Abstract
Purpose
The purpose of this research was to study whether methotrexate (MTX) as treatment for ectopic pregnancy (EP) impacts the future fertility of women undergoing assisted reproductive technology (ART)
Methods
In a systematic review and multi-center retrospective cohort from four academic and private fertility centers, 214 women underwent an ART cycle before and after receiving MTX as treatment for an EP. Measures of ovarian reserve and responsiveness and rates of clinical pregnancy (CP) and live birth (LB) were compared in the ART cycles prior and subsequent to MTX.
Results
Seven studies were identified in the systematic review, and primary data from four institutions was included in the final analysis. Women were significantly older in post-MTX cycles (35.3 vs 34.7 years). There were no differences in follicle stimulating hormone, antral follicle count, duration of stimulation, oocytes retrieved, or fertilization rate between pre- and post-MTX cycles. However, post-MTX cycles received a significantly higher total dose of gonadotropins (4206 vs 3961 IU). Overall, 42 % of women achieved a CP and 35 % achieved a LB in the post-MTX ART cycle, which is similar to national statistics. Although no factors were identified that were predictive of LB in young women, the number of oocytes retrieved in the previous ART cycle and current AFC were predictive of LB (AUC 0.76, 0.75) for the older women.
Conclusions
MTX does not influence ovarian reserve, response to gonadotropin stimulation, and CP or LB rate after ART. MTX remains a safe and effective treatment option for women with asymptomatic EPs.
Keywords: Methotrexate, Ectopic pregnancy, Ovarian reserve, In vitro fertilization, Pregnancy rate
Introduction
Ectopic pregnancy is a significant cause of maternal morbidity and mortality and accounts for 1.5–2 % of all pregnancies [1–3]. Methotrexate (MTX) therapy has emerged as a safe, effective alternative to surgical management of an asymptomatic ectopic pregnancy [4–6]. As a folic acid antagonist and inhibitor of DNA synthesis, MTX functions by targeting actively proliferating cells, and in the case of an ectopic pregnancy, impedes further growth of the fetal cells. However, the impact of MTX on other dividing cells, such as oocytes and granulosa cells, is unclear [7].
During in vitro fertilization (IVF), gonadotropin stimulation of the ovary may amplify metabolically active follicles. Additionally, blood flow to the ovary is increased, thereby theoretically delivering increased quantity of MTX to the ovary and causing direct damage to oocytes and granulosa cells [8]. While data exists on the maintenance of tubal patency, resumption of menses, and clinical pregnancy rates, few studies have evaluated the effects of MTX on ovarian reserve and the effectiveness of future assisted reproductive technology (ART) [9–11]. There is no data available to specifically counsel women with diminished ovarian reserve, who may be at increased risk for the effects of MTX, nor is there information regarding the effects of multiple doses of MTX.
The objective of this study was to compare ovarian reserve parameters, IVF stimulation characteristics, and clinical outcomes in the IVF cycle before and after MTX administration for treatment of an ectopic pregnancy.
Materials and methods
The conduct and reporting of this systematic review closely adhered to guidelines of the preferred reporting items for systematic review (PRISMA) guidelines [12].
Search strategy
Our clinical librarian (SF), trained in systematic reviews, created search strategies for the concepts of MTX and ovarian responsiveness using a combination of standardized terms and keywords harvested from indices, dictionaries, and on-topic articles. To exclude animals, the Human filter for PubMed recommended in Cochrane Handbook for Systematic Reviews of Interventions was used as a model to create filters for the other databases searched [13]. The search strategies were launched in PubMed 1946–, Embase 1947–, Scopus 1823–, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov. Searches were limited to English using database supplied limits. Searches were completed in August 2013. The full strategies for PubMed and Embase are available in the Appendix. All results were exported to EndNote. The automatic duplicate finder was applied, and duplicates were assumed to be accurately identified and removed. The reference list was reviewed and relevant articles were evaluated. Reference lists in the included articles were manually screened for additional, potential publications.
Study selection criteria
Studies that compared ovarian reserve parameters and IVF stimulation characteristics before and after the IVF cycle that resulted in pregnancy were considered. Only original research published in English was included. Study design was not limited.
Study selection and data collection
The results of the systematic search were thoroughly reviewed independently by two authors (CEB and ESJ). Corresponding authors were then contacted and primary data requested. De-identified patient-level data was collected from compliant authors.
Primary data from Washington University’s IVF program was also analyzed for inclusion. All subjects whose IVF cycle resulted in an ectopic pregnancy were treated with MTX and then underwent a subsequent IVF cycle between January 2001 and August 2013 which were included in the analysis. Details of the IVF cycles that resulted in ectopic pregnancy were extracted from the institution’s SART database. Patient characteristics including age, BMI, and race were recorded. Ovarian reserve parameters, specifically antral follicle count (AFC), follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH), were collected. IVF characteristics, such as IVF indication, stimulation protocol, total dose of gonadotropins, duration of stimulation in days, peak estradiol level, number of oocytes retrieved, fertilization rate, number of embryos transferred, number of embryos cryopreserved, and pregnancy outcomes, were also abstracted. Number of doses of MTX, the need for surgical management of the ectopic pregnancy, and time between MTX and the subsequent IVF cycle were also described. The primary outcome was number of oocytes retrieved.
Ethical approval
Authors from each institution obtained ethical approval from their Institutional Review Board. In addition, IRB approval was obtained from Washington University prior to the chart review and data extraction.
