Abstract
Morc3, a member of a highly conserved nuclear matrix protein super-family plays an important part in chromatin remodeling, DNA repair, epigenetic regulation and cellular senescence. However, its role in bone homeostasis is not known. In the present study, a phenotype-driven ENU mouse mutagenesis screen revealed that Morc3mut +/− mice exhibit reduced cortical area and thickness with increased cortical porosity. Morc3mut +/− mice displayed reduced osteoclast numbers and surface per bone surface as well as osteocyte numbers, concomitant with altered gene expressions such as Rankl/Opg and Sost in ex vivo long bones. In vitro experiments revealed a significant increase in the number of Sca-1+/c-kit+ haematopoietic stem cells (HSCs), and a significant reduction in senescence associated β-galactosidase activity in bone marrow macrophages (BMMs). In addition, we observed a decrease in osteoclastogenesis and bone resorption accompanied by upregulation of STAT1 expression in osteoclast lineage cells. Strikingly, Morc3 protein localization within the nuclear membrane was shifted to the cytoplasm in Morc3mut +/− osteoclasts. Further, Morc3mut +/− mice displayed increased osteoblast differentiation and altered gene expression. Collectively, our data show that Morc3 is a previously unreported regulator of cortical bone homeostasis and haematopoietic stem cells niche, accompanied by altered bone cell differentiation.
Bone is a rigid organ, yet highly susceptible to metabolic changes throughout the adult life. Bone homeostasis is continuously maintained by the bone remodeling process which is tightly regulated by two key activities: bone removal by osteoclasts and bone matrix formation by osteoblasts. Imbalances in either bone resorption or bone formation can lead to clinical diseases like osteoporosis, osteopetrosis and Paget’s disease of bone1. Worldwide direct and indirect annual costs of fracture due to osteoporosis have been estimated to be US$20 billion in the USA and about AUD$2.75 billion in Australia2. Despite recent advances in bone biology, the precise molecular mechanisms responsible for pathological bone conditions remain unclear. Therefore, elucidating the molecular mechanisms and novel molecules involved in the maintenance of bone homeostasis is crucial for the better understanding of skeletal health and development of novel therapeutics against various bone diseases.
Morc3 (NXP2/KIAA0136/ZCWCC3) is a member of a highly conserved nuclear protein super-family, with characteristic domains that directly link the Morc proteins to signaling-dependent chromatin remodeling and epigenetic regulation3. Mapping of functional domains revealed it as a nuclear matrix protein with a putative RNA binding site in a nuclear matrix binding domain which is vital for transcription regulation4. Similar to other GHKL (gyrase, Hsp90, histidine kinase, MutL)-ATPase family members, Morc3 forms a homodimer through GHKL-ATPase and coiled-coil domains in an ATP-binding-dependent manner5. It functions as a molecular clamp through the ATPase cycle to form Morc3 nuclear domains in a PML (promyelocytic leukemia)-independent manner. The CW- type Zinc Finger domain of Morc3 is required for proper localization in the nucleus and contains an important histone recognition module specifically for H3K4 methylation6. Expression of Morc3 is ubiquitous, with high levels seen in immune cells7. Global knockout of Morc3 in mice is perinatally lethal, with all Morc3−/− mice dying within 1 day of birth for unknown reasons. Morc3 plays an important role in p53 induced cellular senescence by activating p53 and localizing it to PML nuclear bodies8. It binds to PML through small ubiquitin-like modifier (SUMO) and SUMO-interacting motif (SIM). Association of Morc3 with PML requires modification by SUMO1 at its multiple SUMOylation sites. It also binds to SUMO2 to facilitate SUMO-mediated transcriptional repression9. This evidence suggests that Morc3 is a new player in DNA repair and epigenetic regulation.
Morc3 has been implicated in regulating interferon (IFN)-mediated JAK-STAT signaling networks10. Recently, Morc3 has been identified to interact with tyrosine kinase membrane receptor ROR1. ROR1 co-operates with the pre-B cell receptor through activation of downstream signaling pathways such as AKT and MAPK to promote survival of acute lymphoblastic leukemia11. This suggests that Morc3 is associated with the regulation of cell signaling pathways that control cell survival and proliferation. Morc3 has been identified as an antigen for circulating auto-antibodies in ~25% of patients with juvenile dermatomyositis (JDM)12, an autoimmune dysfunction frequently associated the skin calcinosis (calcium deposition under the skin). Furthermore, calcified lesions in patients with JDM are associated with increased expression of osteogenic markers including OCN, BSP and MGP13. Anti-Morc3 auto-antibodies have also been identified in a subset of adult dermatomyositis (ADM) patients14, and this has been linked to malignancy15.
Overall, Morc3 is a transcriptional regulator of proteins involved in signal transduction pathways (IFN-activated STAT, AKT and MAPK) and calcium homeostasis. However, its role in bone homeostasis and remodeling has not been previously reported. Using a phenotype-driven ENU mouse mutagenesis screen, we identified a heterozygous Morc3 (Morc3mut +/−) mutant mouse strain, which displays altered bone homeostasis. We uncovered that Morc3 mutant mice exhibit reduced cortical area and thickness with increased cortical porosity, accompanied by altered haematopoietic stem cells niche and bone cell differentiation, as well as by the upregulation of IFN-β and STAT1 expression in osteoclast and osteoblast lineage cells.
Results
Morc3mut +/− mice exhibit lower cortical but not trabecular bone mass
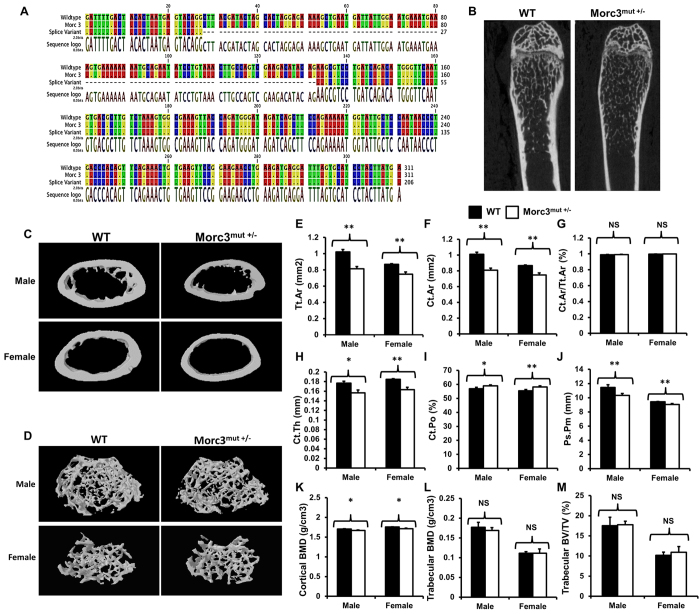
To determine the role of Morc3 in the skeleton, we evaluated the mutant mouse strain with ENU-induced point mutation in the Morc3 gene. The mutation lies in the sixth base pair (bp) of the splice donor site of intron 12 (exon10/11) of the Morc3 gene resulting in a substitution from T to C. Homozygous mutation of Morc3 is embryonically lethal at approximately embryonic day 9 (E9). The heterozygous Morc3 mice (Morc3mut +/−) were born healthy at the predicted Mendelian frequencies with the mutation that resulted in an additional splice variant of Morc3. Sequencing of these splice variants revealed that the mutation in Morc3mut +/− mice resulted in an expression of Morc3 mRNA similar to wild type (WT) along with an additional splice variant with deleted exon 10 (Fig. 1A). Morc3mut +/− mice had a similar body structure to that of WT littermates with no obvious differences observed between Morc3mut +/− and WT controls (Supplementary Fig. 1). Interestingly, micro computed tomography (microCT) analysis revealed that the cortical bone mass and cortical BMD were significantly lower in young Morc3mut +/− mice compared to age- and sex-matched WT littermates (Fig. 1B–K), and these differences persisted in older mutant mice (Supplementary Fig. 2), suggesting defects in cortical bone growth. In comparison, there were no significant differences in trabecular bone parameters between Morc3mut +/− and WT mice (Fig. 1L,M) (Supplementary Fig. 3). In fact, the trabecular bone phenotype remained unaltered even in older Morc3mut +/− male mice when compared to their WT littermates (Supplementary Fig. 4).
