Summary
The goal of this study is to determine the feasibility of intravenous gemcitabine and an intraperitoneal platinum agent in the treatment of patients with ovarian cancer. We performed a retrospective chart review of patients with primary, persistent or recurrent ovarian cancer, who received intravenous gemcitabine and an intraperitoneal platinum agent. Patients received gemcitabine (750 mg/m2) intravenous on days 1 and 8 and cisplatin (100 or 60 mg/m2) intraperitoneal on day 1 every 21 - 28 days. An alternate regimen was composed of gemcitabine (750 mg/m2) intravenous and carboplatin (AUC 5) intraperitoneal on day 1 every 21 days. Dose reductions occurred at the discretion of the prescribing physician.
Intravenous gemcitabine and an intraperitoneal platinum agent were administered to 12 patients with advanced primary or recurrent ovarian cancer. Myelosuppression was the most common toxicity. Grade 3 or 4 thrombocytopenia, neutropenia and anemia occurred in 7, 8 and 2 patients respectively. Dose reductions were required in 7 of 12 patients. 10 of 12 patients received 6 cycles of the regimen. Treatment was discontinued prior to 6 cycles in 2 of 12 patients secondary to progression in one case and to grade 4 neutropenia and thrombocytopenia in another.
The combination of intravenous gemcitabine and an intraperitoneal platinum agent appears to be a feasible regimen in patients with ovarian cancer. The most common toxicity was myelosuppression, which resulted in dose reductions in almost half of the patients.
Keywords: Ovarian cancer, intraperitoneal administration, chemotherapy, toxicity
Introduction
In the United States, ovarian cancer ranks fifth as a cause of cancer-related deaths among females.1 Primary treatment typically includes cytoreductive surgery and chemotherapy. Therapy for recurrences utilizes chemotherapy and in specific cases may include surgery. Regardless of setting, chemotherapy for ovarian cancer has traditionally been given intravenously (IV).
The role for intraperitoneal (IP) chemotherapy in the treatment of ovarian cancer continues to be investigated.2-4 In the majority of ovarian cancer cases, disease is limited to the peritoneal cavity. IP administration of chemotherapy has been advocated for ovarian cancer secondary to the exposure of the peritoneal cavity to sustained high concentrations of antitumor agents. GOG 172 compared IV cisplatin and IV paclitaxel versus IP cisplatin and IV and IP paclitaxel for optimally cytoreduced (<1 cm residual) stage III primary ovarian cancer patients. The investigation demonstrated a statistically significant improvement in overall survival in the IP arm (p=0.03).2 Based on this data, many centers have adopted IP platinum and IP taxane based regimens as preferred treatment for women with optimally cytoreduced primary ovarian cancer.
Unfortunately, a majority of patients with ovarian cancer will recur and will require further treatment with cytotoxic chemotherapy.5 Reported response rates for second line single agent IV therapies for ovarian cancer are low (15 -35%).6-12 The combination of IV cisplatin and gemcitabine may work synergistically and appears to have activity even in platinum resistant disease.13
IP therapy including cisplatin appears to be safe and effective in the treatment of primary ovarian cancer.2 The use of IP gemcitabine has been investigated in women with recurrent ovarian cancer.14,15 When given as a single agent, limited activity was reported with best responses being stable disease.15 Improved activity is noted when given in combination with IP cisplatin. However, toxicity including peritoneal fibrosis limits the feasibility of this combination.14 The goal of this investigation is to determine the feasibility of IV gemcitabine and an IP platinum agent in the treatment of ovarian cancer.
