Abstract
Purpose
The purpose of this study is to review recurrent pregnancy loss (RPL) due to sperm chromosomal abnormalities and discuss the genetic counseling that is required for men with sperm chromosomal abnormalities.
Method
The literature was reviewed, and a genetic counselor lends her expertise as to how couples with RPL and sperm chromosomal abnormalities ought to be counseled. The review of the literature was performed using MEDLINE.
Results
Sperm fluorescence in situ hybridization (FISH) can be used to determine if disomy or unbalanced chromosomal translocations are present. In men with aneuploidy in sperm or who carry a chromosomal translocation, pre-implantation genetic screening (PGS) combined with in vitro fertilization (IVF) and intra-cytoplasmic sperm injection (ICSI) can increase chances of live birth. In men with abnormal sperm FISH results, the degree of increased risk of abnormal pregnancy remains unclear. Genetic counselors can provide information to couples about the risk for potential trisomies and sex chromosome aneuploidies and discuss their reproductive and testing options such as PGS, use of donor sperm, and adoption. The provision of genetic counseling also allows a couple to be educated about recommended prenatal testing since pregnancies conceived with a partner who has had abnormal sperm FISH are considered to be at increased risk for aneuploidy.
Conclusion
We review the literature and discuss genetic counseling for couples with RPL or recurrent implantation failure due to increased sperm aneuploidy.
Keywords: Chromosomal aneuploidy, Male infertility, Sperm FISH, Genetic counseling, Recurrent pregnancy loss
Introduction
Primary infertility, defined as the inability to conceive despite regular unprotected intercourse for 12 months, affects 15 % of couples and 50 % of cases are ascribed to the male [1]. Recurrent pregnancy loss (RPL), which may be defined as two or more pregnancy losses less than 20 weeks after the last menstrual period, is experienced by 1 to 2 % of women [2, 3]. While there are many genetic causes for male factor infertility, for couples who have experienced RPL or repeated in vitro fertilization (IVF) failures, male chromosomal aneuploidy or chromosomal structural aberrations (such as translocation, inversion, and pericentric inversion) are of particular concern [4–8]. Aneuploidy is defined as an abnormal number of chromosomes (too few or too many) in a cell due to abnormal meiosis. Thus, for men whose partners have experienced ≥2 spontaneous abortions or IVF failures, a sperm DNA fluorescence in situ hybridization (FISH) analysis is recommended to look for male sperm aneuploidy [9–12]. In this review, we discuss the importance of genetic counseling to help couples navigate the difficult decisions that accompany pregnancies associated with abnormal sperm aneuploidy.
Sperm aneuploidy
Sperm fluorescent in situ hybridization (FISH) utilizes fluorescent-labeled primers that bind specifically to each chromosome in the sperm sample, and then analyzed under a fluorescent microscope. Increased or decreased fluorescent signals indicate aneuploidy. When compared to fertile men, men whose partners have a history of RPL have a 2.7 times greater rate of sperm with sex chromosome aneuploidy, 3.3 times greater rate of sperm with chromosomal 13 or 21 aneuploidy, and 6 times greater rate of sperm with chromosomal 18 aneuploidy [13]. However, once a successful pregnancy has been conceived (naturally or through IVF), the magnitude of the increased risk introduced to the pregnancy due to known sperm aneuploidy confirmed by FISH remains unknown. Higher rates of chromosomal abnormalities have been found in intra-cytoplasmic sperm injection (ICSI) offspring than in offspring conceived through natural conception (1.4 % compared to 0.3–0.4 % detected through prenatal testing in the general population) [14]. Yet, no studies have defined the threshold for risk of offspring with chromosomal abnormality based on sperm aneuploidy percent [9]. Because of such ambiguity, urologists often refer patients for genetic counseling in an effort to better educate patients of their risk as they feel uncomfortable offering counseling themselves [15].