Figure 1. Mutation in Morc3 results in cortical bone loss.
(A) Sequencing analysis revealed the mutation in Morc3 generated an additional splice variant with excised exon 10. (B) MicroCT analysis of hindlimbs from 12 week old WT and Morc3mut +/− mice revealed reductions in cortical bone area, thickness and size, and an increase in cortical porosity. (C,D) Representative 3D reconstructions of cortical and trabecular bone in age- and sex- matched WT and Morc3mut +/− mice respectively. (E–K) Cortical bone parameters as assessed by microCT; are shown as (E) total cortical area (Tt.Ar; mm2), (F) cortical bone area (Ct.Ar; mm2), (G) cortical area fraction (Ct.Ar/Tt.Ar; %), (H) cortical thickness (Ct.Th; μm), (I) periosteal perimeter (Ps.Pm; mm), (J) cortical porosity (Ct.Po; %) and (K) cortical bone mineral density (cortical BMD; g/cm3) (n = 9). (L,M) MicroCT analysis of trabecular bone parameters; (L) Trabecular bone mineral density (Trabecular BMD; g/cm3) and (M) bone volume per total volume (Trabecular BV/TV; %) (n = 9). Data are presented as mean/fold change ± SEM. NS = non-significant; *P < 0.05; **P < 0.01.
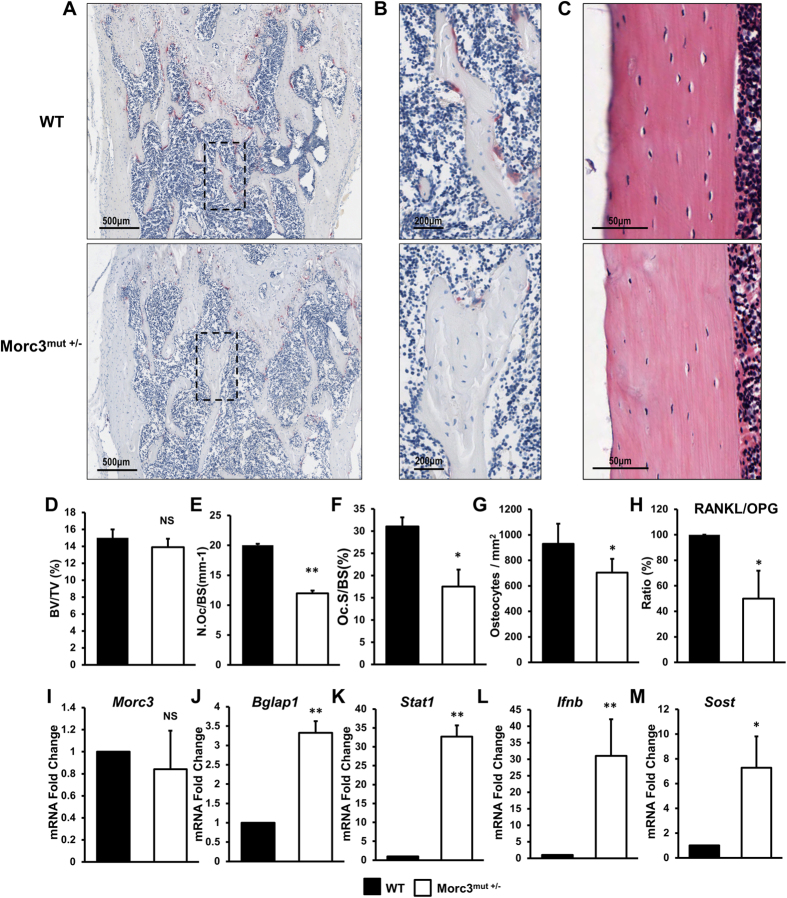
To gain further insight into the in vivo cellular phenotype of the Morc3mut +/− mice, bone histomorphometry was performed on decalcified sections stained for TRAcP activity and with haematoxylin and eosin (Fig. 2A–C). Consistent with micro computed tomography data, histomorphometric analysis of femora from 12 week old Morc3mut +/− mice showed a normal trabecular bone mass when compared to WT mice (Fig. 2D). Analysis of osteoclast parameters using TRAcP stained sections revealed that Morc3mut +/− mice exhibited a significant decrease in the number of osteoclasts per bone surface and osteoclast surface per bone surface (Fig. 2E,F). These findings indicate that Morc3mut +/− mice exhibit reduced osteoclast numbers in vivo. Interestingly, on further investigation of the cortical bone in the Morc3mut +/− mice, we observed a significant reduction in osteocyte density in vivo (Fig. 2C,G). This result suggests that the Morc3 mutation might alter osteocyte formation or survival. Osteocytes play a critical role in adult bone homeostasis through the production of Rankl and other signaling molecules impacting osteoclast and osteoblast functions. To explore whether mutation of Morc3 alters the gene expression levels of critical osteoclast, osteoblast and osteocyte proteins and signaling intermediates in the cortical bone of Morc3mut +/− mice, real time PCR was performed on RNA isolated from long bones (bone marrow removed). The ratio of mRNA expression of Rankl/Opg was significantly reduced in long bones of Morc3mut +/− mice ex vivo (Fig. 2H). We found no changes in the expression of Morc3 in the long bones of Morc3mut +/− mice (Fig. 2I); however, a significant increase in mRNA expression of bone related markers including osteocalcin (Bglap1), Stat1, Ifnb1 and sclerostin (Sost) (Fig. 2J–M) was observed. This result suggests that the Morc3 mutation leads to significant changes in signaling molecule profiles in the cortical bone compartment, possibly to compensate for the decreased cortical bone mass.
Figure 2. Analysis of WT and Morc3mut +/− femurs from 3 month old male mice using histology and qPCR.
(A) Representative low power images of TRAP stained femur sections showing region just below the growth plate (mag = 40X, scale bar = 500 μm). (B) Higher power view of area within dashed box in (A) showing individual TRAP positive (purple stain) osteoclasts at the bone surface (Mag = 100X, scale bar = 200 μm). (C) Representative images of haematoxylin and eosin stained cortical bone (mag = 200X, scale bar = 50 μm). (D–G) Quantitative histomorphometric analysis of bone parameters; (D) trabecular bone volume fraction (BV/TV: %), (E) Number of osteoclasts relative to bone surface (N.OC/BS: mm-1), (F) Osteoclast surface relative to bone surface (Oc.S/BS: %) (G) Osteocyte density relative to cortical bone area (Osteocytes/mm2). (H–M) Total bone RNA isolated from the hindlimbs (bone marrow flushed) of 12 week old WT and Morc3mut +/− mice were subjected to real time-PCR to analyze the gene expression profile of (H) Rankl vs. Opg ratio, (I) Morc3, (J) Bglap1, (K) Stat1 (L) Ifnb1 and (M) Sost was determined. Hprt1 was used as integral housekeeping control (n = 4). Data are presented as mean/fold change ± SEM. NS = non-significant; *P < 0.05; **P < 0.01.