Patients and Methods
Patients
The Institutional Review Board of Johns Hopkins Medical Institutions approved this retrospective chart review. Written informed consent was not deemed necessary for this minimal risk investigation. Chemotherapy records of the Kelly Gynecologic Oncology Service at Johns Hopkins Medical Institutions were reviewed to identify ovarian cancer patients treated between 2003 and 2007 with IV gemcitabine and an IP platinum agent. Medical records were retrospectively reviewed and relevant clinical and pathology data extracted. Eligible patients underwent primary and/or secondary cytoreductive surgery for ovarian cancer by the Kelly Gynecologic Oncology service at Johns Hopkins Medical Institutions. Patients required optimal debulking with residual disease <1 cm at the time of their most recent surgery. All patients were > 18 years of age and had an expected survival of >6 months. none of the patients had received radiation therapy for treatment of their ovarian cancer. Prior chemotherapy was allowed, but was given prior to a patient's most recent surgery. IP ports (BardPort titanium implanted ports with peritoneal catheters) were placed at the discretion of the treating physician either at the time of cytoreductive surgery or as a second procedure. (Figure 1) Port function is assessed by flushing the IP port with 20 ml of normal saline. The infusate should flush easily. This is first performed at the completion of port placement and then prior to each administration of chemotherapy. The treating physician is contacted if there is any difficultly with IP port function.
Figure 1.
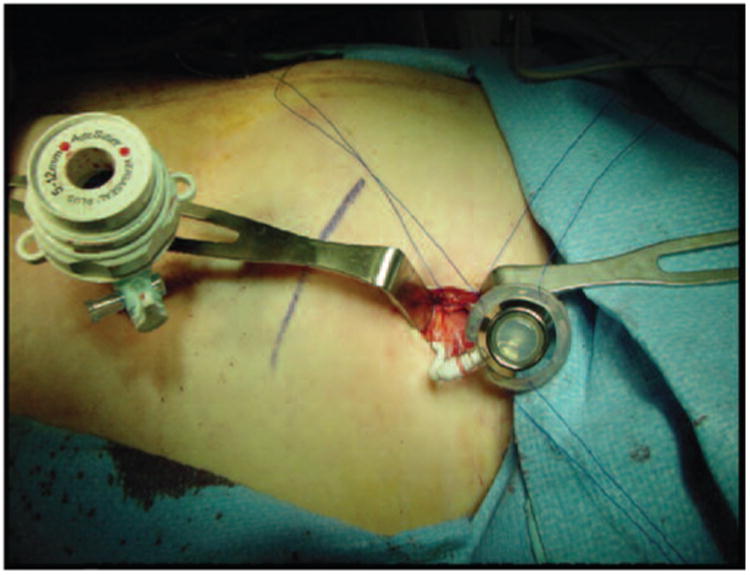
Laparoscopic placement of IP port. IP ports (BardPort titanium implanted ports with peritoneal catheters) were placed at the discretion of the treating physician either at the time of cytoreductive surgery or as a second procedure.
Performance status and toxicity
Patients were assessed prior to chemotherapy. History and physical exam were obtained. Patient had performance status scores of 0 or 1 based on Gynecologic Oncology Group (GOG) criteria. Eligible patients had appropriate hematologic function including a white blood cell count 3,000 cells/mm3 and platelet count 100,000 cells/mm3, renal function with creatinine 1.5 mg/dl and hepatic function including bilirubin 1.5 mg/dl and aspartate aminotransferase and alkaline phosphatase <2 times the upper limit of normal. Patients were evaluated for therapy related toxicities during routine pre chemotherapy visits. Toxicities were reported based on NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE). IP ports were evaluated to confirm continued function.
Chemotherapeutic regimen
Patients received gemcitabine IV (750 mg/m2) on days 1 and 8. Patients received cisplatin IP (100 or 60 mg/m2) via the IP catheter on day 1. The dose of cisplatin was determined at the discretion of the treating physician who had knowledge of toxicity if any with any prior chemotherapy regimens. Chemotherapy was administered on a 21 or 28 days cycle at the discretion of the treating physician. A single patient received an alternative regimen on a 21-day cycle with gemcitabine IV (750 mg/m2) and carboplatin IP (AUC 5) on day 1. The patient did not receive day 8 gemcitabine and the regimen was given on a 21-day cycle.
