Abstract
There is a growing recognition in maternal and child health of the importance of social, behavioral, biological, and genetic factors across the entire life course. Unfortunately, most state maternal and child health surveillance systems are not designed to readily address longitudinal research questions or track and follow children across multiple programs over time. The Virginia Department of Health (VDH) recently integrated its birth defects registry, newborn hearing screening tracking and management system, and electronic birth certificate (EBC) into a robust, Web-based surveillance system called the Virginia Vital Events and Screening Tracking System (VVESTS). Completely redesigning the existing birth defects and newborn hearing screening system (the Virginia Infant Screening and Infant Tracking System—VISITS I) with minimal disruption of ongoing reporting presented a number of challenges. Because VVESTS had different requirements such as required fields and data validations, extensive data preparation was required to ensure that existing VISITS I data would be included in the new system (VISITS II). Efforts included record deduplication, conversion of free text fields into discrete variables, dealing with missing/invalid data, and linkage with birth certificate data. VISITS II serves multiple program needs; improves data quality and security; automates linkages within families, across programs, and over time; and improves the ability of VDH to provide children with birth defects and their families necessary follow-up services and enhanced care coordination.
Keywords: birth defects, congenital anomalies, hearing screening, hearing loss, surveillance, birth certificate, data linkages
Introduction
Accurate, complete, and timely data are the foundation of public health surveillance. The ability of state birth defects surveillance programs to meet various goals and objectives is driven by the manner in which the data are collected and used to improve public health.1 The Virginia Department of Health (VDH) recently integrated its birth defects registry, newborn hearing screening tracking and management system, and electronic birth certificate (EBC) into a robust, Web-based surveillance system called the Virginia Vital Events and Screening Tracking System (VVESTS). A major component of the integrated system is the Virginia Infant Screening and Infant Tracking System (VISITS), which includes the birth defects registry and newborn hearing screening databases. VVESTS serves multiple program needs, improves data quality and security, and lessens the burden of hospital reporting. This manuscript describes the methods, challenges, and opportunities associated with the VISITS component of this innovative solution to public health surveillance.
Program Descriptions
The Virginia birth defects registry, called the Virginia Congenital Anomalies Reporting and Education System (VaCARES), was first mandated by the Code of Virginia in 1985 and later amended in 1986 and 2006 (§ 32.1–69.1 and § 32.1–69.2). VaCARES is a passive compliant surveillance system2 that covers the entire Commonwealth of Virginia. Every hospital, as defined by the Code of Virginia, reports to VaCARES any child under the age of 2 years diagnosed as having one or more of 86 categories of structural, functional, or biochemical abnormalities based on ICD-9-CM coding.3 Hearing loss is also reported to VaCARES, along with inborn errors of body chemistry diagnosed through state-mandated hearing and newborn dried bloodspot screening programs. In addition to serving as a statewide birth defects registry, VaCARES provides condition-specific information to the parents and physicians of children with birth defects.
The Virginia newborn hearing screening program, called the Virginia Early Hearing Detection and Intervention Program (VEHDIP), was established in 1999 in the Code of Virginia (§ 32.1–64.1 and § 32.1–64.2) and operates under Virginia Administrative Code (12 VAC 5-80). The goal of VEHDIP is to reduce the burden of communication disorders resulting from hearing loss. In Virginia, hospitals with infant nurseries or neonatal intensive care services are required to screen the hearing of all newborns prior to discharge. If an infant does not pass the initial screening, the hospital must refer the infant for diagnostic evaluation. Hospitals are mandated to provide results of initial hearing screening tests to VEHDIP for infants who failed their initial screen, infants who were not screened, and infants who passed the initial screen but were identified to be at risk for progressive hearing loss. In 2010, VCH started receiving the hearing results of all infants from hospitals. All persons who provide audiological services must also report the status and/or results of diagnostic evaluations to VEHDIP for infants and children up to 2 years of age.4
Virginia Infant Screening and Infant Tracking System (VISITS)
The primary purpose of VISITS is to create a single record for infants and children reported to VEHDIP and VaCARES so that VDH can provide these children and their families with necessary follow-up services and enhanced care coordination. In addition, child health workers and policy makers can use VISITS to extract aggregate, non-identifiable data for conducting needs assessments, planning services for children with special health care needs, targeting prevention efforts, providing surveillance and evaluation, responding to constituent questions, and satisfying state and federal reporting requirements.