Mutation in Morc3 increases the number of Sca-1+/c-kit+ haematopoietic stem cells (HSCs) and reduces bone marrow macrophage (BMM) senescence
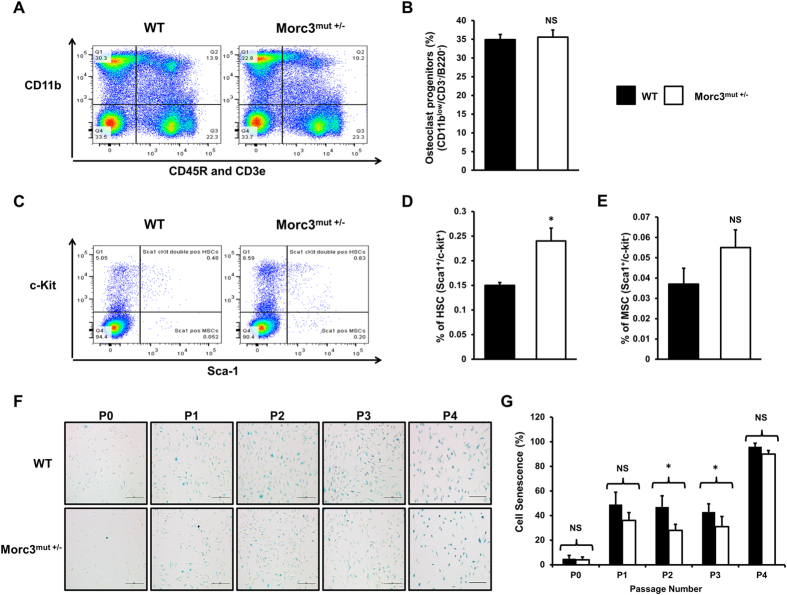
To examine the cellular basis in the bone microenvironment that contributes to the bone phenotype bone marrow populations were analyzed using flow cytometry (Fig. 3A–E). We found a significant increase in the number of putative haematopoietic stem cells (HSCs) (Sca-1+/c-kit+) in the Morc3mut +/− bone marrow (Fig. 3D), but no changes in the proportion of triple negative (CD11blow/−/CD3−/B220−) osteoclast progenitors (Fig. 3B), or putative mesenchymal stem cells (MSCs) (Sca-1+/c-kit−) (Fig. 3E).
Figure 3. Mutation in Morc3 alters bone marrow stem cell niche in HSC lineage and exhibit reduced BMM cell senescence.
Total bone marrow cells were extracted from the hind limbs of age and sex matched WT and Morc3mut +/− mice. The cells were immunostained with anti-CD45R, anti-CD11b and anti-CD3e along with Live/Dead Aqua cell death stain for flow cytometry analysis. Live mononuclear cells were assessed for lineage marker and HSC/MSC marker expression. (A) Representative pseudocolour density plot of CD45R-/CD3e-/CD11b-/low mononuclear cell population (putative osteoclast progenitors – Q4). (C) Representative pseudocolour density plot showing HSC (Sca1+, c-kit+) and MSC (Sca1+, c-kit−) populations from triple negative cells (Q4 of (A)). (B–E) Quantitative analysis of bone marrow cell populations by flow cytometry in Morc3mut +/− mice relative to wildtype littermates; (B) percentage of osteoclast progenitors (CD11blow/−/CD3−/B220−); (D) haematopoietic stem cells (HSC) (Sca1+/c-kit+); and (E) mesenchymal stem cells (MSC) (Sca1+/c-kit−) (n = 3). (F) Representative images of senescence associated β-galactosidase (SA-βgal) activity observed in WT and Morc3mut +/− BMMs over 4 cell culture passages. By P4 most cells were positive for SA-βgal activity in all cultures. Scale bar = 100 μm. (G) Quantification of the percentage of cells staining positive for SA-β-gal activity following sequential passage in tissue culture (n = 3). Data are presented as mean ± SEM. NS = non-significant; *p < 0.05.
Morc3 has an important function in senescence pathways, we next investigated whether BMM isolated from WT and Morc3mut +/− femora varied in their induction of senescence associated β-galactosidase (SA-β-gal) following passaging in vitro. We observed a significant reduction in SA-β-gal activity in cells isolated from Morc3mut +/− mice over time in culture (Fig. 3F,G), indicating alterations in senescence pathways in the bone marrow compartment. Taken together, these results suggest Morc3 mutation alters HSCs populations and BMM senescence or survival in the bone marrow.
Mutation in Morc3 impairs osteoclast formation but promotes osteoclast survival
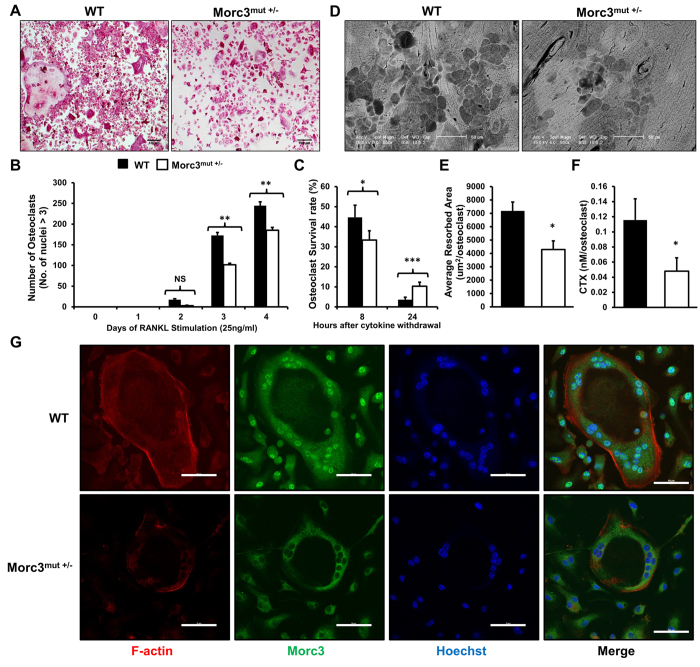
Consistent with the in vivo findings, in vitro quantitative analysis of Rankl induced osteoclast formation from bone marrow monocytes (BMM) isolated from WT and Morc3mut +/− littermates showed that the average number of Morc3mut +/− osteoclasts was significantly reduced as compared to the number of WT osteoclasts (Fig. 4A,B). Further, in vitro mature osteoclast survival assay revealed that Morc3mut +/− osteoclasts exhibit higher survival rates as compared to WT osteoclasts after 24 hours of cytokine withdrawal (Fig. 4C). These results suggest that the mutation in Morc3 impairs osteoclast formation, but promotes osteoclast survival.
Figure 4. Mutation in Morc3 impairs osteoclast formation and bone resorption activity but promotes osteoclast survival.
Osteoclasts were derived from BMM of WT and Morc3mut +/− mice cultured in vitro in the presence of Rankl (25 ng/ml) and M-CSF. (A) Representative images of TRAcP stained WT and Morc3mut +/− bone marrow derived osteoclasts (mag = 100X, scale bar = 100 μm). (B) Quantification of TRAP positive multinucleated cells (nuclei ≥ 3) (n = 4). (C) Osteoclast survival rate as a percentage of osteoclasts remaining following withdrawal of Rankl and M-CSF at day 4. (D) Scanning electron microscopy images of resorption pits formed by WT and Morc3mut +/− bone marrow-derived osteoclasts. (mag = 800X, scale bar = 50 μm). (E) Quantification of area resorbed per osteoclast (μm2/osteoclast) and (F) release of CTX into culture medium from Rankl-stimulated bone marrow cells from WT and Morc3mut +/− mice on bone slices (n = 3). (G) Osteoclasts derived from bone marrow of WT and Morc3mut +/− mice cultured on cover slips and immunostained with F-actin (red), anti-Morc3 antibody (green), counter stained with the nuclear dye Hoechst 33258 (blue), and visualized by confocal microscopy. (mag = 400X, scale bar = 50 μm). Data are presented as mean ± SEM. NS = non-significant; *p < 0.05; **p < 0.01, ***p < 0.001.