IP ports were accessed with a Huber needle. IP chemotherapy was infused to gravity. Patients were given 500 cc of normal saline IP prior to chemotherapy. The IP chemotherapy was then infused in 1000 cc of normal saline. Finally, an additional 500 cc of normal saline were administered IP. IV hydration and antiemetics were given per institutional protocol. Appropriate laboratory tests, including complete blood count, metabolic panel and CA 125, were obtained per routine. An absolute neutrophil count 1,300 cells/mm3 and a platelet count 100,000 cells/mm3 was required prior to initiation of day 1 of chemotherapy. On day 8 of each cycle, patient required an absolute neutrophil count 750 cells/mm3 and platelet count 75,000 cells/mm3.
Dose reduction
Dose reductions were performed for significant toxicity (typically grade 3 or 4 hematologic) at the discretion of the treating physician. Dose reductions typically consisted of a 20% reduction in either the gemcitabine or cisplatin dose. Additionally, day 8 gemcitabine was omitted in some patients. Pegfilgrastim 6mg subcutaneous was administered on day 2 or day 9 for neutropenia at the discretion of the treating physician.
Statistical analysis
Standard statistical methods were employed. Patient demographics were tabulated. The time of diagnosis was considered to be the date of the primary surgical procedure or tissue biopsy. Time from diagnosis to death or last follow-up was calculated. Patient data were otherwise censored at the time of latest contact. Overall survival curves and disease free interval curves were generated using the methods of Kaplan and Meier.
Results
Patient demographics
IV gemcitabine and an IP platinum agent were administered to 12 patients with a history of advanced ovarian cancer. Median age at diagnosis was 57.8 years (range 40 - 77). FiGO stage at diagnosis was stage III for 11 of 12 patients and stage IV for 1 of 12 patients. High grade adenocarcinoma was diagnosed in 11 of 12 patients. A majority of patients (10/12) had serous histology. Performance status was 0 for 11 of 12 patients.
Patients received treatment at the discretion of the treating physician and had a variety of clinical presentations. 9 patients received the IP regimen for primary or persistent disease and 3 patients received treatment for recurrent disease. The specific clinical scenarios are as follows: 1 patient received gemcitabine IV and cisplatin IP as primary treatment after optimal cytoreductive surgery for a low grade serous ovarian carcinoma. 5 patients underwent suboptimal primary debulking surgery followed by IV carboplatin and paclitaxel (3 or 6 cycles). These 5 patients then underwent a second operative procedure (interval optimal cytoreduction of macroscopic disease) followed by gemcitabine IV and cisplatin IP. 2 patients underwent optimal cytoreductive surgery followed by 6 cycles of iV carboplatin and paclitaxel. Despite negative CA 125 and radiographic imaging, microscopic disease was discovered during a second look procedure and both patients were then treated with gemcitabine IV and cisplatin IP. 1 patient was diagnosed by needle biopsy and received 6 cycles of IV platinum based neoadjuvant chemotherapy (4 cycles of carboplatin and paclitaxel and 2 cycles of carboplatin). She then underwent optimal cytoreduction followed by gemcitabine IV and cisplatin IP. The 3 final patients were found to have recurrence greater than 6 months from completing primary therapy (primary cytoreduction followed by 6 – 8 cycles of IV carboplatin and paclitaxel). These patients underwent optimal secondary cytoreductive surgery and were then treated with gemcitabine IV and platinum IP. All 12 patients were optimally cytoreduced with residual disease < 1cm prior to initiation of IP therapy. in 8 of 12 cases, patients were optimally cytoreduced with no residual disease.