The original VISITS application, VISITS I, was released statewide in 2002. This application, developed by VDH through a contractual agreement with Welligent, LLC (formerly Health Informatics of Eastern Virginia Medical School and the Children's Hospital of The King's Daughters), enabled secure online reporting of VaCARES and VEHDIP data to VDH. In 2005, VDH was awarded a 3-year cooperative agreement from the Centers for Disease Control and Prevention (CDC) to redesign the original VISITS I application in order to accomplish the following: 1) minimize the number of VEHDIP infants lost to follow up, 2) expand referrals of identified children with special health care needs to the appropriate source for intervention and/or care coordination, 3) improve the mechanism for identifying infants and children with late onset or progressive hearing loss, 4) link VISITS to birth and death certificate data, 5) respond more efficiently to requests for hearing screening and birth defects registry data, 6) expand integration and linkages with other surveillance systems, 7) ensure high-quality data, and 8) improve efficiency and security.
The current enhanced system, referred to as VISITS II, was released statewide in April 2010. VISITS II is now one reporting module of a larger more comprehensive reporting system, VVESTS. By redesigning VISITS I and integrating the system into VVESTS, which also includes the new EBC, VDH expects to be more successful in providing coordinated and timely follow-up services along with enhanced care coordination where needed for children and families in Virginia.
Methods
The VDH Office of Information Management (OIM) developed VISITS II through an intra-agency contractual agreement primarily using funds provided under a 3-year CDC cooperative agreement. VISITS I had already been brought “in house” and was hosted by OIM, which was also responsible for the development of the new EBC. Early into the development process, VDH decided to integrate VISITS II with the new EBC being developed by OIM. Because the EBC development was not on the same timetable as VISITS II, integration with EBC would necessarily delay the startup of VISITS II. However, it was determined that the benefits of an integrated surveillance system—improved functionality, data quality, and reporting capabilities—justified the delay.
Virginia Infant Screening and Infant Tracking System (VISITS) I Redesign Process
A VISITS redesign network was established that included the following entities: 1) Project Steering Committee, which met monthly to monitor project progress to ensure its success; 2) Project Development Team, which developed and implemented the VISITS II application; and 3) Project User Groups, which reviewed prototypes, participated in VISITS record deduplication and conversion processes, conducted user tests, and participated in user trainings. Project development followed the complete software-development life cycle (SLDC) methodology. This included initial requirement gathering, analysis, design, development, testing, user training, data cleaning and conversion, and implementation and production support. A timeline highlighting key VISITS redesign activities is presented in Table 1.
Table 1. Project timeline for key Virginia Infant Screening and Infant Tracking System (VISITS) redesign activities.
| Year 1 (July 1, 2005–June 30, 2006) |
| Completed memorandum of agreement with Office of Information Management |
| Established project steering committee and development team |
| Drafted VISITS II Requirements Document,5 which defined the scope of work and deliverables, system requirements, and deliverables |
| Completed evaluation of Virginia Congenital Anomalies Reporting and Education System6 based on Centers for Disease Control and Prevention (CDC) guidelines7 and incorporated recommendations in the requirements document5 |
| Year 2 (July 1, 2006–June 30, 2007) |
| Completed the VISITS II Requirements Document5 |
| Completed initial prototypes of VISITS II and initiated testing by in-house user groups |
| Established and convened the VISITS I deduplication team, who were tasked with removing duplicate records and merging data for the same individuals with multiple IDs |
| Year 3 (July 1, 2007–June 30, 2008) |
| Partially completed deduplication of VISITS I records |
| Completed the major programming phase of VISITS II, including integration with the new electronic birth certificate record |
| Completed plans for conversion of VISITS I records into the VISITS II database |
| Initiated VISITS II beta testing with in-house user groups and corrected identified problems |
| Completed plans for allowing automatic referral from VISITS II to Care Connection for Children, a statewide network of centers of excellence for children with special health care needs |
| Completed evaluation of the Virginia Early Hearing Detection and Intervention Progam8 based on CDC guidelines7 and incorporated recommendations in the requirements document5 |
| Year 4 (July 1, 2008–June 30, 2009) |
| Continued multiple rounds of beta testing with in-house user groups and corrected identified problems |
| Completed programming for the automated Care Connection for Children referrals |
| Conducted security testing of VISITS II user roles and privileges |
| Initiated statewide implementation of the new electronic birth certificate record |
| Initiated the process of VISITS I records deduplication for conversion into the VISITS II database according to data deduplication rules |
| Year 5 (July 1, 2009–June 30, 2010) |
| Completed matching of VISITS I records to birth certificate records |
| Completed the last rounds of VISITS II beta testing with in-house user groups and completed corrections to identified problems |
| Completed deduplication process and conversion testing |
| Completed a survey of computers used by hospital users |
| Implemented statewide training on VISITS II for hospital users9 |
| Released VISITS II statewide on April 12, 2010 |
Challenges and Solutions
Any modifications to live applications can present challenges to users, developers, and program staff. Carrying out a complete redesign of VISITS while ensuring minimal disruption in reporting was especially difficult. Converting data from the previous system and integration of VISITS with the new EBC required significant problem solving to overcome a series of major challenges.