Statistical methods
The primary data from contributing authors, including Washington University, were pooled and analyzed as a retrospective cohort study. SPSS (Version 22.0, IBM Corp. in Armonk, NY) was utilized for statistical analysis. Standard bivariate statistics were applied for the entire cohort and for women stratified by age (<35 years, 38, and older) to identify relevant predictors. Parametric and non-parametric testing was used as appropriate (paired t test, Wilcoxon signed rank test, Mann–Whitney U test, and chi square analysis). Receiver operator characteristic (ROC) curves were used to determine the strength of identified predictors (reported as area under the curve (AUC)). A post hoc power analysis was performed using G*Power (Version 3.1.9.2; 2009) to detect a two-tailed difference with 80 % power and 5 % alpha.
Results
Systematic review
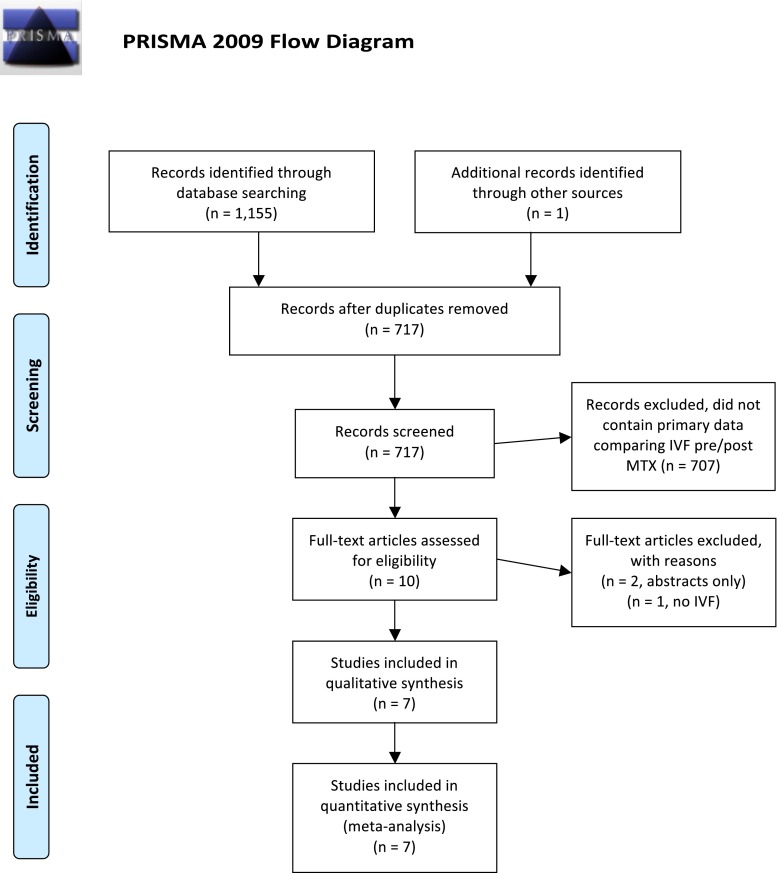
As shown in Fig. 1, the systematic review produced 716 studies. One additional study was included during review of the literature as it was published after the initial systematic search. Seven hundred seven articles were excluded because they did contain primary data comparing IVF cycles before and after receiving MTX for an ectopic pregnancy. The remaining ten articles were then closely reviewed. Two studies by the same author were published as abstracts only and were therefore excluded [14]. One additional study was excluded because none of the patients were undergoing ART [15]. Seven studies met all the inclusion criteria [16–22]. As shown in Table 1, five of the studies were retrospective cohort analyses and two were prospective observational studies [19, 20]. All of the studies used a paired analysis of IVF cycles before and after MTX, but two studies also compared to a control group of patients who underwent salpingectomy [17, 22]. The majority of these studies are limited by their retrospective nature and sample size. Only two studies included more than 50 subjects [16, 17].
Fig. 1.
PRISMA four-phase flow diagram of search yield, screening and inclusion steps
Table 1.
Systematic review
| Study | Location | Study period | Design | Sample size | MTX protocol | Mean age | Mean time since MTX or time between cycles | Ooyctes retrieved post-MTX |
|---|---|---|---|---|---|---|---|---|
| Boots, et al. 2013 | Illinois, USA | 2007–2011 | Retrospective cohort | 66 | Single-dose (50 mg/m2)a | 34.6 | 187 | 13.7 |
| Hill, et al. 2014 | Maryland, USA | 2004–2010 | Retrospective cohort | 153 | Not stateda | 34.3 | 158 | 14 |
| McLaren, et al. 2009 | California, USA | 1999–2005 | Retrospective cohort | 30 | Single-dose (50 mg/m2) | 36.9 | Not stated | 10.8 |
| Oriol, et al. 2008 | Spain | 2005–2006 | Prospective cohort | 14 | Single-dose (1 mg/kg) | 33 | 226 | 10.5 |
| Orvieto, et al. 2007 | Israel | NA | Prospective cohort | 14 | Single-dose | 34 | 171 | 10 |
| Provansal, et al. 2009 | France | 2000–2007 | Retrospective cohort | 11 | Single-dose (1 mg/kg) | 32 | 180 | 5 |
| Wiser, et al. 2013 | Israel | 2005–2012 | Retrospective cohort | 36 | Not stated | 33.8 | 222 | 9.5 |
aAnalyzed effect of multiple doses
Multi-center retrospective cohort
Corresponding authors of these seven studies were contacted and primary data requested. Three corresponding authors responded, and their patient-level data was utilized [16–18]. Complete data was available for 214 subjects (Hill, n = 117; McLaren, n = 23; Boots, n = 66, Washington University, n = 8). Four authors did not provide patient-level data; therefore, the 75 subjects among these four studies were not included in retrospective cohort analysis (Oriol n = 14, Orvieto n = 14, Provansal n = 11, Wiser n = 36). Of the four centers that provided primary data (including our own), three used the single-dose MTX protocol; one center did not comment on protocol type. As shown in Table 1, the individual studies have similar mean ages, time between cycles (or time since MTX), and number of oocytes retrieved post-MTX suggesting their little heterogeneity among them.