IP ports were placed at the time of cytoreductive surgery in 8 of 12 patients. A second procedure was required for placement in 4 of 12 patients. One port failure occurred during the administration of chemotherapy. A return to the operating room was required to re-adjust the port and it functioned without difficulty for the remaining cycles of the regimen. (Table 1)
Table 1. Patient demographics; IP platinum, IV gemcitabine for ovarian cancer.
| Characteristic | |
|---|---|
| Number of Patients | 12 |
| Age at diagnosis (range), years | 57.8 (40-77 |
| No. (%) | |
|
| |
| Stage | |
| III | 11 (92) |
| IV | 1 (8) |
| Histology | |
| Serous | 10 (83) |
| Clear Cell | 1 (8) |
| Mixed Endometrioid/Transitional Cell | 1 (8) |
| Grade | |
| 1 | 1 (8) |
| 3 | 11 (92) |
| Performance Status | |
| 0 | 11 (92) |
| 1 | 1 (8) |
| IP therapy administered for | |
| Primary or persistent disease | 9 (75) |
| Recurrent disease | 3 (25) |
| Placement of IP Port | |
| At the time of cytoreductive surgery | 8 (67) |
| At the time of a second procedure | 4 (33) |
| Residual Disease prior to initiation IP therapy | |
| Macroscopic <1 cm | 4 (33) |
| Microscopic | 8 (67) |
Chemotherapeutic regimens
IV gemcitabine and IP cisplatin regimen was administered to 12 patients with a history of advanced ovarian cancer. The majority of patients (9/12) were prescribed treatment on a 28-day cycle. The remaining 3 patients were prescribed chemotherapy on a 21 day cycle. The majority of patients (11/12) received IP cisplatin. Of these patients, 4 received cisplatin at an initial dose of 100 mg/m2 and 7 received IP cisplatin at an initial dose of 60 mg/m2. At the discretion of the treating physician, 1 patient received IP carboplatin at an initial AUC of 5. All IP platinum was administered on day 1 of a 21 or 28 day cycle. All 12 patients received IV gemcitabine at an initial dose of 750 mg/m2. Gemcitabine was administered on day 1 and 8 for all 9 patients who received the regimen on a 28 day cycle and for 2 of 3 patients who received the regimen on a 21-day cycle. Gemcitabine was administered on day 1 only for 1 of 3 patients who received the regimen on a 21-day cycle. The majority of patients (10/12) received 6 cycles of chemotherapy. (Table 2)
Table 2. Chemotherapy regimens.
| # of Patient | Gemcitabine Dose | Day | Platinum Dose | Day | Cycle Length |
|---|---|---|---|---|---|
| 4 | 750 mg/m2 | 1+8 | Cisplatin 100 mg/m2 | 1 | 28 |
| 5 | 750 mg/m2 | 1+8 | Cisplatin 60 mg/m2 | 1 | 28 |
| 1 | 750 mg/m2 | 1 | Carboplatin AUC 5 | 1 | 21 |
| 2 | 750 mg/m2 | 1+8 | Cisplatin 60 mg/m2 | 1 | 21 |
Toxicities
All patients underwent optimal cytoreductive surgery (primary, interval or secondary) prior to initiation chemotherapy. IV gemcitabine and IP platinum was administered 12 patients. Patients were evaluated for toxicity based on NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE). The majority of patients (10/12) experienced myelosuppression. Gastrointestinal symptoms were noted in 10 of 12 patients. Grade 3 and 4 toxicities were noted in 10 of 12 patients. (Table 3). Pegfilgrastim was administered to 9 of 12 patients. Aprepitant was administered to 6 of 12 patients. Erythropoietic agents were utilized in 5 of 12 patients. significant thrombocytopenia (grade 4) occurred in 3 of 12 patients and was treated with oprelvekin or amifostine. Oprelvekin was initiated 24 hours after chemotherapy and administered every day until the thrombocytopenia resolved. Amifostine was given immediately prior to the administration of chemotherapy. Platelet transfusions were typically administered for counts <20,000 cells/mm3.
