parameters (such as tfullB, texpB1 and texpB2) might be stronger predictors of embryo gender than earlier ones (such as s2 and tM). As embryo development progresses, the range (minimum and maximum values) of morphokinetic variables also gets wider thus facilitating the detection of any existing difference between subgroups which could be one explanation to our slightly discrepant findings.
The main limitation of our study is the very limited number of 81 live birth deliveries analyzed. Interestingly, however, even this moderate sample size which was achieved in a single center during a 2-year study period, has allowed us to discover significant differences that are in concordance with previously published relevant TLM articles. Moreover, it also allows the development and application of an initial, center-specific “gender-prediction” model which later could be improved on as sample size progressively increases [32, 33]. As a considerable strength, by our center’s routine policy, our dataset included only single embryo transfers; thus, we did not have to rely exclusively on KID embryos avoiding any potential bias related to a differential in embryo quality (KID versus non-KID). It is also important to note that in our cohort, TLM variables were not used for enhanced embryo selection (only classical morphological criteria were used); therefore, it is unlikely that the sex ratio observed in our study (49 % were female) could have been affected by TLM technology itself.
In conclusion, in a small cohort of consecutive live births obtained from unselected infertile patients undergoing minimal ovarian stimulation and single blastocyst transfer, female embryo gender was strongly associated with late, expanded blastocyst stage morphokinetic parameters. If confirmed on a larger sample, these could be potentially used to increase the proportion of female embryos among blastocysts that were non-invasively selected using time-lapse technology.
Compliance with ethical standards
Financial support
None.
Footnotes
Capsule
Blastocyst stage morphokinetic variables are predictive of female embryo gender following single blastocyst transfer
References
- 1.Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20(5):617–31. doi: 10.1093/humupd/dmu023. [DOI] [PubMed] [Google Scholar]
- 2.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013;26(5):477–85. doi: 10.1016/j.rbmo.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 4.Dal Canto M, Coticchio G, Mignini Renzini M, De Ponti E, Novara PV, Brambillasca F, et al. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online. 2012;25(5):474–80. doi: 10.1016/j.rbmo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Rubio I, Galan A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102(5):1287–94 e5. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 6.VerMilyea MD, Tan L, Anthony JT, Conaghan J, Ivani K, Gvakharia M, et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod Biomed Online. 2014;29(6):729–36. doi: 10.1016/j.rbmo.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciray HN, Aksoy T, Goktas C, Ozturk B, Bahceci M. Time-lapse evaluation of human embryo development in single versus sequential culture media--a sibling oocyte study. J Assist Reprod Genet. 2012;29(9):891–900. doi: 10.1007/s10815-012-9818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz M, Garrido N, Gadea B, Munoz M, Perez-Cano I, Meseguer M. Oocyte insemination techniques are related to alterations of embryo developmental timing in an oocyte donation model. Reprod Biomed Online. 2013;27(4):367–75. doi: 10.1016/j.rbmo.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Munoz M, Cruz M, Humaidan P, Garrido N, Perez-Cano I, Meseguer M. Dose of recombinant FSH and oestradiol concentration on day of HCG affect embryo development kinetics. Reprod Biomed Online. 2012;25(4):382–9. doi: 10.1016/j.rbmo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Munoz M, Cruz M, Humaidan P, Garrido N, Perez-Cano I, Meseguer M. The type of GnRH analogue used during controlled ovarian stimulation influences early embryo developmental kinetics: a time-lapse study. Eur J Obstet Gynecol Reprod Biol. 2013;168(2):167–72. doi: 10.1016/j.ejogrb.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Wissing ML, Bjerge MR, Olesen AI, Hoest T, Mikkelsen AL. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online. 