Recurrent pregnancy loss
American Society of Reproductive Medicine (ASRM) guidelines currently recommend karyotype analysis of both partners and analysis of the female partner, including uterine structural assessment and evaluation of hormonal and metabolic factors. In their 2012 statement on the evaluation of recurrent pregnancy loss, ASRM does not recommend testing sperm morphology [16]. Their report states that cytogenetic studies of products of conception from couples with recurrent pregnancy loss do not reveal an increased rate of sex chromosome aneuploidy. The study cited for a support of this statement, however, never investigated men with sperm aneuploidy, but rather looked at all couples with recurrent pregnancy loss due to any number of reasons [17]. The other paper cited in the ASRM 2012 statement, in fact, contradicts their statement by demonstrating that couples with abnormal sperm FISH results showed decreased pregnancy and implantation rates and increased miscarriage rates [8]. Thus, until more in-depth studies can be performed to explore this relationship between sperm aneuploidy and pregnancy outcomes, men with recurrent pregnancy loss should be screened for sperm aneuploidy.
Genetic counseling
In all cases of genetic counseling, a three-generation pedigree is obtained looking specifically for a familial history of infertility, recurrent pregnancy loss, birth defects, intellectual disability, and apparent genetic disease. Couples are counseled that all pregnancies have a 3 % risk for birth defects and/or intellectual disability regardless of maternal age, ethnicity, or family history. Couples are advised of the risk for Down syndrome and other chromosomal abnormalities related to maternal age. Finally, couples are counseled about reproductive risk related to ethnic background and family history.
Evaluation through sperm FISH provides results in two general categories—increased aneuploidy of a single chromosome or global elevation of aneuploidy in all tested chromosomes. Increased aneuploidy of a single chromosome may be suggestive of the presence of a balanced chromosomal translocation if a large increase in disomy of one chromosome is present in the sperm FISH results. Balanced chromosomal translocations are a known cause of RPL [18]. For men with reciprocal chromosomal translocations, pre-implantation genetic screening (PGS) is recommended, as this has increased the live birth rate from 4.9 % to more than 80 % in some studies [19–21]. PGS utilizes either FISH or array-based analysis to detect chromosomal abnormalities or genetic defects in embryos undergoing IVF/ICSI [5]. PGS can also be used to identify embryos without aneuploidy or an unbalanced translocation for implantation in the female [22].
Global elevation of chromosomal aneuploidy may be apparent when the percentage of all chromosomes tested is increased, usually 13, 15, 18, 21, X, and Y [5, 23]. Studies have examined additional chromosomes (4, 6, 7, 8, 9, 10, 11, 12, and 17) and while these also show increased aneuploidy, their diagnostic yield is low when compared to the results of testing 13, 18, 21, X, and Y [7]. In this case of global chromosomal aneuploidy, the absolute percentage of aneuploid chromosomes ranges from 2 to 9 %. The basis for the increased aneuploidy most likely is an underlying abnormality of global meiotic division—such as mutation in meiosis-specific genes, environmental factors, or abnormal meiotic recombination [12, 24–28]. Patients with chromosomal abnormalities can consider PGS along with IVF and intra-cytoplasmic sperm injection. No randomized controlled trials have determined that PGS increases live births in part because enrolling patients and ethical issues present problems. No retrospective studies have been performed because of absence of data and lack of standardized protocols. Nevertheless, it seems likely that PGS yields increased live birth rates because doctors attempt to implant only embryos without aneuploidy [9].
While the impact of chromosomal aneuploidy on pregnancy outcome is unclear, it is imperative that the couple receives genetic counseling to understand the implications of aneuploidy for a pregnancy and to discuss that there is some increased risk of trisomy 13, 18, and 21, as well as a variety of sex chromosome aneuploidies. Many couples are not acquainted with these trisomies and what their presence means for a pregnancy and thus genetic counseling can ensure that the couples understand the implications of these conditions. In addition, the couple should receive information about all of their reproductive options, including the option of PGS combined with IVF and ICSI, conceiving naturally, use of donor sperm in combination with IVF/ICSI, or intrauterine insemination (IUI) and adoption.