Table 2 describes the baseline characteristics of the pooled cohort. The mean age of subjects at the start of the pre-MTX IVF cycle was 34.7 ± 4.7 years. Women were slightly, but significantly, older in post-MTX cycles than in pre-MTX cycles, 35.3 ± 4.1 years. When comparing markers of ovarian reserve, there were no differences in FSH or AFC. AMH was not measured in any of the included studies.
Table 2.
Significant predictors of live birth in women 38 years of age and older
| Demographics | Pre-MTX (n = 214) | Post-MTX (n = 214) | P value |
|---|---|---|---|
| Age (years) | 34.7 ± 4.2 | 35.3 ± 4.1 | <0.01 |
| BMI (kg/m2) | 25.7 ± 5.6 | 25.8 ± 5.6 | <0.01 |
| FSH (IU/L) | 7.0 ± 2.9 | 7.1 ± 3.0 | NS |
| AFC | 13.8 ± 7.8 | 13.9 ± 7.2 | NS |
| IVF cycle characteristics | |||
| Duration of stimulation (days) | 11.9 ± 2.9 | 11.9 ± 3.3 | NS |
| Total dose of gonadotropins (IU) | 3961 ± 1786 | 4206 ± 1825 | <0.01 |
| Peak E2 (pg/mL) | 2345 ± 1108 | 2334 ± 1103 | NS |
| Endometrial thickness (cm) | 10.2 ± 2.2 | 10.3 ± 2.5 | NS |
| Number of oocytes | 12.7 ± 6.1 | 12.8 ± 6.3 | NS |
| Fertilization rate | 70 ± 21 % | 71 ± 22 % | NS |
| Number of embryos transferred | 2.4 ± 0.8 | 2.4 ± 1.1 | NS |
| Number of embryos cryopreserved | 0.55 ± 0.25 | 1.05 ± 1.91 | <0.01 |
| IVF outcomes | |||
| Clinical pregnancy | – | 42.1 % (82/195) | |
| Ectopic pregnancy | 100 % (214/214) | 3.1 % (6/195) | |
| Pregnancy loss | – | 14.3 % (28/195) | |
| Live birth | – | 34.8 % (48/138) | |
| Number of MTX doses | 1.52 ± 0.63 (1–3) | ||
| Time between cycles (days) | 161 (81–737)a | ||
Mean ± SD
aMedian (range)
Among the 214 women, 119 (55.6 %) women received a single dose of MTX, while 79 (36.9 %) received two doses, and only 16 (7.5 %) women received three doses of MTX. The median time between the first day of stimulation in the pre- and post-MTX IVF cycles was 161 days, ranging from 81 to 737 days.
In Table 3, the cohort is stratified by features considered to be at high risk for the theoretical effects of MTX on ovarian reserve including the following: advanced age, low AFC, few oocytes retrieved during the initial IVF cycle, multiple doses of MTX, and a short interval between MTX and the subsequent IVF cycle. The pre- and post-MTX IVF cycles of women 38 years of age and older were compared to the cycles of younger women. Both older and younger women received a statistically increased total dose of gonadotropins in the post-MTX cycle. Similarly, when comparing the cycles of women who received two or more doses of MTX to those who received only one dose, a statistically higher total dose of gonadotropins was administered in all the post-MTX cycles as compared to pre-MTX cycles. There were no differences in duration of stimulation, peak estradiol levels, number of oocytes retrieved, fertilization rate, or number of embryos transferred in any of the high-risk categories. Almost every category had more embryos to cryopreserve in the cycle after MTX.
Table 3.
Stratification by high-risk features
| High-risk feature | Duration of stimulation (days) | Total dose of gonadotropins (IU) | Peak E2 (pg/mL) | Number of oocytes | Fertilization rate | Embryos transferred | Embryos Cryopreserved | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | Pre-MTX | Post-MTX | |