2014;28(4):508–14. doi: 10.1016/j.rbmo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 2013;99(3):738–44 e4. doi: 10.1016/j.fertnstert.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Freour T, Dessolle L, Lammers J, Lattes S, Barriere P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99(7):1944–50. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 14.Serdarogullari M, Findikli N, Goktas C, Sahin O, Ulug U, Yagmur E, et al. Comparison of gender-specific human embryo development characteristics by time-lapse technology. Reprod Biomed Online. 2014;29(2):193–9. doi: 10.1016/j.rbmo.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Bronet F, Nogales MC, Martinez E, Ariza M, Rubio C, Garcia-Velasco JA, et al. Is there a relationship between time-lapse parameters and embryo sex? Fertil Steril. 2015;103(2):396–401 e2. doi: 10.1016/j.fertnstert.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Weston G, Osianlis T, Catt J, Vollenhoven B. Blastocyst transfer does not cause a sex-ratio imbalance. Fertil Steril. 2009;92(4):1302–5. doi: 10.1016/j.fertnstert.2008.07.1784. [DOI] [PubMed] [Google Scholar]
- 17.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–4. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Richter KS, Anderson M, Osborn BH. Selection for faster development does not bias sex ratios resulting from blastocyst embryo transfer. Reprod Biomed Online. 2006;12(4):460–5. doi: 10.1016/S1472-6483(10)61999-2. [DOI] [PubMed] [Google Scholar]
- 19.Hentemann MA, Briskemyr S, Bertheussen K. Blastocyst transfer and gender: IVF versus ICSI. J Assist Reprod Genet. 2009;26(8):433–6. doi: 10.1007/s10815-009-9337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, et al. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35. doi: 10.1186/1477-7827-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodri D, Kawachiya S, Kondo M, Kato R, Matsumoto T. Oocyte retrieval timing based on spontaneous luteinizing hormone surge during natural cycle in vitro fertilization treatment. Fertil Steril. 2014;101(4):1001–7 e2. doi: 10.1016/j.fertnstert.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Alpha Scientists in Reproductive M, Embryology ESIGo The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 23.Kato K, Ueno S, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, et al. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod Biomed Online. 2014;29(4):411–6. doi: 10.1016/j.rbmo.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Kirkegaard K, Kesmodel US, Hindkjaer JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28(10):2643–51. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]
- 26.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81(3):551–5. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chang L, Sone Y, Silber S. Minimal ovarian stimulation (mini-IVF) for IVF utilizing vitrification and cryopreserved embryo transfer. Reprod Biomed Online. 2010;21(4):485–95. doi: 10.1016/j.rbmo.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29(12):2650–60. doi: 10.1093/humrep/deu278. [DOI] [PubMed] [Google Scholar]
- 29.Bodri D, Sugimoto T, Serna JY, Kondo M, Kato R, Kawachiya S, et al. Influence of different oocyte insemination techniques on early and late morphokinetic parameters: retrospective analysis of 500 time-lapse monitored blastocysts. Fertil Steril. 2015;104(5):1175–81 e2. doi: 10.1016/j.fertnstert.2015.07.1164. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Time-lapse videography of human embryos: using pronuclear fading rather than insemination in IVF and ICSI cycles removes inconsistencies in time to reach early cleavage milestones. Reprod Biol. 2015;15(2):122–5. doi: 10.1016/j.repbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Chang HJ, Lee JR, Jee BC, Suh CS, Kim SH. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: a systematic review and meta-analysis. Fertil Steril. 2009;91(6):2381–90. doi: 10.1016/j.fertnstert.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 32.Kirkegaard K, Campbell A, Agerholm I, Bentin-Ley U, Gabrielsen A, Kirk J, et al. Limitations of a time-lapse blastocyst prediction model: a large multicentre outcome analysis. Reprod Biomed Online. 2014;29(2):156–8. doi: 10.1016/j.rbmo.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Freour T, Le Fleuter N, Lammers J, Splingart C, Reignier A, Barriere P. External validation of a time-lapse prediction model. Fertil Steril. 2015;103(4):917–22. doi: 10.1016/j.fertnstert.2014.12.111. [DOI] [PubMed] [Google Scholar]