Data duplication
Prior to 2006, Virginia Code did not have a provision that allowed VaCARES hospital users to view data previously entered into VISITS I at another hospital facility. Since VaCARES receives reports of children with birth defects up to 2 years of age, children were frequently assigned duplicate ID numbers whenever they were seen in a hospital other than the original reporting facility (eg, birth hospital). Legislation was passed in 2006 that authorized VISITS users to view certain existing demographic and identifying information fields from the EBC system regardless of hospital of origin to reduce duplication. Programming functions to allow for these searches were not available until VISITS II was released, however, so data duplication continued to be an issue in VISITS I.
Existing duplicate records were identified through a series of 8 queries developed using several different combinations of variables. The first query was a deterministic match on child's and mother's first and last names as well as child's date of birth. The next 7 queries required some degree of manual review or search. These queries looked at various combinations of name, screening hospital, screening medical record number, gender, and contact address. All available records (VaCARES data since 1986 and VEHDIP data since 2000) were included to ensure complete records would be available in the VISITS II application. A total of 20,578 unique client records out of 202,082 clients in the system were identified as potential duplicates and were reviewed. In total, 8,180 client IDs were merged and 1,439 were deleted due to duplicative or missing information, representing 4.8% of all clients in the VISITS I application.
Children from multiple births provided unique data challenges, and often had to be reviewed separately using other data sources for verification. For example, VISITS I frequently had both generic (eg, Baby Boy Smith) and proper names (eg, John Smith) for a set of twins which resulted in 4 clients records for 1 pair of twins. Resolving these involved in-depth use of other data sets and unique circumstance clues. In one common scenario, a twin was transferred from the birth hospital to another facility with a higher level of care. This resulted in VISITS entries from both the birthing and transfer hospitals with differing medical record numbers. The birth certificate match could be narrowed down to 2 choices in twins; however, hospital medical record numbers were not available for linkage prior to 2007. The birth certificate record could in some cases be used to uniquely identify a twin if only 1 had been transferred. The birth certificate records contained some other potentially unique fields such as birth weight or anomalies specific to only 1 multiple. In some cases, newborn blood-spot data matched to birth certificate records as part of newborn screening follow up could be used to verify medical record numbers and birth weights.
Another challenge for deduplication was the sequence with which other data cleaning processes were occurring. In some cases, records would show up on a file indicating no date of birth. Staff would then research and correct dates of birth in a separate data quality exercise. Once this type of data correction was made, the client would qualify and show up in a deduplication query. Thus, deduplication queries had to be run multiple times. Data deduplication corrections were applied to the live system as they were identified; however, new duplicates were also being generated until the VISITS II data conversion.
Data conversion
Extensive data cleaning and preparation of VISITS I data was completed prior to conversion to improve data quality and reliability going forward in VISITS II. Identified issues related to data quality included missing data, illogical data related to dates, and conversion of free text into discrete fields. Over 16,000 VISITS I records were missing important data fields such as birth hospital or mother's name. Matches to birth certificate data and in some cases matches to death certificate data were used to populate required missing data fields. In over 2,000 cases, there were impossible date relationships between date of birth, admission or screening date, and discharge date. The first stage of data cleaning was to verify the date of birth with a birth or death certificate match. Then, other missing dates could be corrected or reasonably estimated.
Risk indicators for hearing loss are mandated to be collected and reported to VEHDIP. In VISITS I, these were entered as free text. In VISITS II, risk indicators were modeled after the most recent Joint Committee on Infant Hearing recommendations,10 and the reporting format was changed to a forced choice field for general and subcategories of risk. A total of 19,780 free text risk indicators were mapped to the new system as categorical risk variables, converted as an activity note, or deleted as they were no longer considered valid. In all, approximately 467,000 individual data fields were modified to improve data quality.
Linking existing records to birth certificate data
In order to include existing data into the new VVESTS system, VISITS I client data needed to be matched to Virginia Vital Records birth certificate data. Records for VaCARES clients between 1985 and 1999 had previously been matched to birth certificate data; however, records for VEHDIP clients had never been matched. Matching to vital records was the last major data exercise conducted. First, OIM staff completed an analysis and categorization of VISITS I records into 1 of 4 categories:
Category 1: Records previously linked to birth certificate records (28%)
Category 2: Records that could be linked on an exact match of infant's first name, infant's last name, child's date of birth, gender, and mother's first name (29%)
Category 3: Records with generic names such as BB or Baby Boy to be matched (11%)
Category 4: Records with other names and sufficient fields to be matched (32%)
Records in the first 2 categories were subjected to data quality exercises and given at least 1 manual review. Subsets were identified due to mismatching names between VISITS and the current vital records information. Some cases could not remain linked (n=1,717) due to discrepancies that were likely due to amendments, adoptions, or prior error.