Table 3. Toxicities.
| Event | Any | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Number (%) | ||||
| Any | 11 (92) | 1 (8) | 5 (42) | 5 (42) |
| Anemia | 10 (83) | 8 (67) | 2 (17) | 0 (0) |
| Neutropenia | 9 (75) | 1 (8) | 6 (50) | 2 (17) |
| Thrombocytopenia | 9 (75) | 2 (17) | 4 (33) | 3 (25) |
| Fever | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Nausea & Vomiting | 3 (25) | 3 (25) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neuropathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Dose reductions
Dose reductions were prescribed in 7 of 12 patients. Of the 4 patients receiving gemcitabine 750 mg/m2 IV on day 1 and 8 and cisplatin 100 mg/m2 IP on day1 on a 28-day cycle, 2 patients required no dose reductions. A dose reduction to gemcitabine 600 mg/m2 and cisplatin 80 mg/m2 was made in 1 patient. Chemotherapy was discontinued in 1 patient secondary to progression. Of the 5 patients, who received gemcitabine 750 mg/m2 IV on day 1 and 8 and cisplatin 60 mg/m2 IP on day 1 on a 28-day cycle, 1 patient required no dose reductions. Gemcitabine was reduced to 600 mg/m2 in 1 patient. A dose reduction to gemcitabine 600 mg/m2 and cisplatin 48 mg/m2 was made in 1 patient. Day 8 gemcitabine was dropped in 2 patients. in 1 patient, the cycle was shortened to 21 days. in 1 patient, pegfilgrastim was added during cycle 2 and day 8 gemcitabine was reinstituted without subsequent need for dose alteration. The 1 patient, who received gemcitabine 750 mg/m2 IV on day 1 and carboplatin AUC 5 iP on day 1 on a 21-day cycle, required no dose reductions. Of the 2 patients receiving gemcitabine 750 mg/m2 IV on day 1 and 8 and cisplatin 60 mg/m2 IP on day 1 on a 21-day cycle, 1 patient required a dose reduction to gemcitabine 600 mg/m2 and cisplatin 48 mg/m2. Day 8 gemcitabine was dropped in the other patient and after 5 cycles chemotherapy was stopped secondary to pancytopenia. Dose reductions occurred in 5 of 9 patients on a 28-day cycle and in 2 of 3 patients on a 21-day cycle. As mentioned, chemotherapy was discontinued after 4 cycles in 1 patient secondary to disease progression and after 5 cycles in another patient secondary to pancytopenia. (Table 4)
Table 4. Chemotherapy regimens dose reductions.
| # of Pts | Gemcitabine Dose | Platinum Dose | Cycle (days) | Dose Reduction |
|---|---|---|---|---|
| 4 | 750 mg/m2 d 1+8 | Cisplatin 100 mg/m2 d 1 | 28 | Gem/CDDP reduced 600/80 mg/m2 (1 pt) Regimen discontinued 2° to progression (1 pt) |
| 5 | 750 mg/m2 d 1+8 | Cisplatin 60 mg/m2 d 1 | 28 | Gem reduced 600 mg/m2 (1 pt) Gem/CDDP reduced 600/48 mg/m2 (1 pt) D8 Gem dropped; cycle shortened 21 d (1 pt) D8 Gem held first cycle only (1 pt) |
| 1 | 750 mg/m2 d 1 | Carboplatin AUC 5 d 1 | 21 | None |
| 2 | 750 mg/m2 d1+8 | Cisplatin 60 mg/m2 d 1 | 21 | Gem reduced 600 then 480 mg/m2 (1 pt) D8 Gem dropped (1 pt) |
Patient outcomes
The majority of patients (10/12) received all 6 prescribed doses of IP platinum and IV gemcitabine. Treatment was discontinued after 4 cycles in 1 patient secondary to radiographic evidence of progression of disease. Treatment was discontinued in another patient secondary to grade 4 neutropenia and thrombocytopenia. Evaluation after completion of 6 cycles of therapy demonstrated progressive disease in 2 patients. Of the remaining 8 patients, 7 patients demonstrated a complete chemical response with normalization of CA 125 levels and negative radiogaphic imaging. A single patient had normalization of her CA 125, but residual disease was noted on PET/CT scan. Patients were followed for a median of 2.0 years from the initiation of IP platinum and IV gemcitabine. Median overall survival was 2.74 years. Median survival from initiation of IP platinum and IV gemcitabine was 2.06 years. 3 of 12 patients are alive without evidence of disease. 2 patients are alive with disease. The majority of patients (7/12) have died of disease. Kaplan Meier curve demonstrating overall survival from the time of initial diagnosis for the group is shown. (Figure 2)
Figure 2.