For some couples, the increased risk of chromosomal abnormalities in children is not a risk that they are willing to take; thus, the option of using donor sperm or adopting might be more appealing. In a retrospective study of 407 couples with male factor infertility who discontinued treatment without conceiving, 11 % pursued adoption and 1 % of couples used donor sperm. The role of the genetic counselor is to inform the patients of all of their options so that couples can make informed decisions about future reproductive attempts [29].
Prenatal testing
Recommended post-conception screening and testing: natural conception
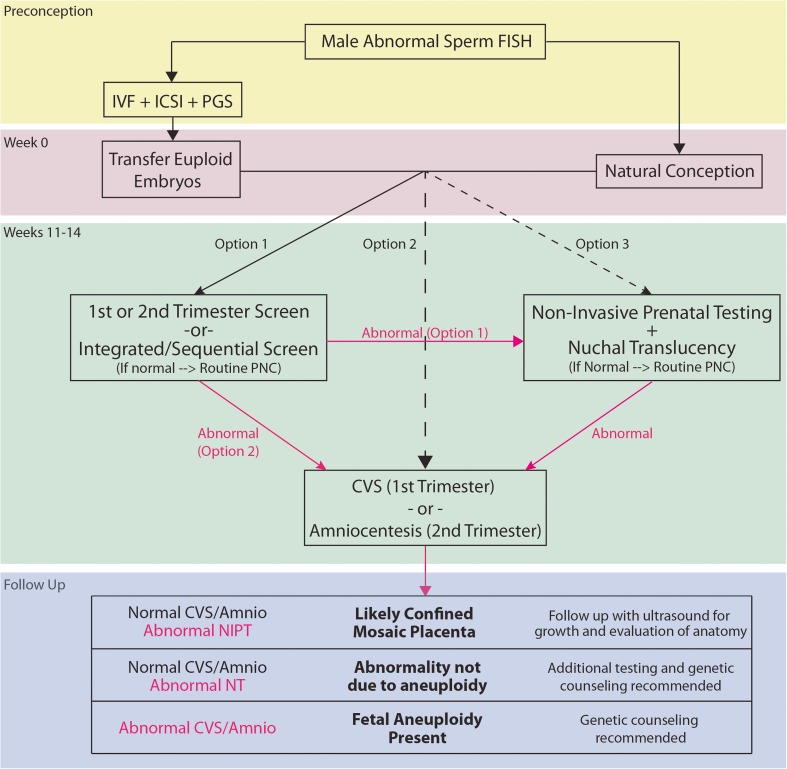
If a couple conceives naturally when the man has known chromosomal translocation, inversion, or aneuploidy in the sperm, the couple should be offered screening or diagnostic testing during the first or second trimesters to assess fetal chromosomal abnormalities. Maternal serum analytes combined with ultrasound measurement of nuchal translucency are used to screen for chromosomal aneuploidy during the first and second trimesters (Table 1) [30]. Positive screening test results can be followed up with non-invasive pregnancy testing (NIPT) or with chorionic villus sampling or amniocentesis plus cytogenetic analysis, which provide conclusive prenatal diagnosis. The provided guideline (Fig. 1) may be useful for clinical decision-making. However, the option(s) that any given couple may pursue can be limited by local availability.
Table 1.
Screening tests for aneuploidy
| Screening test | Gestational age (weeks) | NT | PAPP-A | Free ß-hCG or hCG | MSAFP | uE3 | Inh A |
|---|---|---|---|---|---|---|---|
| First trimester | 9–14 | x | x | x | |||
| Second trimester triple | 15–22 | x | x | x | |||
| Second trimester quad | 15–22 | x | x | x | x | ||
| Full integrated | First trimester tests at 9–14, followed by Second trimester tests at 15–22 weeks | x | x | x | x | x | x |
| Serum integrated | x | x | x | x | x | ||
| Step-wise sequential | x | x | x | x | x | x |
NT ultrasound assessment of nuchal translucency, PAPP-A pregnancy-associated plasma protein A, ß-hCG beta human chorionic gonadotropin, MSAFP maternal serum alpha-fetoprotein, uE3 unconjugated estriol, Inh A, inhibin A
Fig. 1.