| Advanced age | |||||||||||||||
| ≥38 years | 61 | 11.9 ± 3.1 | 12.4 ± 3.2 | 4745 ± 1681 | *5111 ± 1736 | 2230 ± 1145 | 2155 ± 1138 | 11.5 ± 5.7 | 10.8 ± 6.1 | 73 % ± 19 % | 73 % ± 20 % | 2.8 ± 0.83 | 2.8 ± 1.3 | 0.44 ± 1.1 | 0.58 ± 1.3 |
| <38 years | 153 | 11.9 ± 2.9 | 11.8 ± 3.3 | 3648 ± 1734 | *3845 ± 1738 | 2387 ± 1096 | 2398 ± 1088 | 13.2 ± 6.2 | 13.6 ± 6.3 | 69 % ± 22 % | 70 % ± 22 % | 2.2 ± 0.8 | 2.3 ± 1.0 | 0.60 ± 1.3 | *1.2 ± 2.1 |
| Low AFC | |||||||||||||||
| <10 | 61 | 11.8 ± 2.8 | 11.7 ± 2.7 | 4532 ± 1867 | 4778 ± 1773 | 2092 ± 1004 | 2059 ± 912 | 11.0 ± 5.6 | 11.1 ± 5.9 | 71 % ± 20 % | 70 % ± 23 % | 2.4 ± 0.79 | 2.4 ± 1.1 | 0.27 ± 0.63 | *0.62 ± 1.2 |
| ≥10 | 129 | 11.9 ± 3.0 | 12.0 ± 3.5 | 3519 ± 1671 | *3780 ± 1795 | 2468 ± 1142 | 2456 ± 1167 | 14.0 ± 6.2 | 14.5 ± 6.2 | 69 % ± 22 % | 71 % ± 21 % | 2.3 ± 0.82 | 2.3 ± 1.1 | 0.62 ± 1.4 | *1.3 ± 2.1 |
| Few oocytes retrieved | |||||||||||||||
| ≤5 | 21 | 11.3 ± 1.9 | 11.4 ± 2.8 | 4917 ± 1680 | 5027 ± 1516 | 1757 ± 1419 | 1577 ± 748 | 3.9 ± 1.1 | *7.6 ± 5.4 | 82 % ± 21 % | 78 % ± 25 % | 2.4 ± 0.61 | 2.3 ± 0.89 | 0 ± 0 | *0.95 ± 1.8 |
| >5 | 193 | 11.9 ± 3.0 | 12.0 ± 3.3 | 3857 ± 1770 | *4117 ± 1837 | 2408 ± 1056 | 2414 ± 1106 | 13.7 ± 5.6 | 13.4 ± 6.2 | 69 % ± 21 % | 70 % ± 21 % | 2.4 ± 0.87 | 2.4 ± 1.2 | 0.61 ± 1.3 | *1.1 ± 1.9 |
| No. of MTX doses | |||||||||||||||
| ≥2 | 95 | 11.8 ± 2.5 | 12.0 ± 3.4 | 3891 ± 1805 | *4149 ± 1885 | 2377 ± 1232 | 2304 ± 1140 | 12.5 ± 6.5 | 12.9 ± 6.1 | 68 % ± 23 % | 69 % ± 22 % | 2.3 ± 0.86 | 2.4 ± 1.1 | 0.46 ± 1.2 | *1.0 ± 1.8 |
| <2 | 119 | 12.0 ± 3.2 | 11.9 ± 3.1 | 4017 ± 1776 | *4252 ± 1783 | 2320 ± 1001 | 2358 ± 1077 | 12.9 ± 5.8 | 12.7 ± 6.6 | 72 % ± 20 % | 72 % ± 22 % | 2.4 ± 0.84 | 2.4 ± 1.1 | 0.63 ± 1.3 | *1.1 ± 2.0 |
| Time between cycles | |||||||||||||||
| <180 days | 128 | 11.8 ± 2.6 | 11.7 ± 2.7 | 4112 ± 1798 | 4336 ± 1787 | 2158 ± 1058 | 2249 ± 1160 | 12.4 ± 6.2 | 12.8 ± 6.5 | 71 % ± 21 % | 71 % ± 24 % | 2.4 ± 0.8 | 2.5 ± 1.1 | 0.43 ± 1.2 | *1.0 ± 1.9 |
| ≥180 days | 86 | 12.1 ± 3.4 | 12.3 ± 3.9 | 3736 ± 1129 | *4013 ± 1875 | 2641 ± 1129 | 2457 ± 1001 | 13.2 ± 6.0 | 12.8 ± 6.1 | 68 % ± 21 % | 71 % ± 19 % | 2.4 ± 0.9 | 2.3 ± 1.2 | 0.73 ± 1.3 | 1.1 ± 1.9 |
| Obesity | |||||||||||||||
| BMI ≥ 30 kg/m2 | 46 | 12.3 ± 3.7 | 11.6 ± 3.1 | 3715 ± 1828 | *4088 ± 1863 | 2263 ± 1086 | 2004 ± 1025 | 12.9 ± 5.5 | 12.4 ± 5.5 | 67 % ± 23 % | 67 % ± 22 % | 2.3 ± 0.9 | 2.4 ± 1.2 | 0.39 ± 1.0 | 0.65 ± 1.5 |
| BMI < 30 kg/m2 | 144 | 11.8 ± 2.6 | 12.0 ± 3.3 | 3916 ± 1793 | *4141 ± 1860 | 2366 ± 1120 | 2436 ± 1113 | 13.0 ± 6.3 | 13.6 ± 6.5 | 71 % ± 20 % | 72 % ± 22 % | 2.3 ± 0.8 | 2.3 ± 1.1 | 0.57 ± 1.3 | 1.2 ± 2.0 |
Bold and * indicate significant findings
Mean ± SD
*P < 0.05
Information regarding clinical outcomes was available for 214 women (Table 2). Nineteen women did not undergo embryo transfer. At least four women delayed transfer due to planned comprehensive chromosome screening with embryo vitrification. Three women had failed fertilization, one elected to freeze embryos and transfer at a later date for personal reasons, and one transfer was cancelled for ovarian hyperstimulation syndrome. Reasoning for the decision not to proceed with transfer was not available in the remaining ten subjects. Of note, four of these women had at least one embryo cryopreserved.
