Introduction
The approval by the US Food and Drug Administration of ipilimumab (Yervoy; Bristol-Myers Squibb, Princeton, New Jersey) expanded the therapeutic options for treating patients with metastatic melanoma. However, for patients with a history of an autoimmune disorder or organ transplantation, options for therapy are more limited. Here, we describe the successful administration of ipilimumab to two patients with metastatic melanoma, each of whom had previously undergone kidney transplantation. In both cases, the patients remained on low-dose immunosuppression during ipilimumab therapy. Kidney function was monitored closely and remained stable throughout treatment. Both patients experienced benefit from ipilimumab.
Case Reports
Case 1.
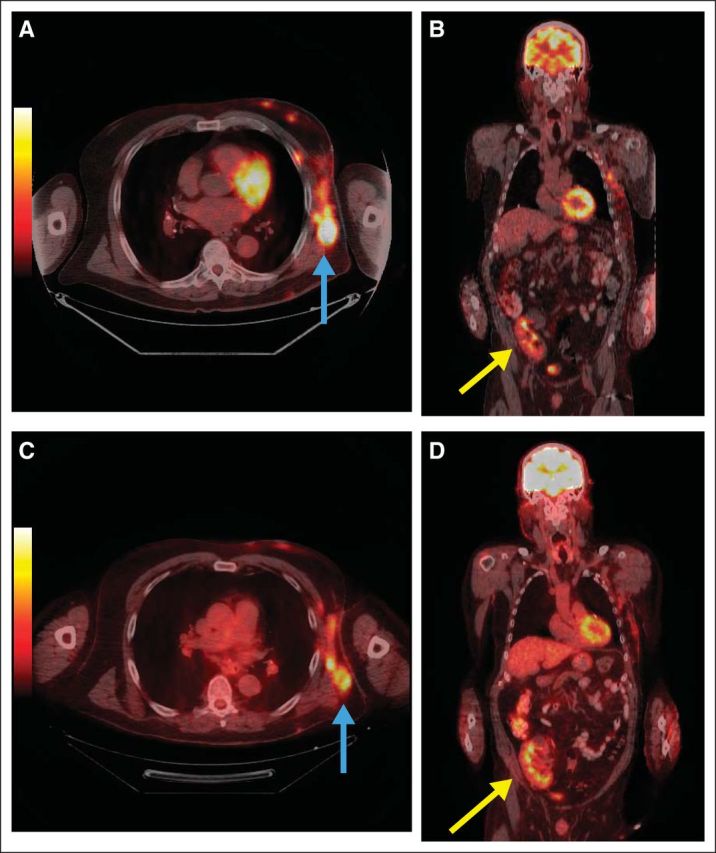
A 72-year-old man underwent deceased donor kidney transplantation in October 2000 for end-stage kidney disease due to hypertension. The remainder of his past medical history was only remarkable for hypercholesterolemia. After transplantation, his kidney function remained stable with a baseline serum creatinine of 1.2 mg/dL (GFR = 82 mL/min) on an immunosuppressive regimen consisting of prednisone and tacrolimus. In 2008 the patient was found to have a ≥ 8 mm ulcerated melanoma on his left chest. After a wide local excision with a left axillary sentinel lymph node biopsy revealing a 2 mm deposit of melanoma in one lymph node, the patient underwent a completion left axillary node dissection. Subsequently, two regional recurrences were treated with surgery and radiotherapy. A positron emission tomography/computed tomography (PET/CT) scan performed in January 2011 revealed unresectable left chest wall metastases and a new liver lesion which subsequently progressed through temozolomide and a platinum-based regimen. Tacrolimus was stopped and the patient remained on prednisone monotherapy at 5 mg daily. Six weeks later, in August 2011, ipilimumab was initiated. His serum creatinine was 1.2 mg/dL. The patient tolerated therapy well, and PET/CT scans in November 2011 revealed decreased abnormal metabolic activity corresponding to subcutaneous soft tissue lesions in the left lateral and anterior chest wall (Fig 1, blue arrows; Figs 1A and 1B, immediately before ipilimumab; Figs 1C and 1D, after ipilimumab) and near resolution of the previously seen abnormal [18F]-fluorodeoxyglucose (FDG) uptake in the left lobe of the liver. Also seen was normal FDG uptake in the transplanted kidney in right pelvic region (Fig 1, yellow arrows). Repeat PET/CT scans in April and October 2012, and January 2013, demonstrated a continued partial response to therapy. The patient's serum creatinine remained stable after therapy.
Fig 1.
Case 2.