Flow chart for high-risk pregnancy associated with abnormal male sperm FISH
Because of the increased risk of fetal chromosomal abnormalities, the couple may opt to skip initial serum analyte/ultrasound screening in favor of improved screening using non-invasive pregnancy testing (NIPT) or invasive testing [31]. Invasive testing uses either chorionic villus sampling (CVS) to obtain material from the placenta at 10–14 weeks gestation or amniocentesis to obtain amniotic fluid at or after 15 weeks gestation. Cytogenetic analysis is performed on the sample collected through CVS and amniocentesis using karyotyping or chromosomal microarray [32]. Invasive cytogenetic testing has a higher sensitivity than serum analyte screening of NIPT for trisomies, monosomies, mosaicism, and deletions or duplications. However, the process of obtaining cellular material increases risk of preterm premature rupture of the membranes (PPROM), pre-viable delivery, and fetal injury. NIPT identifies extracellular fetal nucleic acids as well as maternal nucleic acids circulating within maternal blood (cell-free DNA), and thus can screen for trisomies 13, 18, and 21, and sex chromosome aneuploidies. NIPT is more sensitive and specific than serum/ultrasound screening (Table 2), even in the first trimester, and is a safe alternative to invasive testing. NIPT screening is currently only recommended in high-risk populations as the increased prevalence of fetal chromosomal aneuploidy improves the positive predictive value (PPV) of the screening. The true risk of fetal aneuploidy with increased levels of sperm aneuploidy is currently unknown, but it is likely that risk is elevated sufficiently above baseline to permit use of NIPT as a primary screening tool.
Table 2.
Sensitivity and specificity of NIPT testing for various chromosomal abnormalities
| NIPT sensitivity | NIPT specificity | False-positive (1-specificity) | |
|---|---|---|---|
| Trisomy 21 | 99.1–100 % | 99.8–100 % | <1 % |
| Trisomy 18 | 97.4–100 % | 99.6–100 % | <1 % |
| Trisomy 13 | 80–100 % | 99.7–100 % | <1 % |
| Monosomy X | 92–95 % | – | – |
Unless the couple strongly wishes to avoid invasive diagnostic testing, CVS or amniocentesis is recommended for confirmation of all positive NIPT results. After a positive NIPT, if cytogenetic testing with CVS or amniocentesis is normal, this suggests that the abnormal level of cell-free DNA in maternal serum is not likely the result of a fetal chromosome abnormality but instead likely the result of confined placental mosaicism. Given the high NPV of NIPT, any negative result is reassuring that fetal aneuploidy is unlikely and only routine prenatal care is indicated from that point forward.
Unfortunately, up to 12 % of samples do not yield an interpretation in NIPT, even with repeat testing. The level of cell-free fetal DNA in maternal blood (fetal fraction) is decreased when the fetus is aneuploid and so these pregnancies may be more likely to not yield an NIPT result; thus, paradoxically, women who receive no result are at increased risk of aneuploidy compared to baseline.
Recommended post-conception screening and testing: IVF with PGS
The couple with known male sperm aneuploidy may choose to pursue in vitro fertilization with pre-implantation genetic screening, rather than attempting to conceive naturally. After ovarian stimulation and oocyte retrieval, intra-cytoplasmic sperm injection (ICSI) can be performed using either fresh or cryopreserved and thawed testicular sperm, as outcomes have been shown to be equivalent with either method [33]. Trophectoderm cells are retrieved from the blastocyst 5 to 6 days after fertilization to provide material for screening before embryo selection and transfer. Pre-implantation genetic screening refers to screening embryos for aneuploidy when the parents are chromosomally normal, or presumed to be, whereas pre-implantation genetic diagnosis uses probes to identify specific heritable gene mutations or chromosomal abnormalities that have been transmitted from a known genetically abnormal parent [34].
After a successful transfer and implantation of a screened embryo, routine blood work is recommended at the first prenatal visit, followed by ultrasound assessment of nuchal translucency at 11–14 weeks and NIPT or maternal serum screening. If these screening tests are normal, the pregnancy can be considered low-risk and followed with routine prenatal care. If the nuchal translucency, NIPT, or maternal serum screening are abnormal, then diagnostic cytogenetic testing using CVS or amniocentesis should be offered to the couple.