Records in the last 2 categories were selected to be matched through a customized Microsoft Access based application that permitted combinations of exact and probabilistic matches based on estimated probabilities.11 Multiples were excluded from this matching process when infant first name was missing or non-specific (eg, “Baby Girl1”). The software ran through a series of deterministic linkage passes and identified records which matched on 4 key fields:
Pass 1: Infant's first name, infant's last name, mother's first name, mother's last name
Pass 2: Infant's first name, infant's last name, infant's date of birth, mother's last name
Pass 3: Infant's last name, infant's date of birth, mother's first name, mother's last name
Pass 4: Infant's first name, infant's date of birth, mother's first name, mother's last name
Pass 5: Infant's first name, infant's last name, infant's date of birth, mother's first name
A second set of deterministic matches was conducted on the remaining records to identify records that were missed in the preceding steps due to missing data, data that had changed since the child's birth (eg, mother's name changed due to marriage/divorce), or data entry errors. The following linkage passes required 3 key fields to match and were also verified manually:
Pass 6: Infant's first name, infant's last name, infant's date of birth
Pass 7: Infant's first name, infant's date of birth, mother's first name
For the third category, singleton infants with generic names, the software successfully matched 16,075 records or 75.6% of the generic name cohort. For the fourth category, a total of 44,053 matches were produced or 74.5% of that cohort.
Staff also worked on matching records throughout the data conversion process by manually searching for matching birth records while researching other data issues; creating queries to match records on a variety of name, date of birth, hospital, address, and medical record numbers that were done on ad-hoc basis; and continuing use of matching software. In addition, other datasets, such as death certificates and newborn blood-spot data previously matched with birth certificates were used to research cases, especially when trying to distinguish matches for multiple births. An additional 6,319 cases were matched through these processes, and over 32,000 matches were researched and verified or discounted through a combination of these activities.
Integrating VISITS II with the new electronic birth certificate system
Since VISITS II shares the child record with the new EBC record early on in the process as part of the development of the requirements document, a series of meetings were held with staff from the Divisions of Vital Records and Health Statistics. The purpose of these meetings was to come to an agreement regarding such issues as which fields would be required to be entered in the child demographic record, how the data would be entered (eg, free text or forced choice from a list), and what values were considered valid for a particular field. Decision rules also had to be made regarding how the system would translate specific ICD-9-CM codes into the general categories of birth defects recorded in the EBC system. It was decided to show congenital anomalies information from birth certificate on the VaCARES screen. However, most birth certificate anomalies are not specific to a single ICD-9-CM code; therefore, they are not automatically populated.
Training
Two months before the statewide release of VISITS II, eight 3-hour training sessions for 135 hospital users were convened throughout Virginia on 4 separate dates. Fifty-six out of 64 reporting hospitals (86%) sent at least 1 representative for training. Training was provided by VDH staff and was targeted to anyone who entered data for either VEHDIP or VaCARES. Some hospitals indicated they may change and streamline some of their business processes since the basic demographics of each child would already be entered by the birth registrar. User training included the following:
Exercises on resetting the user's password
Registering a child entirely through VISITS II
Searching for a child that was already registered as an electronic birth certificate client
Entering discharge summary data
Entering initial hearing screening data
Entering hearing rescreening data and viewing a summary page
Running hospital hearing screening reports
Entering birth defects data
Running VaCARES hospital reports
Creating a new client record
Creating or associating a mother to a VISITS II client
Viewing summary data for a VISITS II client
Entering client transfer data
A take-home training packet was provided to each participant, which included the following items:
Welcome letter
Information systems security access agreement form
Instructions to log on to the VISITS II practice Web site
User training exercises
VISITS II hospital training evaluation form