Osteoclast function was assessed by culturing mature osteoclasts derived from BMM of WT and Morc3mut +/− mice on bovine bone slices (Fig. 4D). When normalized against osteoclast numbers, the area of bone resorbed by Morc3mut +/− osteoclasts was significantly decreased as compared to the area resorbed by WT osteoclasts (Fig. 4E). Consistent with these findings, the levels of c-terminal fragments of collagen type 1 (CTX) released into the culture media during bone resorption by Morc3mut +/− osteoclasts was significantly reduced as compared to WT osteoclasts (Fig. 4F).
Confocal microscopy analysis showed Morc3 protein localization within the nuclear membrane was shifted to the cytoplasm in Morc3mut +/− osteoclasts as compared to WT controls (Fig. 4G). Intact F-actin rings were formed by Morc3mut +/− osteoclasts, suggesting that osteoclast polarization was not significantly altered by Morc3 mutation in mature osteoclasts.
Mutation in Morc3 displays activated STAT1 signaling pathway and altered Stat1 and Ifnb1 gene expression during osteoclastogenesis
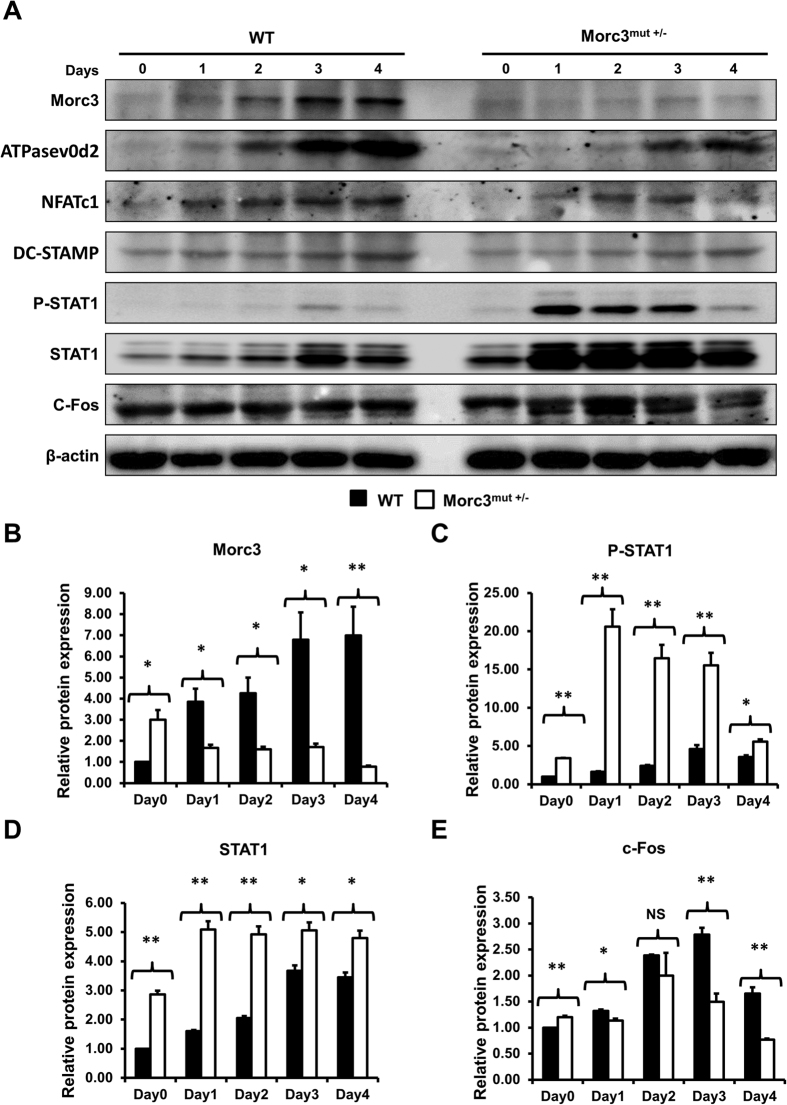
Mutation in Morc3 leads to impaired osteoclast formation and bone resorption activity. Consistently, we found that expression of the osteoclast associated proteins, ATPasev0d2, NFATc1 and DC-STAMP were reduced in Morc3mut +/− cells by western blot analysis (Fig. 5A). Notably, protein expression of Morc3 was also significantly reduced during osteoclastogenesis in Morc3mut +/− osteoclasts as compared to WT (Fig. 5A,B). Interestingly, a remarkable increase in phosphorylated STAT1 (P-STAT1) and total STAT1 protein expression was observed during osteoclast differentiation in Morc3mut +/− mice as compared to WT (Fig. 5A,C,D). In contrast, significant reductions in c-FOS protein levels were detected during osteoclastogenesis in Morc3mut +/− osteoclasts as compared to WT osteoclasts (Fig. 5E). C-Fos is an essential transcriptional regulator of osteoclastogenesis, which auto-inhibits itself through upregulation of IFN-β16. Further analysis by real time PCR showed that Ifnb1 and Stat1 gene expression are increased during osteoclastogenesis in Morc3mut +/− cells (Supplementary Fig. 5). These results suggest that mutation in Morc3 leads to inhibition of osteoclastogenesis through upregulation of IFN-β/STAT1 signaling pathway.
Figure 5. Mutation in Morc3 inhibits Rankl-induced osteoclastogenesis through upregulation of STAT1 signaling pathway.
Whole cell lysates extracted from osteoclast cultures were analyzed by Western blots. (A) Representative western blot image of Morc3, ATPasev0d2, NFATc1, DC-STAMP, P-STAT1, STAT1, c-FOS and β–catenin protein levels during WT and Morc3mut +/− osteoclast differentiation. Quantitative analysis of (B) Morc3, (C) phosphorylated STAT1 (PSTAT1), (D) STAT1 and (E) c-FOS protein expression relative to β-actin and further normalized to WT day 0 control by densitometry (n = 3). Data are presented as fold change ± SEM. N.S = non-significant; *P < 0.05; **P < 0.01.
Mutation in Morc3 leads to increased osteoblast differentiation and altered osteoblastic gene expression
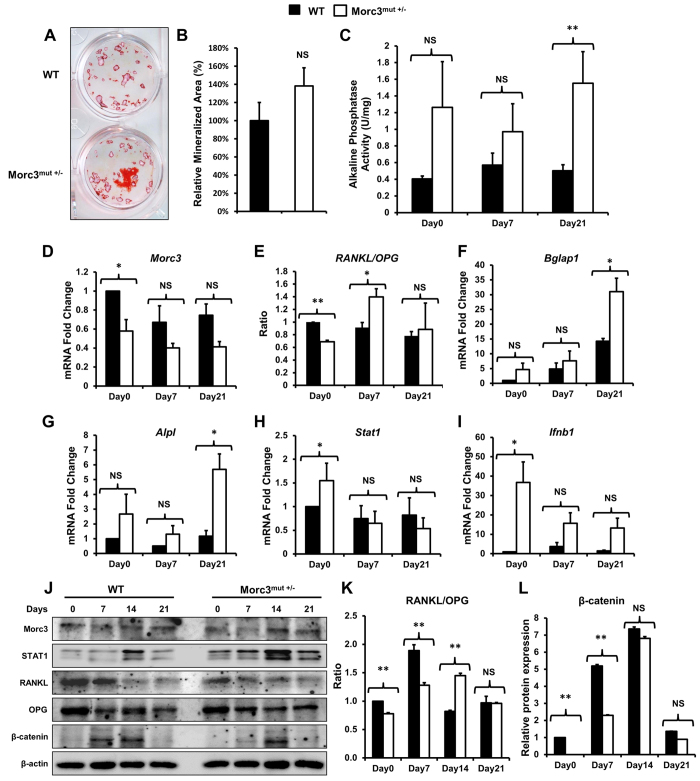
The bone mineralization activity of osteoblasts derived by outgrowth from Morc3mut +/− long bones was not altered relative to WT osteoblasts, as determined by alizarin red S staining at day 21 of culture (Fig. 6A,B). Increased ALP activity was observed in Morc3mut +/− osteoblasts at day 21 of culture as compared to WT osteoblasts (Fig. 6C). These results suggest that osteoblast differentiation was altered but bone mineralization activity appears to be unaffected in osteoblasts derived from Morc3mut +/− mice.