Conclusion
For couples who struggle with RPL and repeated IVF failure, it is essential that the male is examined for either chromosomal aneuploidy or chromosomal structural abnormalities. While it is difficult to assess the precise risk associated with increased levels of sperm aneuploidy, genetic counseling is imperative to educate couples about the associated risks and inform them of all of their reproductive and testing options. Further research is needed to investigate the relationship between sperm chromosomal aneuploidy and reproductive outcomes as well as the somatic health of the male patient. Most importantly, for pregnancies conceived with a partner with increased sperm aneuploidy appropriate prenatal screening and testing should be offered, even when PGS has been performed, as these pregnancies are considered to be at increased risk for aneuploidy.
Footnotes
Capsule We review the literature and discuss genetic counseling for couples with RPL or recurrent implantation failure due to increased sperm aneuploidy.
References
- 1.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23(25):1–160. [PubMed] [Google Scholar]
- 2.Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstetrics Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Medicine PCotASfR Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99(1):63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Burrello N, Vicari E, Shin P, Agarwal A, De Palma A, Grazioso C, et al. Lower sperm aneuploidy frequency is associated with high pregnancy rates in ICSI programmes. Hum Reprod. 2003;18(7):1371–6. doi: 10.1093/humrep/deg299. [DOI] [PubMed] [Google Scholar]
- 5.Hwang K, Weedin JW, Lamb DJ. The use of fluorescent in situ hybridization in male infertility. Ther Adv Urol. 2010;2(4):157–69. doi: 10.1177/1756287210373758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicopoullos JDM, Gilling-Smith C, Almeida PA, Homa S, Nice L, Tempest H, et al. The role of sperm aneuploidy as a predictor of the success of intracytoplasmic sperm injection? Hum Reprod. 2008;23(2):240–50. doi: 10.1093/humrep/dem395. [DOI] [PubMed] [Google Scholar]
- 7.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14(5):1266–73. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- 8.Rubio C, Gil-Salom M, Simón C, Vidal F, Rodrigo L, Mínguez Y, et al. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod. 2001;16(10):2084–92. doi: 10.1093/humrep/16.10.2084. [DOI] [PubMed] [Google Scholar]
- 9.Ramasamy R, Besada S, Lamb DJ. Fluorescent in situ hybridization of human sperm: diagnostics, indications, and therapeutic implications. Fertil Steril. 2014;102(6):1534–9. doi: 10.1016/j.fertnstert.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collodel G, Giannerini V, Antonio Pascarelli N, Federico MG, Comodo F, Moretti E. TEM and FISH studies in sperm from men of couples with recurrent pregnancy loss. Andrologia. 2009;41(6):352–60. doi: 10.1111/j.1439-0272.2009.00936.x. [DOI] [PubMed] [Google Scholar]
- 11.Vidal F, Blanco J, Egozcue J. Chromosomal abnormalities in sperm. Mol Cell Endocrinol. 2001;183(Supplement 1):S51–S4. doi: 10.1016/S0303-7207(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 12.Hwang K, Lipshultz LI, Lamb DJ. Use of diagnostic testing to detect infertility. Curr Urol Rep. 2011;12(1):68–76. doi: 10.1007/s11934-010-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, Lipshultz LI. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril. 2015;103(4):906–9.e1. doi: 10.1016/j.fertnstert.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, et al. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17(10):2600–14. doi: 10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. Cambridge: Cambridge University Press; 2009.