VISITS II hospital user logon request form, which designates the role(s) that will be assigned to hospital users (eg, basic logon, hospital hearing, hospital VaCARES)
Hospital training CD, which included a copy of all training materials
Results
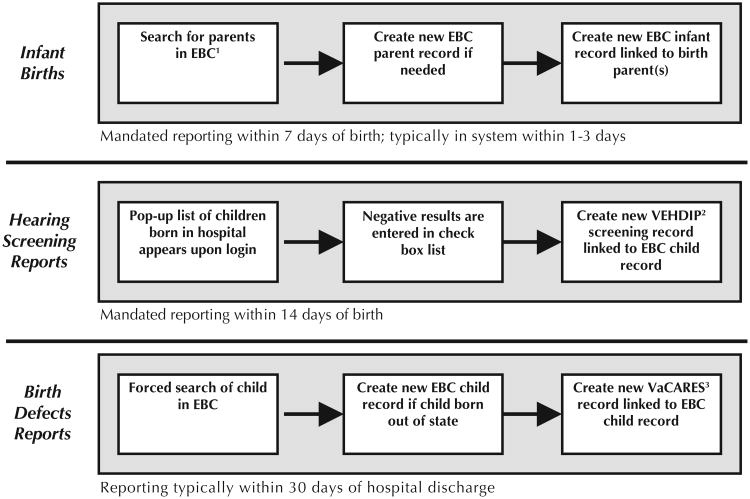
Following the statewide phase-in implementation of the VVESTS EBC module, VISITS II was released statewide on April 12, 2010, at which time all reporting hospitals switched from entering VaCARES and VEHDIP data into VISITS I to VISITS II, and VDH staff switched from uploading Virginia Newborn Screening Program data into VISITS I to VISITS II. A summary of major differences between the VISITS I and VISITS II applications is shown in Table 2. In VISITS II, Virginia birth defects and newborn hearing screening surveillance data are fully integrated with the state's electronic birth certificate system (see Figure 1).
Table 2. Major differences between Virginia Infant Screening and Infant Tracking System (VISITS) I and VISITS II applications.
| VISITS I | VISITS II | |
|---|---|---|
| Data Linkages | Linkages with birth certificate data had to be completed on an ad-hoc basis. | Users enter/select child and family demographics directly from the Electronic Birth Certificate system. |
| Child Search | Only included persons from logon facility resulting in duplicates and confusion about transfers. | Includes basic demographics data from all entries in electronic birth certificate data. |
| Data Entry | Many fields were free text (e.g., transfer hospital, hearing loss risk indicators). | Whenever possible, users are provided with a list of values to choose. |
| Data Validation | Had no validations of date fields. | Date validations do not permit wrong or illogical dates to be entered. |
| Birth Defects Recorded | Accepted any ICD-9-CM code and did not allow the same codes to be reported from multiple hospitalizations. | Accepts only mandated ICD-9-CM codes and does accept the same code from two different hospitalizations reducing confusion about data entry. |
| Case Status | Users had to scroll down to see case status information. | Users have a child summary at the top of the screen with current case status. Important information such as “child closed in system” or “deceased” will be easy to see. |
| Event history | Client summary was not in chronological order of events. | Client summary is in chronological order of events, which will help users easily understand history and next steps. |
| Hearing Status | Hearing hospital users had to compile their own statistics for reporting to VDH. | Children with unknown hearing screening status automatically pop up so that hospitals know exactly which children need follow-up or need results entered. |
| Transfer of Hearing Screens | Multiple “initial” hearing screenings could be entered for children going to multiple facilities. | Initial hearing screening can only be entered once. Upon transfer the record is locked except to the transfer hospital which is responsible for next screening entry. |
| Hearing Loss Risk Indicators | Users had to search to view all risk indicators, but was unclear if risk indicators were current. | Risk indicators are viewable on every screening and can be modified as needed. |
Figure 1. Data flow in Virginia Infant Screening and Infant Tracking System (VISITS) II.
1Electronic Birth Certificate. 2Virginia Early Hearing Detection and Intervention Program.
3Virginia Congenital Anomalies Reporting and Education System.
Although a formal evaluation of the impact of the VISITS II system will not take place until summer 2011, significant progress has already been made towards each of the 8 goals of the redesign in its first nine months of operation. Progress to date related to the 8 goals are detailed below:
Goal 1: Ensure High-Quality Data
The new VISITS II application incorporates a number of key features that improve data quality. First, hospital users are required to search the EBC prior to entering birth defects or hearing screening information. This has virtually eliminated the data duplication that was pervasive in the VISITS I application. Second, the VISITS II application includes a wide range of data validations and requirements not present in VISITS I, which prevents inaccurate record abstracting and data entry. These include range and logic checks (eg, entry is limited to list of possible values; reported events do not occur before birth date), automated calculations and conversions (eg, low birth weight group and date-based fields such as age of child are computed), and forced choice from drop-down lists (eg, accepts only mandated ICD-9-CM codes). Third, the VISITS II application has built-in date-posting fields to monitor timeliness and maintains a transaction log, which tracks and dates all additions, deletions, and changes to the database that allows staff to recreate a “snapshot” of the data at any given time.