Kaplan-Meier analysis of overall survival for patients with ovarian cancer treated with IV gemcitabine and IP platinum. The x-axis indicates overall survival in years after initial diagnosis. The y-axis indicates the proportion of patients surviving with uncensored data.
Discussion
Despite aggressive surgical management followed by platinum and taxane based chemotherapy, many women with ovarian cancer will recur. Options for recurrent ovarian cancer have limited effectiveness. novel therapies are needed. As ovarian cancer is often confined to the peritoneal cavity, IP chemotherapy has been suggested as an innovative approach.
IP administration of chemotherapy results in a higher IP concentration of chemotherapeutic agent than can be achieved with standard IV doses. Miyagi and colleagues16 sampled serum and intraperitoneal fluid from patients receiving either IP (n=11) or IV (n=11) carboplatin. Regardless of route of administration, the carboplatin was dosed at an AUC of 6, calculated by the Calvert formula. The measured area under the curve (AUC) for free platinum in the serum was not statistically different for the iP and the iV group, with a mean AUC (mg × min/ml) of 2.86 and 2.40, respectively. However, the measured AUC for free platinum in the intraperitoneal space was 17 times higher for the IP group as compared to the IV group, mean AUC (mg × min/ml) of 53.31 and 3.10, respectively.
The utilization of IP chemotherapy in the treatment of primary ovarian cancer has been associated with promising results. GOG 172, a randomized prospective phase 3 trial, demonstrated improved survival for optimally cytoreduced stage III ovarian cancer patients, enrolled in the IP arm as compared to those patients who received treatment in the IV only arm.2 Further investigation both of additional IP regimens other than platinum and taxane and of IP administration for recurrent ovarian cancer is warranted.
Gemcitabine has shown significant activity in the treatment of ovarian cancer7 and its use in IP regimens for the treatment of ovarian cancer is reasonable. However, results from prior investigations of gemcitabine containing IP regimens were not promising. single agent IP gemcitabine provided limited activity.15 Although activity was improved with IP cisplatin and IP gemcitabine, the utilization of this combination was limited by toxicity.14
Our data show the combination of IV gemcitabine and an IP platinum agent to be a feasible regimen for the treatment of ovarian cancer. The most common toxicity was myelosuppression, occurring in 10 of 12 patients. Dose reductions were required in over half of patients (7/12) with the majority of patients (9/12) requiring pegfilgrastim. Overall, IP platinum and IV gemcitabine was well tolerated without long term sequela.
Our investigation is limited by small numbers, a heterogenous patient population and its retrospective nature. Given these limitations, the activity of IP platinum and IV gemcitabine is difficult to determine. At the completion of treatment 7 of 12 patients had no evidence of disease with normalization of their CA 125 and no radiographic evidence of malignancy. Currently, 3 patients are without evidence of disease.