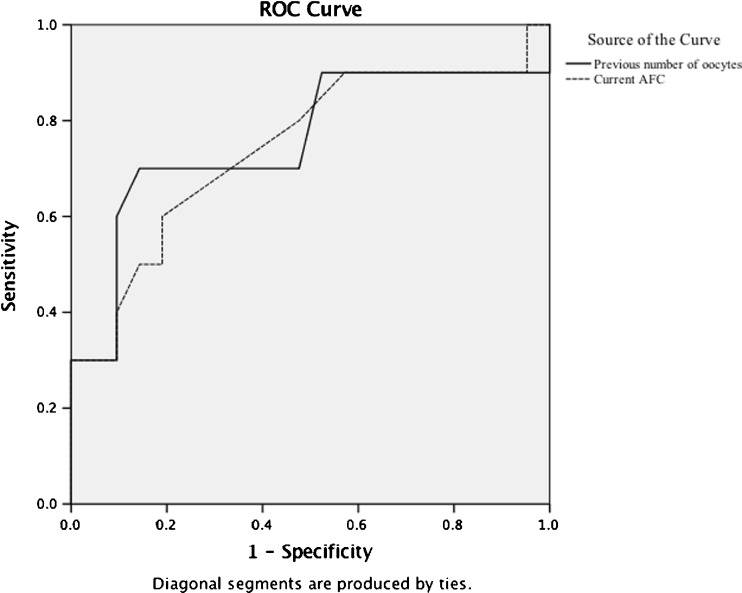
Forty-two percent (82/195) of women who had at least one embryo transferred achieved a clinical pregnancy (CP) in the post-MTX ART cycle. This is similar to national data published by the Society of Assisted Reproductive Technology demonstrating a pregnancy rate per transfer of 53 % in women less than 35 years of age and 37 % in women 38–40 years [23]. Six (3.1 %) women had another ectopic pregnancy. Live birth (LB) data was available for 138 women. Of these women, 34.8 % achieved a live birth. The probability of LB in women younger than 35 years of age (42.6 %) was again comparable to SART reports (46 %). No factors were identified that were predictive of CP or LB for these women. The probability of LB was lower in women 38 years of age and older (29.3 %), but not different from age-matched national reports (27.3 %). To assess cycle characteristics’ predictive value of LB in women 38 years of age and older, an ROC analysis was performed. As illustrated in Fig. 2, numbers of oocytes retrieved and AFC served as reasonable predictors of live birth. For example, 10 oocytes retrieved in the previous ART cycle predicted LB with 83 % sensitivity and 55 % specificity whereas 13 oocytes predicted live birth with 67 % sensitivity and 86 % sensitivity (AUC 0.76). Current AFC of 11 predicted LB in these older women with 80 % sensitivity and 52 % specificity, and AFC of 13 predicted LB with 60 % sensitivity and 81 % specificity (AUC 0.75).
Fig. 2.
Diagonal segments are produced by ties
When comparing ovarian reserve and response characteristics in women who did and did not achieve a LB in the post-MTX, there were few differences noted (Table 4). Women with a successful LB were younger, required a lower total dose of gonadotropins, and had significantly more oocytes retrieved in the cycle that resulted in LB. There were no other differences between the two cohorts, including no difference in the number of doses of MTX received or in the time interval between cycles.
Table 4.
Comparison of women whose post-MTX ART cycle resulted in live birth
| Pre-MTX | Live birth (n = 48) | No live birth (n = 90) | P value |
|---|---|---|---|
| Age (years) | 33.6 ± 4.3 | 35.3 ± 4.2 | 0.03 |
| BMI (kg/m2) | 25.8 ± 5.3 | 26.1 ± 5.6 | NS |
| AFC | 14.2 ± 8.0 | 12.1 ± 7.8 | NS |
| Duration of stimulation (days) | 10.8 ± 1.9 | 10.9 ± 1.7 | NS |
| Total dose of gonadotropins (IU) | 3273 ± 1559 | 4337 ± 1739 | <0.01 |
| Peak E2 (pg/mL) | 2374 ± 1404 | 2142 ± 957 | NS |
| Number of oocytes | 14.0 ± 6.7 | 12.5 ± 5.5 | NS |
| Fertilization rate | 69 ± 23.4 % | 64 ± 19.6 % | NS |
| Number of MTX doses | 1.58 ± 0.68 | 1.64 ± 0.68 | NS |
| Time between cycles (days) | 153 (81–554)a | 162 (94–522)a | NS |
| Post-MTX | |||
| Age (years) | 34.3 ± 4.2 | 35.8 ± 4.3 | 0.05 |
| BMI (kg/m2) | 25.9 ± 5.3 | 26.2 ± 5.6 | NS |
| AFC | 14.7 ± 7.3 | 12.7 ± 7.4 | NS |
| Duration of stimulation (days) | 10.9 ± 1.9 | 10.9 ± 1.7 | NS |
| Total dose of gonadotropins (IU) | 3506 ± 1714 | 4395 ± 1754 | <0.01 |
| Peak E2 (pg/mL) | 2487 ± 1294 | 2189 ± 1041 | NS |
| Number of oocytes | 15.7 ± 5.8 | 11.6 ± 5.7 | <0.01 |
| Fertilization rate | 74 ± 17.1 % | 66 ± 19.3 % | 0.03 |
| Number of embryos transferred | 2.4 ± 0.9 | 2.6 ± 1.1 | NS |
| Number of embryos cryopreserved | 1.4 ± 2.2 | 0.5 ± 1.4 | 0.02 |
Mean ± SD
aMedian (range)
Discussion
Ectopic pregnancy is an undesired yet common outcome after ART therapy. Treatment options include expectant management, surgical intervention, and medical management with MTX [24]. Limited published evidence exists on the impact of MTX on future ART success to help guide in their decision making. In this large multi-center study, MTX appears to remain a safe and effective treatment option for women with asymptomatic ectopic pregnancies. MTX does not influence ovarian reserve, response to gonadotropin stimulation, CP, or live birth rate after IVF. With a median time of less than 6 months between cycles, there were no differences in AFC, FSH, duration of stimulation, maximum estradiol levels, fertilization rate, or number of embryos transferred. With adequate power, there was also no difference in the primary outcome of the number of oocytes retrieved. This conclusion was illustrated in the analysis of all the data as well as in stratified analyses.
The only consistently different parameter was an increased dose of total gonadotropins in the post-MTX cycle. Analysis of all the data as well as analysis of the stratified data demonstrated this finding. It is important to note that similar to women with high-risk features, women considered low risk for the effects of MTX (<38 years old, AFC ≥ 10, >5 oocytes retrieved, only one MTX dose, and ≥180 days between cycles) also received higher doses of medication in the second cycle. Women may be requiring higher doses due to the impact of MTX on their ovarian reserve, but without a non-paired control group, we cannot eliminate an effect of MTX. However, the most likely explanation for the subtle increase in dosage is a combination of the passage of time and physicians’ natural tendency to increase dosage in subsequent cycles.