Goal 2: Respond More Efficiently to Data Requests
Prior to VISITS II, datasets were not routinely made available to external researchers because of data quality concerns and the time required to prepare a cleaned and deduplicated dataset. Internally, these same concerns could introduce long delays in the preparation of reports. Key fields for hearing loss, for example, often needed to be abstracted from notes entered into free text fields in the database.
The most critical data reporting needs by hospitals and VDH program staff were programmed into the VISITS II application so that hospitals are able to generate their own reports as needed. The following reports are available to hospital VaCARES users:
City and county
Confirmation of diagnosis
Deceased infants
Diagnosis
Health districts
Hospital reporting
Hospital reporting time
Infants who have a specific risk indicator identified but do not have the corresponding birth defect (ICD-9-CM code)
Interstate exchange
Monthly automatic report for hospitals
No cases reported
Parents contact
Race and ethnicity
Source of report
The following reports are available to VEHDIP hospital users:
Children pending hearing/discharge information
Hospital infant status report
Monthly screening rates
Monthly screening results
Monthly screening results (listing of all hospitals by month)
Referral centers report
Report of reasons not screened
VEHDIP staff can also directly query the back-end data in the VISITS II application to create ad-hoc reports. An example of this is a quarterly report sent to each hospital that summarizes reporting compliance for that facility compared to state totals from all hospitals during that quarter.
Goal 3: Improve Efficiency and Security
It is expected that hospitals will be able to streamline their data-entry processes and take less time to submit records, but the extent to which this occurs will not be known until the formal evaluation is completed. Initial results are promising, however. Despite being allowed up to 14 days following discharge to report hearing screening results, the median time for reporting across all hospitals the first full quarter that VISITS II was live (3rd quarter 2010) was only 5 days. At VDH, the data quality improvements and built-in reports detailed above have resulted in immediate improvements in staff efficiency. Since the conversion to VISITS II, data cleaning efforts have shifted from an extensive, ongoing data deduplication and cleaning process to one of periodic verification of data. Staff epidemiologists have now been able to focus efforts beyond basic surveillance reporting and have started to address critical research questions (eg, risk indicators for hearing loss, factors related to loss to follow-up, and birth defects comorbidities).
VISITS II has implemented a number of security improvements that were not part of VISITS I. Since the application was developed by VDH, all Virginia Information Technologies Agency security standards were met.12 Examples of security enhancements include logons and passwords that are role-based, user accounts that lock out if not used for 30 days, and Web-site access that is limited to computers with installed security certificates.
Goal 4: Minimize the Number of VEHDIP Infants Lost to Follow-up
In VISITS II, hospitals are notified automatically via a pop-up screen regarding all children in their care needing hearing screening results entered or follow-up. VISITS II is also programmed to ensure VDH and hospitals are clear about an infant's hearing screening status when a hospital transfer occurs because transfers may result in delayed or missed hearing screens.13 In cases of infant transfer, reporting responsibility in VISITS II will be transferred to the new hospital. The initial hearing screening can only be entered once, and only the assigned hospital can enter follow-up hearing screening data.
Goal 5: Expand Referrals of Children with Special Health Care Needs
Care Connection for Children is a statewide network of centers of excellence in Virginia that provides care coordination and other support services for children with special health care needs. Records for infants who screened positive for any of the 28 disorders tested by the state laboratory as part of the Virginia newborn bloodspot screening program14 are uploaded into the VISITS II application on a weekly basis, and VISITS II is programmed to make automatic electronic referrals to Care Connection for Children for these infants.
Goal 6: Improve the Mechanism for Identifying Cases of Progressive Hearing Loss
Hospitals now report screening results and risk factors for progressive hearing loss to VISITS II for all infants, regardless of the outcome. In the first 6 months of operation (April 13 to October 12, 2010), 51,121 unique infant records were created in VISITS II, which represents 99.6% of in-state resident live birth events during that period. By comparison, only 7,315 infant records (13.5% of all resident births) were created during the same 6-month period of the preceding year in VISITS I. In VISITS II, risk indicators for progressive hearing loss are viewable on every screening and can be modified as needed. The risk factor information across all infants can be used by VDH to increase efforts to ensure these at-risk families get their child retested per the recommended schedule.