Figure 6. Delayed early stage osteoblast differentiation in Morc3mut +/− mice.
Osteoblasts from the long bones of 12 week old WT and Morc3mut +/− mice were cultured in osteogenic media with dexamethasone (10 nM), β-glycerophosphate (10 mM) and ascorbate (50 μg/ml). (A) Representative images of alizarin red S stained mineralised nodules. (B) Quantitative analysis of mineralised nodule area expressed as the relative % of wild type control (n = 6). (C) Alkaline phosphatase activity (units per mg of protein; U/mg) was measured in whole cell lysates from osteoblast cultures at day 0, 7 and 21. (n = 3) (D–I) Real Time-PCR analysis of gene expression in Morc3mut +/− long bone osteoblast cultures expressed as fold change relative to wild type at day 0 control; (D) Morc3, (E) Rankl/Opg (expression ratio), (F) Osteocalcin (Bglap1), (G) Alkaline phosphatase (Alpl), (H) Stat1, and (I) Ifnb1. Hprt1 was used as integral housekeeping control. (J) Representative western blot image of Morc3, STAT1, Rankl, OPG and β–catenin protein levels during WT and Morc3mut +/− osteoblast differentiation. (K,L) Quantitative analysis of protein levels by densitometry, bands were normalized to β-actin loading control and compared to WT day 0 control (n = 3); (K) Rankl/OPG (expressed as a ratio) and (L) β-catenin. Data are presented as fold change ± SEM. NS = non-significant; *p < 0.05; **p < 0.01.
Real-time PCR was performed to determine whether osteoblast marker genes were differentially expressed between WT and Morc3mut +/− osteoblasts. The mRNA levels of Morc3 were significantly reduced on day 0 in Morc3mut +/− osteoblasts, but were comparable to WT controls during the late stages of osteoblast differentiation (Fig. 6D). Similarly the ratio of Rankl/Opg mRNA levels in Morc3mut +/− osteoblasts was reduced on day 0, but remarkably upregulated on day 7 and normalized during the late stages of osteoblast differentiation (Fig. 6E). In contrast, the mRNA expression of osteoblast marker genes Bglap1 and Alpl were comparable in WT and Morc3mut +/− osteoblasts during early stages of osteoblast differentiation, but were significantly increased on day 21 in Morc3mut +/− osteoblasts, consistent with ALP enzyme activity (Fig. 6F,G). Interestingly, we found increased mRNA expression of Stat1 and Ifnb1 in Morc3mut +/− osteoblasts on day 0, which stabilized during the late stages of osteoblast differentiation (Fig. 6H,I). Consistent with this observation an increase in STAT1 protein expression was observed during Morc3mut +/− osteoblast differentiation as compared to WT (Fig. 6J). Western blot analysis of protein expression of Morc3, Rankl and OPG in Morc3mut +/− osteoblasts showed consistent patterns as observed in their gene expression profile during osteoblast differentiation (Fig. 6J), with the Rankl/OPG ratio significantly reduced in favor of increased OPG levels during early time points (Fig. 6K). Interestingly, protein expression of β-catenin, an essential osteoblast differentiation marker17, was reduced during the early stage of osteoblastogenesis (day 0–7) in Morc3mut +/− osteoblasts as compared to WT (Fig. 6L). Hence, these results suggest that the mutation in Morc3 leads to altered expression patterns of essential osteoblast marker genes, as well as Stat1 and Ifnb1 during osteoblast differentiation when compared to wild type mice.
Discussion
Bone homeostasis is accomplished by tight regulation of bone resorption and bone formation activities by three key bone cells; the osteoclasts, osteoblasts and osteocytes. However, disruptions to these balanced activities leads to several pathological bone conditions, including osteoporosis, osteopetrosis and Paget’s disease. Identification of novel genes and molecular pathways that regulate bone homeostasis may help us to develop new therapeutic strategies against bone diseases. Using a phenotype driven ENU mutagenesis screening approach we have demonstrated that partial loss of Morc3 results in reduced cortical bone mass and thickness with increased cortical porosity associated with the upregulation of inflammatory molecules including IFN-β/STAT1 and sclerostin. To our knowledge, this is the first study to implicate a direct role for Morc3 in regulation of bone homeostasis.
Our study utilized heterozygous Morc3 mutant mice as homozygous mutants did not survive past embryonic day 9, which prevented us from further analysis of its role in the adult skeleton. This is consistent with the mutation causing a loss of function as the global deletion of Morc3 results in early postnatal lethality8. The mutation occurs at the splice donor site of intron 12 of Morc3 resulting in the generation of a splice variant of Morc3 mRNA with deleted exon 10. Exon 10 is predicted to be important for the integrity of the zinc finger domain which is critical for Morc3 DNA and nucleosome interactions8. Interestingly, we observed very little Morc3 in the nucleus of Morc3mut +/− cells, suggesting that the mutation interfered with nuclear localization of the Morc3 protein. The previously described functions of Morc3 all require nuclear localization4,5,8,9; hence the loss of Morc3 activity is likely due, at least in part, to the altered localization of the protein.
Morc3mut +/− mice displayed normal body length and body size, but a significantly reduced cortical BMD and thickness with increased cortical porosity when compared to WT controls; however, the trabecular bone mass and growth plate were unaffected in both male and female Morc3mut +/− mice. There is increasing evidence that cortical and trabecular bone undergoes differential regulation18,19,20. These results suggest a role for Morc3 in the regulation of cortical bone homeostasis, a function that has been attributed to osteocytes21. It is possible that the single WT Morc3 allele present in the heterozygous mutants limited the trabecular phenotype and that complete ablation of Morc3 in bone cells would affect both cortical and trabecular bone, a conditional knockout of Morc3 in bone cells would clarify this issue.
Osteocyte lacunar density was reduced in Morc3mut +/− femurs from both male and female mice. Reduced osteocyte lacunar density is associated with aging, and can impact the ability of bone to respond to microfractures22,23. The underlying reason for the reduced osteocyte density is not clear, although direct effects of the mutation on osteocyte function or survival due to the failure of senescence pathways are possible. The absence of empty osteocyte lacunae, and the enhanced survival of mutant osteoclasts suggest that apoptosis is unlikely to be affected; changes to osteoblast senescence pathways resulting in reduced osteocyte formation are more likely. It has been previously observed that osteocyte density changes are associated with reduced remodeling of femoral bone in humans22. It has also been reported that high sclerostin expression is associated with more deeply embedded mature osteocytes24. The reduced number of osteoclasts observed in the Morc3mut +/− mice is consistent with a reduced remodeling phenotype in this mouse model. Based on these results, we hypothesize that impaired bone turnover rates lead to reduced cortical bone mass and potentially increased fracture susceptibility in Morc3mut +/− mice.