IP chemotherapy appears to provide a survival advantage, when given as part of primary treatment for ovarian cancer in select patients. Our data suggest that iP platinum and IV gemcitabine is a feasible regimen for the treatment of ovarian cancer. Our retrospective study did not contain sufficient numbers to determine the activity of this regimen or a population of ovarian cancer patients most likely to benefit. This regimen may be most appropriate in patients with recurrent ovarian cancer who undergo optimal secondary cytoreductive surgery and did not receive IP chemotherapy as part of primary treatment.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 3.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 5.Eisenkop SM, Friedman RL, Spirtos NM. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer. 2000;88(1):144–53. doi: 10.1002/(sici)1097-0142(20000101)88:1<144::aid-cncr20>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Gershenson DM, Kavanagh JJ, Copeland LJ, Stringer CA, Morris M, Wharton JT. Re-treatment of patients with recurrent epithelial ovarian cancer with cisplatin-based chemotherapy. Obstet Gynecol. 1989;73(5 Pt 1):798–802. [PubMed] [Google Scholar]
- 7.Shapiro JD, Millward MJ, Rischin D, Michael M, Walcher V, Francis PA, et al. Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecol Oncol. 1996;63(1):89–93. doi: 10.1006/gyno.1996.0284. [DOI] [PubMed] [Google Scholar]
- 8.Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to national Cancer institute Treatment Referral Center 9103. J Clin Oncol. 1993;11(12):2405–10. doi: 10.1200/JCO.1993.11.12.2405. [DOI] [PubMed] [Google Scholar]
- 9.Piccart MJ, Gore M, Ten Bokkel Huinink W, Van Oosterom A, Verweij J, Wanders J, et al. Docetaxel: an active new drug for treatment of advanced epithelial ovarian cancer. J Natl Cancer Inst. 1995;87(9):676–81. doi: 10.1093/jnci/87.9.676. [DOI] [PubMed] [Google Scholar]
- 10.Bookman MA, Malmstrom H, Bolis G, Gordon A, Lissoni A, Krebs JB, et al. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase ii study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998;16(10):3345–52. doi: 10.1200/JCO.1998.16.10.3345. [DOI] [PubMed] [Google Scholar]
- 11.Hoskins PJ, Swenerton KD. Oral etoposide is active against platinum-resistant epithelial ovarian cancer. J Clin Oncol. 1994;12(1):60–3. doi: 10.1200/JCO.1994.12.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998;16(2):405–10. doi: 10.1200/JCO.1998.16.2.405. [DOI] [PubMed] [Google Scholar]
- 13.Rose PG, Mossbruger K, Fusco N, Smrekar M, Eaton S, Rodriguez M. Gemcitabine reverses cisplatin resistance: demonstration of activity in platinum- and multidrug-resistant ovarian and peritoneal carcinoma. Gynecol Oncol. 2003;88(1):17–21. doi: 10.1006/gyno.2002.6850. [DOI] [PubMed] [Google Scholar]
- 14.Sabbatini P, Aghajanian C, Leitao M, Venkatraman E, Anderson S, Dupont J, et al. Intraperitoneal cisplatin with intraperitoneal gemcitabine in patients with epithelial ovarian cancer: results of a phase I/II Trial. Clin Cancer Res. 2004;10(9):2962–7. doi: 10.1158/1078-0432.ccr-03-0486. [DOI] [PubMed] [Google Scholar]
- 15.Morgan RJ, Jr, Synold TW, Xi B, Lim D, Shibata S, Margolin K, et al. Phase I trial of intraperitoneal gemcitabine in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Clin Cancer Res. 2007;13(4):1232–7. doi: 10.1158/1078-0432.CCR-06-1735. [DOI] [PubMed] [Google Scholar]
- 16.Miyagi Y, Fujiwara K, Kigawa J, Itamochi H, Nagao S, Aotani E, et al. intraperitoneal carboplatin infusion may be a pharmacologically more reasonable route than intravenous administration as a systemic chemotherapy. A comparative pharmacokinetic analysis of platinum using a new mathematical model after intraperitoneal vs. intravenous infusion of carboplatin—a Sankai Gynecology Study Group (SGSG) study. Gynecol Oncol. 2005;99(3):591–6. doi: 10.1016/j.ygyno.2005.06.055. [DOI] [PubMed] [Google Scholar]