Goal 7: Link VISITS II to Birth and Death Certificate Data
Since VISITS II is a module of Virginia's EBC system, it shares the same client data. Any infant born in Virginia can be identified by hospital staff through the required search screen (see Figure 1) and will be automatically linked to the birth certificate data through use of a common unique identifier. The only VISITS II records not automatically linked to birth certificate data will be those of infants born out of state and Virginia resident births that occurred outside of Virginia. Since VDH has data sharing arrangements with surrounding states, the majority of these infants are expected to have a record in the system by the time a Virginia hospital reported an event to VISITS II. During the first 6 months of VISITS II operation, 99.7% (50,957/51,121) of all VISITS II clients were originally created by the EBC system and automatically linked to the VISITS II data.
When funding becomes available, death certificate data will become part of the VVESTS system and VISITS II linkage with these data will become automated. Currently, the VDH Divisions of Vital Records and Health Statistics routinely links infant deaths to births. Live births in the VVESTS system are flagged and the matching death certificate number is stored in the birth certificate data, providing an easy link to death certificates.
Goal 8: Expand Integration and Linkages with Other Surveillance Systems
Housing the VISITS II application within VDH and ensuring the data are linked to birth certificate records leaves VISITS II well positioned to expand integration and linkage with other surveillance systems. The VDH Maternal and Child Health (MCH) Data Mart uses birth certificate records as its core dataset and contains several datasets linked to births on an annual basis (eg, Special Supplemental Nutrition Program for Women, Infants and Children (WIC), hospital discharge, and maternally-linked pregnancy history data). Every 2 weeks a copy of the current provisional birth and death certificate database from VVESTS is imported into the VDH Data Warehouse. Every week a copy of the VISITS II database is also imported into the Data Warehouse, which enables linkage with any dataset previously linked to birth certificate data using the EBC unique identifier shared across the all of the linked data.
VDH is currently determining the feasibility of integrating VISITS II and the Virginia Immunization Information System to improve follow-up for infants and children with hearing loss. Since this immunization registry populates its client table with the same bi-weekly extract of birth certificate data that is imported into the Data Warehouse, linkage for infants born in Virginia would be readily matched using the EBC unique identifier. If the systems were integrated, VEHDIP could improve follow-up efforts by updating parent contact information and notifying providers about the status of their patients' hearing screening at the time immunization records are entered.
Discussion
The benefits of integrating the birth defects and newborn hearing screening surveillance with the EBC in Virginia are clear. Although a formal evaluation has not been completed, a number of benefits were evident in the first 6 months the integrated system was in operation. The most immediate and obvious improvements are the quality of data and subsequent efficiency of reporting. Epidemiologists can now quickly produce basic surveillance reports and utilize time previously spent on data cleaning and deduplication to conduct more sophisticated analyses, create additional data linkages, and participate in collaborative efforts with external research partners. Of course, it is expected improved surveillance data will ultimately improve program effectiveness (eg, reduce loss to follow-up and more efficient communication with providers and families).
Although some of the specific details of the Virginia's transition to an integrated system may not apply to all states, the lessons learned across Virginia's experience can help other states interested in this type of integration assess their readiness and position themselves for success.
For any new system, there has to be a commitment to expend the staff time needed to bring any existing data up to the quality standards of the new system. The required effort depends on the quality of existing data and how many years of historic data need to be moved into the new system. In Virginia it took multiple staff over 2 years to go through the tedious data-cleaning process. A successful redesign needs a project manager who understands and can balance technical requirements, program needs, and policy issues. A good working relationship with information technology staff needs to be developed in order to translate program needs into the specific details included in a formal requirements document. Of course, support for the public health benefits of an integrated system from Vital Records is essential. In Virginia, Vital Records had to allow VISITS II users to search birth records and be confident that VISITS II would not affect their system's performance or compromise security. This would not have happened without a good prior collaborative relationship with Vital Records and a series of discussions that occurred early in the EBC redesign process.
Future Directions
The Virginia Department of Health plans to develop and administer a formal user satisfaction survey to hospital users within 12 months of the release of VISITS II. During the same time period, a formal review and analysis of the VISITS II hospital training evaluation forms, which were completed by training participants, will be done to identify areas needing improvement. Improvements to VISITS II will be planned and implemented using available funds based on the results of this survey and feedback from VDH staff who work with the application. VDH continues to explore the feasibility of integrating and linking VISITS II data with other child-specific public health surveillance systems, such as the Virginia Immunization Information System and the Individuals with Disabilities Education Act Part C Early Intervention Program.
Conclusion
There is a growing recognition in maternal and child health of the importance of social, behavioral, biological, and genetic factors across the entire life course.15 There is a substantial body of evidence indicating that some of these factors may even influence birth outcomes across multiple generations.16–17 Unfortunately, most state maternal and child health surveillance systems are not set up to readily address longitudinal research questions or track and follow children across multiple programs over time. By integrating birth defects and newborn hearing screening systems with an electronic birth certificate record, states will be well positioned to fill critical gaps in information that can guide program and policy decisions. Integration with the electronic birth certificate also enables automatic linkages with other surveillance data that are commonly linked to births such as WIC, death certificate records, and Pregnancy Risk Assessment Monitoring System (PRAMS) data.