Women were significantly older in the post-MTX cycle, though the mean age at the post-MTX cycle was relatively young (35.3 years) with a mean difference of only 0.6 years. Whether this significant increase in age is due to time patients are counseled to wait after MTX before proceeding with another cycle [25, 26] or it is influenced by other factors such as cost is unknown. After review of the first cycle, physicians may increase the dose and/or choose a more aggressive protocol with the aim of retrieving more oocytes and a better outcome. Prior to MTX, 43 % of the cycles utilized a luteal phase agonist protocol and 34 % utilized an antagonist protocol. In the subsequent cycles, 26 % were luteal phase agonists and 47 % antagonists.
Conclusions from this analysis are in agreement with a recently published meta-analysis [27]. However, the strength of this study lies in the utilization of primary rather than secondary data. Primary data allows for more detailed and stratified analyses of potentially high-risk women. Additionally, the sample size is powered to detect a clinically significant difference in number of oocytes, which is the best representation of ovarian reserve and is a predictor of pregnancy and live birth in older women. Finally, the nature of a multi-center collection of data improves generalizability.
Limitations of this analysis include its retrospective study design. In the systematic review of all studies evaluating the effects of MTX on subsequent ovarian reserve, only one of seven studies was prospectively analyzed [19]. It is possible that a significant number of women who receive MTX do not seek or complete an additional ART cycle and are therefore not included in these retrospective analyses. An additional limitation of this systematic review and pooled cohort lies in the possibility of a publication bias. Although publication bias is more typically noted with positive rather than negative results, only one of all the published studies noticed a difference in the ART cycle following MTX. This difference was only noted in the number of oocytes if the subsequent cycle occurred within 180 days of MTX administration .
Although the sample size and power are adequate to detect the primary outcome of oocytes retrieved in the overall cohort, the possibility of a type 2 error among the stratification data cannot be excluded. A final limitation is the relatively short follow-up after MTX administration. The longest time between MTX and the subsequent IVF cycle was approximately 2 years with a median interval of 161 days. Long-term follow-up on the effect of ovarian function many years after receiving MTX is still unknown.
Conclusions
The main finding of this study is the absence of a negative effect of MTX on subsequent IVF outcomes. Women with a history of ART-related ectopic pregnancy have a good chance of LB in a subsequent ART cycle, and repeated doses of MTX do not impact this chance. For women 38 years of age and older, prior response to gonadotropins and current AFC may be helpful tools to predict chance of CP and LB in future ART cycles. Because this is a paired analysis of women whose initial IVF cycle resulted in an ectopic pregnancy, there is no control group to compare outcomes in the post-MTX cycle. However, the pregnancy and live birth rates after MTX were equivalent to those reported by SART in the national data summary during this time. In conclusion, the findings of this large, multi-center pooled cohort are consistent with the findings of nearly all the individual studies as well as a meta-analysis and support the continued use of MTX in the medical management of ectopic pregnancy.
Disclaimer
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of Health and Human Services, Department of Defense, or the US Government.
Acknowledgments
C.E.B. received support from the National Research Training Program in Reproductive Medicine sponsored by the National Institute of Health (T32 HD040135-13) and the Scientific Advisory Board of Vivere Health. E.S.J. received support from the Women’s Reproductive Health Research Program sponsored by the National Institute of Health (K12 HD063086), the Institute of Clinical and Translational Sciences at Washington University (UL1 TR000448), the Barnes Jewish Hospital Foundation, and the March of Dimes. This work was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH, Bethesda, MD.
Appendix
Embase
8/27/2013, Limits: English, 546 Results