Bone nodule formation rates were comparable in WT and Morc3mut +/− osteoblasts. Interestingly, we observed a delay in activation of β-catenin signaling during osteoblast differentiation. Increased mRNA levels of sclerostin in the cortical bone compartment can explain the consistent inhibition of β–catenin and thus delayed osteoblast differentiation and bone formation25. It has recently been shown that the response to mechanical loading by osteocytes is dependent on the activation of Wnt/β-catenin within osteocytes, which results in reduced sclerostin expression and subsequent bone formation at the bone surface26. We observed a relative increase in sclerostin expression in the Morc3mut +/− mice; further work is required to clarify whether Morc3 is involved in regulating osteocyte sclerostin production. Collectively, these alterations in Morc3mut +/− osteoblast and osteocyte lineages could in part contribute to the observed bone phenotype in vivo.
It has been previously shown that loss of Morc3 function prevents activation of p53 mediated cell senescence pathways8. Consistent with this observation we found that senescence was significantly reduced in bone marrow monocytes passaged in vitro from the Morc3mut +/− mice. In vitro osteoclastogenesis assays showed that although Rankl-induced osteoclast formation was significantly reduced in cultures of BMMs of Morc3mut +/− mice, a significant increase in the survival rates of mature Morc3mut +/− osteoclasts after Rankl and M-CSF withdrawal was observed. Reduced senescence of bone marrow monocyte precursor populations might account for the enhanced survival that we observed in osteoclast cultures. Significantly, in vitro bone resorption assay revealed a significant reduction in the area resorbed by Morc3mut +/− osteoclasts as compared to the area resorbed by WT osteoclasts. The altered bone resorption activity of mature Morc3mut +/− osteoclasts could be accounted for by increased survival rates at lower levels of Rankl when compared to WT osteoclasts.
Increased mRNA expression of Stat1 and Ifnb1 during in vitro osteoblast differentiation and a significant reduction in protein expression of β-catenin in Morc3mut +/− pre-osteoblasts revealed a delay in early osteoblastogenesis in Morc3mut +/− mice as compared to WT. These findings are similar to previous studies which demonstrate that IFN-β inhibits osteoblast bone mineralization by affecting the early stages osteoblast differentiation27. The Rankl/Opg ratio was significantly reduced in the cortical bones of Morc3mut +/− mice as compared to WT long bones. Since the heterozygous mutation in Morc3 leads to activated IFN-β/STAT1 pathway, which is involved in Rankl-induced auto-inhibition of c-FOS during osteoclastogenesis, mature Morc3mut +/− osteoclasts appear to be more susceptible to these autoregulatory mechanisms at higher levels of Rankl. These results suggest that the mature Morc3mut +/− osteoclasts in the cortical bone compartment survive longer and therefore, potentially resorb more at lower levels of Rankl/Opg ratio. This might explain the differences between the trabecular and cortical bone mass in Morc3mut +/− mice.
Recent studies suggest that osteocytes, not osteoblasts, are the major source of Rankl that regulate osteoclast formation and function28. Osteocyte-derived IFN-β negatively regulates osteoclastogenesis29. The role of Morc3 in regulating IFN expression has not been directly shown, however it is interesting to note that increased IFN-β expression is found in dermatomyositis patients30, a subset of which display autoantibodies targeting Morc312. The evidence suggests a link between Morc3 and IFN signaling pathways which requires future investigation.
In summary, we have shown that Morc3mut +/− mice have reduced cortical bone mass which is a critical factor towards increased osteoporotic fracture risk in humans. Our data indicate that Morc3 mutation leads to altered nuclear localization of Morc3 protein, and upregulation of the IFN-β/STAT1 pathway, which plays a critical role in the maintenance of bone homeostasis and is a major therapeutic target for the treatment of osteolytic bone diseases31. Our findings establish Morc3 as a novel regulator of bone homeostasis and opens up new avenues for identifying potential treatments targeting bone metabolic disorders.
Materials and Methods
Generation of Mice- Morc3mut +/− mice used in the present study were generated by the Australian Phenomics Facility at the Australian National University in Canberra, Australia. This strain is available from the Australian Phenome Bank. The mutant mice were produced by ENU-induced mutagenesis as described previously32. The mutant C57BL/6 mice were outcrossed to a mapping strain (NOD) to produce F1 carrier mice. The wild type and Morc3mut +/− mutant mice used in this study are from the F10 to F16 progeny. Animal studies were carried out in accordance with protocols approved by the University of Western Australia animal ethics committee and the Australian National University animal ethics committee.
X-ray Microcomputed Tomography (Micro-CT)
The hindlimbs were dissected from the age- and sex-matched WT and mutant mice, fixed in 10% formalin for 24 hours at room temperature and stored in 70% ethanol. Then the hindlimbs were wrapped in tissue and placed in a 1.5 ml microcentrifuge tube and scanned in the Skyscan 1176 microCT machine (Skyscan). The distal femur or tibia was imaged using an X-ray tube voltage of 50 kV and at current of 500 μA with a 0.5 mm aluminium filter. The resolution was set to 6.03 μm and 931 tomographic sections were acquired for each CT scan. 3D images of the scans were reconstructed in NRecon program (Skyscan). Trabecular bone analysis was performed on the secondary spongiosa region (500 μm below the growth plate with a total height of 1 mm towards the mid shaft) of the distal femur. Cortical bone analysis was performed in the mid shaft (4 mm below the growth plate with a height of 1 mm). 3D analysis of trabecular and cortical bone was performed in CT Analyzer program (Skyscan). 3D images were generated in CTvol program (Skyscan).
Bone Histomorphometric Analysis
Trabecular bone and in vivo osteoclast parameters were generated from formalin-fixed, decalcified and paraffin-embedded femurs stained with Hematoxylin and Eosin (H&E) or Tartrate-resistant acid phosphatase (TRAcP). Histomorphometric analysis was performed using BioQuant Osteo software (BioQuant). Slides were scanned with the Scanscope XT machine (Aperio) at 20× objective. Trabecular bone region of interest was measured 500 μm below the growth plate and 1 mm in height at the distal femur. Cortical bone analysis was performed 4 mm below the growth plate with a height of 1 mm.
Osteoclast Cultures
Osteoclasts were generated from freshly isolated bone marrow cells as described previously33. Cells were fixed at the indicated times with 4% paraformaldehyde and stained for TRAcP. After the osteoclasts were generated, both Rankl and M-CSF were removed from the culture (time 0) and osteoclasts were cultured for 8 and 24 hours. At the end of indicated time points (0, 8 and 24 hours) the cells were fixed and stained with TRAcP. The survival rate of the cells was estimated as the percentage of morphologically intact TRAcP positive multinucleated cells compared with those at time 0. Bone resorption assay was performed as described previously33. The number of TRAcP positive osteoclasts was scored prior to assessment of resorptive activity. Resorption pits were visualized by scanning electron microscopy, and the area of bone resorbed was measured using ImageJ software. C-terminal collagen cross-links (CTX) in medium were determined using CrossLaps for Culture ELISA kit (Immunodiagnostic Systems) according to the manufacturer’s instruction.
Immunofluorescence
Osteoclasts cultured on cover slips were fixed with 4% paraformaldehyde and permeabilised using in 0.1% Triton X-100. The osteoclasts were then incubated with primary anti-mouse Morc3 antibody (MBL International. Japan). F-actin was stained with rhodamine-conjugated phalloidin (Molecular Probes, USA). The nuclei were stained with Hoechst 33258 (Molecular Probes, USA). The samples were then incubated with a FITC-conjugated secondary anti-mouse IgG antibody (Sigma-Aldrich, USA) and mounted onto glass slides with Prolong Gold antifade mounting medium (Invitrogen). Morc3 protein and F-actin stain were visualized and imaged using the Nikon Ti-E inverted motorized microscope with Nikon A1Si spectral detector confocal system (Nikon) running on the NIS-Elements C software (Nikon).