Acknowledgments
This project was funded in part by a Centers for Disease Control and Prevention Early Hearing Detection and Intervention Tracking, Surveillance, and Integration Cooperative Agreement (Grant Number: UR3/CCU324750). We would like to acknowledge Janet Rainey, Director of the Virginia Office of Vital Records and the following key staff in the VDH Division of Child and Family Health: Michelle Ballard, Joanne Boise, Nancy Bullock, Tahnee Causey, Pat Dewey, Darlene Donnelly, Ruth Frierson, Ashleigh Howard, Gayle Jones, Sue Lau, Rafael Randolph, Allison Schreiber, Shuhui Wang, and Sharon Williams. We would also like to acknowledge the efforts of VDH Office of Information Management who played a key role in the VISITS II development process: Susan Ann Glass, Tobin Joseph, Bob Klisch, Raj Kocherlakota, Diana Malik, Sharma Puella, Krishnakumar Ramachandran, and Vipul Thakker.
Footnotes
Disclosures: No authors on this paper have any financial relationships or conflicts of interest relevant to this article to disclose.
References
- 1.Sever LE, editor. Guidelines for Conducting Birth Defects Surveillance. Atlanta, GA: National Birth Defects Prevention Network, Inc; Jun, 2004. National Birth Defects Prevention Network (NBDPN) [Google Scholar]
- 2.Kirby RS. Analytical resources for assessment of clinical genetics services in public health: current status and future prospects. Teratology. 2000;61:9–16. doi: 10.1002/(SICI)1096-9926(200001/02)61:1/2<9::AID-TERA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Bodurtha J, Ford N. Virginia Congenital Anomalies Reporting and Education System: Birth Defect Surveillance Data 1989–1998. [Accessed October 3, 2010]; Available at: http://www.vahealth.org/gns/vaCares.htm.
- 4.Virginia Department of Health. Protocols for Diagnostic Audiological Assessment: Follow-up for Newborn Hearing Screening. [Accessed October 3, 2010]; Available at: http://www.vahealth.org/hearing/audiologists.htm.
- 5.Virginia Department of Health. VISITS II Requirements Document. [Accessed October 9, 2010];2008 Mar; Available at: http://www.vahealth.org/gns/visits/visits.htm.
- 6.Virginia Department of Health. An Evaluation of VaCARES. [Accessed October 9, 2010];2006 Jul; Available at: http://www.vahealth.org/gns/visits/visits.htm.
- 7.Centers for Disease Control and Prevention. Updated guidelines for evaluating public health surveillance systems: recommendations from the guidelines working group. MMWR. 2001;50(RR-13):4–25. [PubMed] [Google Scholar]
- 8.Virginia Department of Health. An Evaluation of the Virginia Early Hearing Detection and Intervention Program. [Accessed October 9, 2010];2008 Jul; Available at: http://www.vahealth.org/gns/visits/visits.htm.
- 9.Virginia Department of Health. VISITS II Training Materials. [Accessed October 9, 2010]; Available at: http://www.vahealth.org/gns/visits/visits.htm.
- 10.American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 Position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 11.Mason CA, Tu S. Data linkage using probabilistic decision rules: A primer. Birth Defects Res A Clin Mol Teratol. 2008;82(11):812–821. doi: 10.1002/bdra.20510. [DOI] [PubMed] [Google Scholar]
- 12.Virginia Department of Health. Virginia Information Technology Agency Security Standards. [Accessed January 19, 2011]; Available at: http://www.vita.virginia.gov/library/default.aspx?id=537#securityPSGs.
- 13.Vohr BR, Moore PE, Tucker RJ. Impact of family health insurance and other environmental factors on universal hearing screen program effectiveness. J Perinatol. 2002;22(5):380–385. doi: 10.1038/sj.jp.7210750. [DOI] [PubMed] [Google Scholar]
- 14.Virginia Department of Health. Virginia Core Panel of Disorders. [Accessed January 19, 2011]; Available at: http://www.vahealth.org/vnsp/
- 15.Lu MC, Halfon H. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel I. Maternal health during childhood and later reproductive performance. Ann N Y Acad Sci. 1986;477:29–39. doi: 10.1111/j.1749-6632.1986.tb40318.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapman DA, Scott KG. Intergenerational risk factors and child development. Dev Rev. 2001;21:305–325. [Google Scholar]