‘methotrexate’/exp OR ‘methotrexate’ OR ‘mtx’ OR ‘4 amino 10 methylfolic acid’ OR ‘4 amino 10 methylpteroylglutamic acid’ OR ‘4 amino n10 methylpteroylglutamic acid’ OR ‘a methopterine’ OR ‘abitrexate’ OR ‘amethopterin’ OR ‘amethopterine’ OR ‘ametopterine’ OR ‘antifolan’ OR ‘biotrexate’ OR ‘canceren’ OR ‘cl 14377’ OR ‘cl14377’ OR ‘emtexate’ OR ‘emthexat’ OR ‘emthexate’ OR ‘emtrexate’ OR ‘enthexate’ OR ‘farmitrexat’ OR ‘farmitrexate’ OR ‘farmotrex’ OR ‘folex’ OR ‘ifamet’ OR ‘imeth’ OR ‘lantarel’ OR ‘ledertrexate’ OR ‘maxtrex’ OR ‘metex’ OR ‘methoblastin’ OR ‘methohexate’ OR ‘methotrate’ OR ‘methotrexat’ OR ‘methotrexato’ OR ‘methoxtrexate’ OR ‘methrotrexate’ OR ‘methylaminopterin’ OR ‘methylaminopterine’ OR ‘meticil’ OR ‘metoject’ OR ‘metothrexate’ OR ‘metotrexat’ OR ‘metotrexate’ OR ‘metotrexin’ OR ‘metrex’ OR ‘mexate’ OR ‘mpi 5004’ OR ‘mpi5004’ OR ‘neotrexate’ OR ‘novatrex’ OR ‘nsc 740’ OR ‘nsc740’ OR ‘reumatrex’ OR ‘rheumatrex’ OR ‘texate’ OR ‘texorate’ OR ‘trexall’ OR ‘xaken’ OR ‘zexate’ AND (‘ovarian reserve’/exp OR ‘oocyte development’/exp OR ‘ovary function’/de OR ‘ovary follicle’/exp OR ‘ovary cycle’/exp OR ‘follitropin’/exp OR ‘muellerian inhibiting factor’/exp OR ‘oocyte reserve’ OR ‘ovarian reserve’ OR ‘ovarian responsiveness’ OR ‘ovarian stimulation’ OR ‘ovarian cycle’ OR ‘ovulation cycle’ OR ‘reproductive cycle’ OR ‘ovarian activity’ OR ‘ovarian function’ OR ‘ovarium function’ OR ‘egg development’ OR ‘oocyte growth’ OR ‘oocytogenesis’ OR ‘oogenesis’ OR ‘ovogenesis’ OR ‘ovum development’ OR ‘oocyte maturation’ OR ‘egg maturation’ OR ‘follicle maturation’ OR ‘fertiline’ OR ‘fertinom p’ OR ‘follicle stimulating hormone’ OR ‘follicotropin’ OR ‘folliculostimulating hormone’ OR ‘follitrophin’ OR ‘follitropine’ OR ‘folltropin’ OR ‘fsh’ OR ‘ovagen’ OR ‘super ov’ OR ‘ovarian follicles’ OR ‘ovarian follicle’ OR ‘graafian follicle’ OR ‘graafian follicles’ OR ‘atretic follicle’ OR ‘atretic follicles’ OR ‘hfsh’ OR ‘anthrogon’ OR ‘antral follicle count’ OR ‘afc’ OR ‘anti-mullerian hormone’ OR ‘amh’ OR ‘anti mullerian hormone’ OR ‘antimuellerian hormone’ OR ‘antimullerian hormone’ OR ‘muellerian inhibiting substance’ OR ‘muellerian inhibitor’ OR ‘mullerian inhibiting factor’ OR ‘mullerian inhibiting substance’ OR ‘mullerian inhibitor’ OR ‘mullerian inhibiting hormone’ OR ‘mullerian-inhibitory substance’ OR ‘mullerian inhibitory substance’ OR ‘mullerian-inhibiting factor’ OR ‘mullerian-inhibiting hormone’ OR ‘anti-mullerian factor’ OR ‘anti mullerian factor’ OR ‘mullerian regression factor’) NOT ([animals]/lim NOT [humans]/lim) AND [english]/lim
PubMed
8/27/2013, Limits: English, 89 Results
(“Methotrexate”[Mesh] OR “methotrexate” OR “MTX” OR “4 amino 10 methylfolic acid” OR “4 amino n10 methylpteroylglutamic acid” OR “a methopterine” OR “amethopterin” OR “amethopterine” OR “ametopterine” OR “emthexat” OR “emtrexate” OR “folex” OR “ledertrexate” OR “metex” OR “methotrexat” OR “methoxtrexate” OR “methrotrexate” OR “methylaminopterin” OR “metoject” OR “metothrexate” OR “metotrexat” OR “metotrexate” OR “metrex” OR “mexate” OR “neotrexate” OR “nsc 740” OR “rheumatrex” OR “texate”) AND (“Ovarian Follicle”[Mesh] OR “Follicle Stimulating Hormone”[Mesh] OR “Anti-Mullerian Hormone”[Mesh] OR “ovarian reserve” OR “oocyte development” OR “ovary function” OR “ovary follicle” OR “ovary cycle” OR “follitropin” OR “Mullerian inhibiting factor” OR “oocyte reserve” OR “ovarian reserve” OR “ovarian responsiveness” OR “ovarian stimulation” OR “ovarian cycle” OR “ovulation cycle” OR “reproductive cycle” OR “ovarian activity” OR “ovarian function” OR “egg development” OR “oocyte growth” OR “oocytogenesis” OR “oogenesis” OR “ovogenesis” OR “ovum development” OR “oocyte maturation” OR “egg maturation” OR “follicle maturation” OR “fertiline” OR “follicle stimulating hormone” OR “follicotropin” OR “folliculostimulating hormone” OR “follitrophin” OR “follitropine” OR “folltropin” OR “FSH” OR “ovagen” OR “super ov” OR “Ovarian Follicles” OR “Ovarian Follicle” OR “Graafian Follicle” OR “Graafian Follicles” OR “Atretic Follicle” OR “Atretic Follicles” OR “hFSH” OR “Anthrogon” OR “Antral follicle count” OR “AFC” OR “Anti-Mullerian Hormone” OR “AMH” OR “anti mullerian hormone” OR “antimullerian hormone” OR “muellerian inhibiting substance” OR “mullerian inhibiting substance” OR “mullerian inhibitor” OR “Mullerian Inhibiting Hormone” OR “Mullerian-Inhibitory Substance” OR “Mullerian Inhibitory Substance” OR “Mullerian-Inhibiting Factor” OR “Mullerian-Inhibiting Hormone” OR “Anti-Mullerian Factor” OR “Anti Mullerian Factor” OR “Mullerian Regression Factor”) NOT ((“Animals”[Mesh]) NOT (“Animals”[Mesh] AND “Humans”[Mesh]))
Compliance with ethical standards
Authors from each institution obtained ethical approval from their Institutional Review Board. In addition, IRB approval was obtained from Washington University prior to the chart review and data extraction.
Conflict of interest
There are no conflicts of interest to declare.
Footnotes
Capsule As treatment for an ectopic pregnancy, methotrexate does not affect ovarian reserve, response to gonadotropin stimulation, clinical pregnancy, or live birth rates in subsequent assisted reproductive tenchology cycles.