A 58-year-old man underwent live donor kidney transplantation in 2004 for advanced kidney failure caused by polycystic kidney disease. After transplantation, his kidney function stabilized with a serum creatinine of 2.0 mg/dL (GFR = 58 mL/min) on an immunosuppressive regimen consisting of prednisone, tacrolimus and mycophenolate mofetil. In 2011, he was found to have a 4.2 mm nodular melanoma on his forehead, later found to be BRAF and C-KIT wild type. He underwent a wide local excision, superficial parotidectomy and right neck dissection, which demonstrated melanoma in four lymph nodes. Out of concern that the patient's immunosuppressive medication regimen might promote tumor progression,1 tacrolimus and mycophenolate mofetil were discontinued, and the patient was maintained on prednisone monotherapy at 5 mg daily. A PET/CT scan performed in January 2012 revealed metastatic disease, including bilateral FDG-avid pulmonary nodules and mesenteric lymphadenopathy. The patient began systemic therapy with three cycles of temozolomide, after which a PET/CT scan demonstrated progression of lymph node and lung metastases, as well as new bone lesions. Ipilimumab was initiated in May 2012. He continued on 5 mg of prednisone daily. His creatinine remained stable at 2.0 mg/dL over the course of therapy. Adverse effects included a grade 2 colitis, which responded well to an increased dose of oral corticosteroids followed by a gradual taper. A PET/CT scan performed after his fourth dose of ipilimumab demonstrated disease regression in several areas, including a decrease in size and FDG avidity of multiple bilateral pulmonary lesions. He was monitored for 7 weeks, after which a repeat PET/CT scan demonstrated disease progression. Reinduction therapy was not administered out of concern for provoking a relapse of the colitis that occurred during induction therapy.
Discussion
Clinical trials of the efficacy of ipilimumab before its approval by the US Food and Drug Administration in 2011 excluded patients with active autoimmune disease or those receiving systemic immunosuppression for organ transplantation.2,3 As a result, there is a paucity of information about the safety of administering the drug to these patient populations.
Ipilimumab is a fully humanized monoclonal antibody directed against cytotoxic T-lymphocyte antigen-4 (CTLA-4), a member of the CD28-B7 superfamily.4 CTLA-4 is an inhibitory receptor present on T cells. After recognition of a peptide antigen, ligation of CTLA-4 by B7.1 (CD80) and/or B7.2 (CD86) inhibits T-cell activation, thus preventing proliferation and effector function.5,6
Because T-cell activation leads to upregulation of CTLA-4, blockade by ipilimumab potentially increases the activity of T cells against cancer cells and other cells expressing foreign antigens, such as the donor antigens expressed by the allografts of solid organ transplantation recipients. In acute kidney transplantation rejection, the primary immune event is recognition of donor antigens by T cells. Full activation of T cells is then accomplished by the interaction of costimulatory molecules, such as the binding of CD28 and its ligands.7 Costimulation blockade is an immunosuppression alternative for kidney transplantation recipients. For example, belatacept (Nulojix; Bristol-Myers Squibb, Princeton, New Jersey) is a fusion protein that binds to CD80 and CD86 and prevents kidney transplantation rejection.
One possible explanation as to why CTLA-4 blockade did not bring about acute cellular rejection for the two patients described above is that both underwent kidney transplantation several years before ipilimumab administration and graft acceptance may have already occurred. Indeed, both patients required only low doses of prednisone to maintain renal function. In addition, the expression and activation of donor antigens may differ in patients with stable versus declining allograft function. Giaretta et al8 recently described higher cell-surface and lower intracellular expression of CTLA-4 on T cell populations in kidney transplantation recipients with good allograft function in comparison to those with chronic rejection. Additionally, the in vivo balance between regulatory T cells and effector T cells can vary among different anatomic compartments, the peripheral blood, the tumor, and the allograft. This variation could also help explain why the allograft function of the two patients described above remained stable despite ipilimumab.9
Although further study in a larger patient cohort is required, these two cases illustrate that ipilimumab may be a safe and effective option for patients with metastatic melanoma who have previously undergone solid organ transplantation. This is of particular importance given that the incidence of melanoma is higher among solid organ transplantation patients than in the general population.10 Future studies might include characterization of intratumoral immune infiltrates, including a description of immune cell subpopulations. Additionally, the utility of the reduction of immunosuppressive therapy11 and its relative contribution to the overall antitumor effect should be investigated.
ACKNOWLEDGMENT
Support was provided by National Cancer Institute Cancer Center Support Grant No. P30 CA006973.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: William H. Sharfman, Merck (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
REFERENCES
- 1.Dapprich DC, Weenig RH, Rohlinger AL, et al. Outcomes of melanoma in recipients of solid organ transplant. J Am Acad Dermatol. 2008;59:405–417. doi: 10.1016/j.jaad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Esensten JH, Martinez-Llordella M, et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 8.Giaretta F, Bussolino S, Beltramo S, et al. Different regulatory and cytotoxic CD4+ T lymphocyte profiles in renal transplants with antibody-mediated chronic rejection or long-term good graft function. Transpl Immunol. 2013;28:48–56. doi: 10.1016/j.trim.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87:991–1003. doi: 10.1016/j.mayocp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwald FO, Christenson LJ, Billingsley EM, et al. Melanoma in solid organ transplant recipients. Am J Transplant. 2010;10:1297–1304. doi: 10.1111/j.1600-6143.2010.03078.x. [DOI] [PubMed] [Google Scholar]