Senescence-associated β-galactosidase (SA-β-gal) activity
When BMMs reached confluence, the monolayers were trypsinised and divided into two groups. First group of cells were seeded at a density of 5 × 104 in 24 well tissue culture plates for cytochemical detection SA-β-gal activity, while the remaining cells were passaged further. The above two steps were repeated till the BMMs were unable to grow in the presence of M-CSF or 100% senescent. To visualize cell senescence in fixed BMMs they were incubated with a chromogenic staining solution containing β-gal substrate X-gal at 37 °C in the dark for 16–24 hrs34. The proportion of cells positive for SA-β-gal activity were scored by counting the number of blue cells in the total population.
Flow cytometry analysis of bone marrow stem cell niche
The BD Biosciences protocol was used to immunostain mouse bone marrow cells. In brief, bone marrow cells were extracted from mice hindlimbs. Red blood cells (RBCs) were lysed in ammonium chloride lysis buffer (0.15 M NH4Cl, 10 mM Tris-HCl, 0.1 mM EDTA) and the cells were resuspended in ice cold wash buffer (1% FBS, 0.1% NaN3 in PBS) at a concentration of 2 × 107/ml. Bone marrow cell suspension (106 cells) was incubated with the 50 μl of diluted (0.5 μg in 50 μl) conjugated antibodies: CD45R/B220-PE-Cy7, CD3e-PE-Cy7, CD11b-APC, cKit-BV421, Sca-1-FITC, for 45 minutes in the dark on the ice. The stained cells were washed and resuspended in 500 μl of BD Stabilizing Fixative and stored overnight at 4 °C in flow cytometer tubes. Compensation beads (BD Biosciences)/Single stains and unstained controls were used for compensation. For live/dead stain positive control, 50% of the cells were freeze-thawed in liquid nitrogen and mixed with the other 50% live cell population. LIVE/DEAD Fixable Aqua Dead Cell Stain kit (1 μl/ml of cell suspension) was added to the samples and incubated for 30 minutes in the dark on the ice. The cells were washed and fixed in BD Stabilizing Fixative solution before flow cytometric analysis. The BD FACSCanto II flow cytometer was used for analysis. Data was acquired and compensated in the BD FACSDiva software. FlowJo software was used to import and analyze the acquired data.
Osteoblast Cultures
For osteoblastogenesis assays, osteoblast precursors from adult calvaria or long bones were obtained as outgrowth from collagenase-treated bone pieces as described previously35. The cells were plated into culture dishes at a cell density of 1 × 106 cells/ml in complete Dulbecco’s Modified Eagle’s Medium (Dulbecco’s Modified Eagle’s Medium supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 g/ml streptomycin). When confluent, the osteogenic media (complete Dulbecco’s Modified Eagle’s Medium, 10 nM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/ml ascorbate) was added. After 21 days, the cells were fixed and stained with 1% alizarin red36. ImageJ software was used to measure the mineralized area37. Whole cell lysates were harvested in 0.1% Triton X-100 at different time points, as indicated, to assess the effect of mutation on the alkaline phosphatase activity during osteoblast differentiation in WT and mutant osteoblast cultures.
Immunoblotting
The cells designated for protein extraction from in vitro osteoclastogenesis and osteoblast differentiation assays were directly lysed in the tissue culture plates at different time points using RIPA Cell Lysis Buffer. Western blotting was performed as described previously33. Antibodies used were as follows: Anti-mouse Morc3 (MBL International. Japan); Anti-mouse NFATc1 (BD Biosciences, USA); Anti-mouse V-ATPase d2 subunit (Produced for the Centre for Orthopaedic Research, UWA38); Anti-mouse DC-STAMP (Merck Millipore, Germany); Anti-rabbit c-FOS (Cell Signaling Technology, USA); Anti-rabbit Phospho-STAT1 (Tyr 701) (Cell Signaling Technology, USA); Anti-rabbit STAT1 (Cell Signaling Technology, USA); Anti-rabbit Rankl (R&D systems, China); Anti-goat OPG (R&D systems, China); Anti-rabbit β-catenin (Cell Signaling Technology, USA) and Anti-mouse β-Actin (JLA-20) (Developmental Studies Hybridoma Bank. USA). Detection was done by respective peroxidase-conjugated antibodies (Sigma-Aldrich, USA) and chemiluminescence reagent (PerkinElmer Life Sciences).
PCR Analysis
Total RNA was extracted from the hindlimb (Bone marrow flushed) or cultured cells at the indicated times, from wild type and Morc3mut +/− mice using TRIzol (Invitrogen) and phenol/chloroform extraction. RNA was transcribed into cDNA using an oligo (dT) primer and Moloney murine leukemia virus reverse transcriptase (Promega). Real-time PCR was performed using SYBR Green PCR Master Mix (Qiagen). Primers used are listed in Table 1. Each sample was analyzed in triplicate and normalized Hypoxanthine guanine phosphoribosyl transferase (HPRT).
Table 1. Primer sequences used for PCR analysis.
| Primer | Forward sequence (5′ to 3′) | Reverse sequence (3′ to 5′) |
|---|---|---|
| HPRT | CAGTCCCAGCGTCGTGATTA | TGGCCTCCCATCTCCTTCAT |
| Morc3 | AGTTGGAGGCAAACAACATGGGT | TCGCCACTTTAGACAAGCGT |
| STAT1 | TTCCGACACCTGCAACTGAA | ACGACAGGAAGAGAGGTGGT |
| IFN-β | GTCCTCAACTGCTCTCCACT | CCTGCAACCACCACTCATTC |
| Rankl | CATCCCATCGGGTTCCCATAA | GCAAATGTTGGCGTACAGGT |
| OPG | ACAGTTTGCCTGGGACCAAA | TCACAGAGGTCAATGTCTTGGA |
| Alkaline phosphatase | AACCCAGACACAAGCATTCC | GCCTTTGAGGTTTTTGGTCA |
| Osteocalcin | GCGCTCTGTCTCTCTGACCT | ACCTTATTGCCCTCCTGCTT |
| Sclerostin | CAGACCATGAACCGGGCGGAG | CACTGGCCGGAGCACACCAAC |
Statistics
All data presented are expressed as the mean ± standard error of the mean (SEM). The results are representative of at least three independent experiments. Single comparison tests between wild type and Morc3mut +/− were done by using paired Student’s t-test in Microsoft Excel. For comparisons between multiple means, a one-way analysis of variance statistical analysis (Bonferroni post-hoc test) was used in SPSS. Statistical significance was determined at P values < 0.05.
Additional Information
How to cite this article: Jadhav, G. et al. Morc3 mutant mice exhibit reduced cortical area and thickness, accompanied by altered haematopoietic stem cells niche and bone cell differentiation. Sci. Rep. 6, 25964; doi: 10.1038/srep25964 (2016).
Supplementary Material
Acknowledgments
This study was supported in part by the Australian Health and Medical Research Council (NHMRC, Nos 1107828, 1027932, 1010420, 1010256), Arthritis Foundation of Australia (The H J & G J McKenzie grant), and University of Western Australia Research Collaboration Awards. This work was also supported by a Guangxi Nature and Science Foundation Research Grant (2015GXNSDA139019). The authors also acknowledge technical suggestions from Vincent Kuek, Mingli Yang, Lin Zhou, and equipment operation assistance from the Centre for Microscopy, Characterization and Analysis (CMCA) of The University of Western Australia, a facility funded by the University, State and Commonwealth Governments. Mr. Gaurav Jadhav was a recipient of UWA scholarship (2012–2015), and Dr. Jennifer Tickner was a recipient of a Raine fellowship from the Raine Medical Research Foundation of Western Australia (2014–2015). The authors thank Prof. Christopher Goodnow from Australian National University for providing the ENU mice for screening. This work was also supported in part by a UWA UQ Bilateral Research Collaboration Award. The authors thank Professor Emma Duncan and Dr. Paul Leo for their insightful suggestions for this study.