Contributor Information
Christina E. Boots, Phone: 314.286.2400, Email: bootsc@wudosis.wustl.edu
Micah J. Hill, Email: hillmicah@mail.nih.gov
Eve C. Feinberg, Email: eve.feinberg@integramed.com
Ruth B. Lathi, Email: rlathi@stanford.edu
Susan A. Fowler, Email: fowlers@wusm.wustl.edu
Emily S. Jungheim, Email: jungheime@wudosis.wustl.edu
References
- 1.Zane SB, Kieke BA, Kendrick JS, Bruce C. Surveillance in a time of changing health care practices: estimating ectopic pregnancy incidence in the United States. Matern Child Health J. 2002;6:227–36. doi: 10.1023/A:1021106032198. [DOI] [PubMed] [Google Scholar]
- 2.Society of Assisted Reproductive Technology and the American Society for Reproductive Medicine Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steri. 2004;81:1207–20. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstet Gynecol. 2005;105:1052–7. doi: 10.1097/01.AOG.0000158860.26939.2d. [DOI] [PubMed] [Google Scholar]
- 4.Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med. 1993;329:1174–81. doi: 10.1056/NEJM199307083290224. [DOI] [PubMed] [Google Scholar]
- 5.Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974–8. doi: 10.1056/NEJM199912233412604. [DOI] [PubMed] [Google Scholar]
- 6.Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754–7. [PubMed] [Google Scholar]
- 7.Benian A, Guralp O, Uzun DD, Okyar A, Sahmay S. The effect of repeated administration of methotrexate (MTX) on rat ovary: measurement of serum antimullerian hormone (AMH) levels. Gynecol Endocrinol. 2013;29:226–9. doi: 10.3109/09513590.2012.738725. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–54. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 9.Stovall TG, Ling FW, Buster JE. Reproductive performance after methotrexate treatment of ectopic pregnancy. Am J Obstet Gynecol. 1990;162:1620–3. doi: 10.1016/0002-9378(90)90928-Z. [DOI] [PubMed] [Google Scholar]
- 10.Keefe KA, Wald JS, Goldstein DP, Bernstein M, Berkowitz RS. Reproductive outcome after methotrexate treatment of tubal pregnancies. J Reprod Med. 1998;43:28–32. [PubMed] [Google Scholar]
- 11.Buster JE, Krotz S. Reproductive performance after ectopic pregnancy. Sem Rep Med. 2007;25:131–3. doi: 10.1055/s-2007-970052. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011
- 14.Singer T, Kofinas J, Huang JY, Elias R, Schattman GL, Rosenwaks Z. Anti-Mullerian hormone serum levels and reproductive outcome are not affected by methotrexate or laparoscopic salpingectomy for the treatment of ectopic pregnancy in IVF patients. Fertil Steril. 2011;96:S197. doi: 10.1016/j.fertnstert.2011.07.764. [DOI] [Google Scholar]
- 15.Uyar I, Yucel OU, Gezer C, Gulhan I, Karis B, Hanhan HM, et al. Effect of single-dose methotrexate on ovarian reserve in women with ectopic pregnancy. Fertil Steril. 2013;100:1310–3. doi: 10.1016/j.fertnstert.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Boots CE, Gustofson RL, Feinberg EC. Does methotrexate administration for ectopic pregnancy after in vitro fertilization impact ovarian reserve or ovarian responsiveness? Fertil Steril. 2013;100:1590–3. doi: 10.1016/j.fertnstert.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Hill MJ, Cooper JC, Levy G, Alford C, Richter KS, DeCherney AH, et al. Ovarian reserve and subsequent assisted reproduction outcomes after methotrexate therapy for ectopic pregnancy or pregnancy of unknown location. Fertil Steril. 2014;101:413–9. doi: 10.1016/j.fertnstert.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren JF, Burney RO, Milki AA, Westphal LM, Dahan MH, Lathi RB. Effect of methotrexate exposure on subsequent fertility in women undergoing controlled ovarian stimulation. Fertil Steril. 2009;92:515–9. doi: 10.1016/j.fertnstert.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Oriol B, Barrio A, Pacheco A, Serna J, Zuzuarregui JL, Garcia-Velasco JA. Systemic methotrexate to treat ectopic pregnancy does not affect ovarian reserve. Fertil Steril. 2008;90:1579–82. doi: 10.1016/j.fertnstert.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Orvieto R, Kruchkovich J, Zohav E, Rabinson J, Anteby EY, Meltcer S. Does methotrexate treatment for ectopic pregnancy influence the patient’s performance during a subsequent in vitro fertilization/embryo transfer cycle? Fertil Steril. 2007;88:1685–6. doi: 10.1016/j.fertnstert.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 21.Provansal M, Agostini A, Lacroix O, Gerbeau S, Grillo JM, Gamerre M. Ultrasound monitoring in patients undergoing in-vitro fertilization after methotrexate treatment for ectopic pregnancy. Ultrasound Obstet Gynecol. 2009;34:715–9. doi: 10.1002/uog.7344. [DOI] [PubMed] [Google Scholar]
- 22.Wiser A, Gilbert A, Nahum R, Orvieto R, Haas J, Hourvitz A, et al. Effects of treatment of ectopic pregnancy with methotrexate or salpingectomy in the subsequent IVF cycle. Reprod Biomed Online. 2013;26:449–53. doi: 10.1016/j.rbmo.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26:245–52. doi: 10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]
- 24.Practice Committee of American Society for Reproductive Medicine Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638–44. doi: 10.1016/j.fertnstert.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Svirsky R, Rozovski U, Vaknin Z, Pansky M, Schneider D, Halperin R. The safety of conception occurring shortly after methotrexate treatment of an ectopic pregnancy. Reprod Toxicol. 2009;27:85–7. doi: 10.1016/j.reprotox.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Charache S, Condit PT, Humphreys SR. Studies on the folic acid vitamins, and the persistence of amethopterin in mammalian tissues. Cancer. 1960;13:236–40. doi: 10.1002/1097-0142(196003/04)13:2<236::AID-CNCR2820130205>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Ohannessian A, Loundou A, Courbière B, Cravello L, Agostini A. Ovarian responsiveness in women receiving fertility treatment after methotrexate for ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod. 2014;29:1949–56. doi: 10.1093/humrep/deu174. [DOI] [PubMed] [Google Scholar]