- 16.Practice Committee of the American Society for Reproductive M Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17(2):446–51. doi: 10.1093/humrep/17.2.446. [DOI] [PubMed] [Google Scholar]
- 18.Stern C, Pertile M, Norris H, Hale L, Baker HW. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum Reprod. 1999;14(8):2097–101. doi: 10.1093/humrep/14.8.2097. [DOI] [PubMed] [Google Scholar]
- 19.Keymolen K, Staessen C, Verpoest W, Liebaers I, Bonduelle M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Hum Genet. 2012;20(4):376–80. doi: 10.1038/ejhg.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munné S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril. 2000;73(6):1209–18. doi: 10.1016/S0015-0282(00)00495-7. [DOI] [PubMed] [Google Scholar]
- 21.Kohn TP, Clavijo R, Ramasamy R, Hakky T, Candrashekar A, Lamb DJ, et al. Reproductive outcomes in men with karyotype abnormalities: case report and review of the literature. Can Urol Assoc J = Journal de l’Association des urologues du Canada. 2015;9(9–10):E667–70. doi: 10.5489/cuaj.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio C, Buendía P, Rodrigo L, Mercader A, Mateu E, Peinado V, et al. Prognostic factors for preimplantation genetic screening in repeated pregnancy loss. Reprod Biomed Online. 2009;18(5):687–93. doi: 10.1016/S1472-6483(10)60015-6. [DOI] [PubMed] [Google Scholar]
- 23.Pastuszak AW, Lamb DJ. The genetics of male fertility—from basic science to clinical evaluation. J Androl. 2012;33(6):1075–84. doi: 10.2164/jandrol.112.017103. [DOI] [PubMed] [Google Scholar]
- 24.Baarends WM, van der Laan R, Grootegoed JA. DNA repair mechanisms and gametogenesis. Reproduction. 2001;121(1):31–9. doi: 10.1530/rep.0.1210031. [DOI] [PubMed] [Google Scholar]
- 25.Egozcue J, Sarrate Z, Codina-Pascual M, Egozcue S, Oliver-Bonet M, Blanco J, et al. Meiotic abnormalities in infertile males. Cytogenet Genome Res. 2005;111(3-4):337–42. doi: 10.1159/000086907. [DOI] [PubMed] [Google Scholar]
- 26.Martin RH. Meiotic chromosome abnormalities in human spermatogenesis. Reprod Toxicol. 2006;22(2):142–7. doi: 10.1016/j.reprotox.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Mroz K, Hassold TJ, Hunt PA. Meiotic aneuploidy in the XXY mouse: evidence that a compromised testicular environment increases the incidence of meiotic errors. Hum Reprod. 1999;14(5):1151–6. doi: 10.1093/humrep/14.5.1151. [DOI] [PubMed] [Google Scholar]
- 28.Sarrate Z, Blanco J, Anton E, Egozcue S, Egozcue J, Vidal F. FISH studies of chromosome abnormalities in germ cells and its relevance in reproductive counseling. Asian J Androl. 2005;7(3):227–36. doi: 10.1111/j.1745-7262.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 29.Walschaerts M, Bujan L, Parinaud J, Mieusset R, Thonneau P. Treatment discontinuation in couples consulting for male infertility after failing to conceive. Fertil Steril. 2013;99(5):1319–23. doi: 10.1016/j.fertnstert.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 30.De Domenico R, Faraci M, Hyseni E, Di Prima FAF, Valenti O, Monte S, et al. Increased nuchal translucency in normal karyotype fetuses. J Prenat Med. 2011;5(2):23–6. [PMC free article] [PubMed] [Google Scholar]
- 31.American College of O, Gynecologists ACOG practice bulletin no. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110(6):1459–67. doi: 10.1097/01.AOG.0000291570.63450.44. [DOI] [PubMed] [Google Scholar]
- 32.American College of O, Gynecologists Committee on G Committee opinion no. 581: the use of chromosomal microarray analysis in prenatal diagnosis. Obstet Gynecol. 2013;122(6):1374–7. doi: 10.1097/01.AOG.0000438962.16108.d1. [DOI] [PubMed] [Google Scholar]
- 33.Ohlander S, Hotaling J, Kirshenbaum E, Niederberger C, Eisenberg ML. Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction: a meta-analysis. Fertil Steril. 2014;101(2):344–9. doi: 10.1016/j.fertnstert.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Practice Committee of the American Society for Reproductive M Preimplantation genetic testing: a practice committee opinion. Fertil Steril. 2008;90(5 Suppl):S136–43. doi: 10.1016/j.fertnstert.2008.08.062. [DOI] [PubMed] [Google Scholar]