Footnotes
Author Contributions G.J. carried out most of the experiments and data analysis, and participated in drafting the manuscript. D.T., J.K. and J.T. provided technical assistance, and data analysis. Dr. J.T. and Prof. J.X. supervised the project and experimental designs, and revised the manuscript.
References
- Boyle W. J., Simonet W. S. & Lacey D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003). [DOI] [PubMed] [Google Scholar]
- Sambrook P. & Cooper C. Osteoporosis. Lancet 367, 2010–2018, doi: 10.1016/S0140-6736(06)68891-0 (2006). [DOI] [PubMed] [Google Scholar]
- Li D. Q., Nair S. S. & Kumar R. The MORC family: new epigenetic regulators of transcription and DNA damage response. Epigenetics 8, 685–693, doi: 10.4161/epi.24976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y. et al. The newly identified human nuclear protein NXP-2 possesses three distinct domains, the nuclear matrix-binding, RNA-binding, and coiled-coil domains. J. Biol. Chem. 277, 20611–20617, doi: 10.1074/jbc.M201440200 (2002). [DOI] [PubMed] [Google Scholar]
- Mimura Y., Takahashi K., Kawata K., Akazawa T. & Inoue N. Two-step colocalization of MORC3 with PML nuclear bodies. J. Cell Sci. 123, 2014–2024, doi: 10.1242/jcs.063586 (2010). [DOI] [PubMed] [Google Scholar]
- He F. et al. Structural insight into the zinc finger CW domain as a histone modification reader. Structure 18, 1127–1139, doi: 10.1016/j.str.2010.06.012 (2010). [DOI] [PubMed] [Google Scholar]
- Su A. I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067, doi: 10.1073/pnas.0400782101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. et al. Dynamic regulation of p53 subnuclear localization and senescence by Morc3. Mol. Biol. Cell 18, 1701–1709, doi: 10.1091/mbc.E06-08-0747 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendorff A. et al. NXP-2 association with SUMO-2 depends on lysines required for transcriptional repression. Proc. Natl. Acad. Sci. USA 103, 5308–5313, doi: 10.1073/pnas.0601066103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K. H. et al. Functional transcriptome analysis of the postnatal brain of the Ts1Cje mouse model for Down syndrome reveals global disruption of interferon-related molecular networks. BMC genomics 15, 624, doi: 10.1186/1471-2164-15-624 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicocca V. T. et al. Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia. Cancer Cell 22, 656–667, doi: 10.1016/j.ccr.2012.08.027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena H. et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 60, 1807–1814, doi: 10.1002/art.24547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urganus A. L., Zhao Y. D. & Pachman L. M. Juvenile dermatomyositis calcifications selectively displayed markers of bone formation. Arthritis Rheum. 61, 501–508, doi: 10.1002/art.24391 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceribelli A. et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res. Ther. 14, R97, doi: 10.1186/ar3822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y. et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann. Rheum. Dis. 71, 710–713, doi: 10.1136/annrheumdis-2011-200697 (2012). [DOI] [PubMed] [Google Scholar]
- Takayanagi H. et al. Rankl maintains bone homeostasis through c-FOS-dependent induction of interferon-beta. Nature 416, 744–749 (2002). [DOI] [PubMed] [Google Scholar]
- Hill T. P., Spater D., Taketo M. M., Birchmeier W. & Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. cell 8, 727–738, doi: 10.1016/j.devcel.2005.02.013 (2005). [DOI] [PubMed] [Google Scholar]
- Rhee Y. et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J. Bone Miner. Res. 26, 1035–1046, doi: 10.1002/jbmr.304 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A. et al. Overexpression of DMP1 accelerates mineralization and alters cortical bone biomechanical properties in vivo. J. Mech. Behav. Biomed. Mater. 5, 1–8, doi: 10.1016/j.jmbbm.2011.08.026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N. A. & Vrahnas C. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch. Biochem. Biophys. 561, 22–28, doi: 10.1016/j.abb.2014.05.015 (2014). [DOI] [PubMed] [Google Scholar]
- Kerschnitzki M. et al. Architecture of the osteocyte network correlates with bone material quality. J. Bone Miner Res. 28, 1837–1845, doi: 10.1002/jbmr.1927 (2013). [DOI] [PubMed] [Google Scholar]
- Busse B. et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9, 1065–1075, doi: 10.1111/j.1474-9726.2010.00633.x (2010). [DOI] [PubMed] [Google Scholar]
- Vashishth D., Verborgt O., Divine G., Schaffler M. B. & Fyhrie D. P. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone 26, 375–380, doi: 10.1016/S8756-3282(00)00236-2 (2000). [DOI] [PubMed] [Google Scholar]
- Shah A. D., Shoback D. & Lewiecki E. M. Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int. J. Womens Health 7, 565–580, doi: 10.2147/IJWH.S73244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarski K. H., Galus R., Brodzikowska A. & Wlodarski P. K. Sclerostin, an osteocytes-derived bone-forming inhibitor. Polish Orthop. Traumat. 78, 151–154 (2013). [PubMed] [Google Scholar]
- Lara-Castillo N. et al. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 76, 58–66, doi: 10.1016/j.bone.2015.03.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeckel V. J. et al. IFNbeta impairs extracellular matrix formation leading to inhibition of mineralization by effects in the early stage of human osteoblast differentiation. J. Cell Physiol. 227, 2668–2676, doi: 10.1002/jcp.23009 (2012). [DOI] [PubMed] [Google Scholar]
- Xiong J. et al. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the Rankl Required for Osteoclast Formation in Remodeling Bone. PloS one 10, e0138189, doi: 10.1371/journal.pone.0138189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida C. et al. Osteocytes produce interferon-beta as a negative regulator of osteoclastogenesis. J. Biol. Chem. 289, 11545–11555, doi: 10.1074/jbc.M113.523811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshanapalli A., Shah M., Veerula V. & Somani A. K. The role of type I interferons and other cytokines in dermatomyositis. Cytokine 73, 319–325, doi: 10.1016/j.cyto.2014.11.026 (2015). [DOI] [PubMed] [Google Scholar]
- Abraham A. K., Ramanathan M., Weinstock-Guttman B. & Mager D. E. Mechanisms of interferon-beta effects on bone homeostasis. Biochem. Pharmacol. 77, 1757–1762, doi: 10.1016/j.bcp.2009.01.007 (2009). [DOI] [PubMed] [Google Scholar]
- Nelms K. A. & Goodnow C. C. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity 15, 409–418 (2001). [DOI] [PubMed] [Google Scholar]
- Khor E. C. et al. Loss of protein kinase C-delta protects against LPS-induced osteolysis owing to an intrinsic defect in osteoclastic bone resorption. PloS one 8, e70815, doi: 10.1371/journal.pone.0070815 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F., Erusalimsky J. D., Campisi J. & Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806, doi: 10.1038/nprot.2009.191 (2009). [DOI] [PubMed] [Google Scholar]
- Bakker A. D. & Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. In Bone Research Protocols (eds Helfrich M. & Ralston S.) 816, 19–29, doi: 10.1007/978-1-61779-415-5_2 (Humana Press, 2012). [DOI] [PubMed] [Google Scholar]
- Chim S. M. et al. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. J. Biol. Chem. 286, 22035–22046, doi: 10.1074/jbc.M110.187633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H. et al. Myocyte enhancer factor 2 and microphthalmia-associated transcription factor cooperate with NFATc1 to transactivate the V-ATPase d2 promoter during Rankl-induced osteoclastogenesis. J. Biol. Chem. 284, 14667–14676, doi: 10.1074/jbc.M901670200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.