Abstract
Objectives
To determine prognostic factors for survival inovarian cancer patients treated with intraperitoneal (IP) chemotherapy using ancillary data from cooperative group clinical trials.
Methods
Data were collected from 428 patients with stage III ovarian cancer who underwent optimal surgical cytoreduction (<1 cm) followed by IP paclitaxel/platinum chemotherapy. Primary endpoints were progression free survival (PFS) and overall survival (OS). Potential prognostic variables were included in Cox proportional hazard regression models. Multivariate analysis was conducted to identify independent prognostic factors.
Results
Median PFS was 24.9 months (95% CI, 23.0–29.2) and median OS was 61.8 months (95% CI, 55.5– 69.8). Predictors for PFS were histology, surgical stage and residual disease. Age, histology, and residual disease were prognostic for OS. There were no differences in the hazard ratio for death or progression between patients with positive, negative, or unknown lymph node status. For patients receiving IP chemotherapy (n = 428), 36% of patients had no residual disease with median PFS of 43.2 months (95% CI 32.5–60.4) and median OS of 110 months (95% CI, 60.0–161.3).
Conclusions
Age, histology, and extent of residual disease were predictors of OS in stage III patients treated with IP chemotherapy following optimal cytoreduction. Patients with no residual disease following primary surgery that are treated with adjuvant platinum based IP chemotherapy have survival measures that exceed any rates previously seen in this population.
Keywords: Epithelial ovarian cancer, Intraperitoneal chemotherapy
Introduction
Epithelial ovarian carcinoma (EOC) is the leading cause of death among patients with gynecologic malignancies with an estimated 22,240 new cases and 14,030 deaths for 2013 [1]. Unfortunately, most ovarian cancer patients present with advanced stages due to the absence of effective screening methods coupled with the natural biology of the disease [2]. In the absence of tools for screening or preventive strategies, the best approach for improving clinical outcome is to optimize management after diagnosis. Advances in survival have been achieved with aggressive cytoreductive surgery, followed by platinum and taxane-based chemotherapy, but the majority of patients will experience recurrence and ultimately die from their disease [3,4]. The most promising improvements in survival in recent years have been identified thru clinical trials for patients treated with intraperitoneal (IP) chemotherapy [5–7]. Armstrong et al. documented the longest median survival to date at 66 months for stage III EOC patients treated with IP chemotherapy. The rationale for IP therapy is that higher concentrations of cytotoxic agents can be infused into the peritoneal cavity than would be tolerated systemically. This allows the predominant site of tumor to have sustained exposure to antitumor agents while normal tissues, such as the bone marrow, are relatively spared [8]. Drugs delivered by IP route penetrate only to a depth of a few millimeters beneath the tumor surface, so patients with small volume residual disease are expected to benefit the most [9]. It has not been determined whether IP chemotherapy is of benefit to patients with disease outside of the peritoneal cavity, specifically those with retroperitoneal disease.
There have been three large randomized clinical trials conducted by the Gynecology Oncology Group (GOG) which have demonstrated a clinically significant survival advantage associated with IP chemotherapy compared to intravenous (IV) chemotherapy. Table 1 summarizes patient eligibility, treatment arms, and overall survival (OS) data from the three trials. The objective of this ancillary data analysis was to review pooled data collected from GOG trials using IP chemotherapy and identify subsets of patients within this group that had better or worse clinical outcome measures.
Table 1.
Summary of Gynecologic Oncology Group phase III clinical trials comparing intraperitoneal (IP) and intravenous (IV) chemotherapy in patients with advanced epithelial ovarian cancer (EOC).
| Clinical trial | Patient eligibility | IP Arm | IV Arm | IP OS (months) | IV OS (months) | P value |
|---|---|---|---|---|---|---|
| Alberts (1996) (GOG 104) |
Stage III EOC <1 cm residual disease |
IP cisplatin (100 mg/m2) IV cyclophosphamide (600 mg/m2) |
IV cisplatin (100 mg/m2) IV cyclophosphamide (600 mg/m2) |
49 | 41 | 0.02 |
| Markman (2001) (GOG 114) |
Stage III EOC <1 cm residual disease |
IV carboplatin AUC 9 Q 28 days × 2 cycles IV paclitaxel 135 mg/m2 over 24 h IP cisplatin 75 mg/m2 |
IV cisplatin (75 mg/m2)IV paclitaxel (135 mg/m2) | 62 | 53 | 0.05 |
| Amrstrong (2006) (GOG 172) |
Stage III EOC <1 cm residual disease |
IV paclitaxel 135 mg/m2 over 24 h IP cisplatin 100 mg/m2 IP paclitaxel 60 mg/m2 |
IV cisplatin (75 mg/m2) IV paclitaxel (135 mg/m2) |
65 | 49 | 0.03 |
Methods
The current study was a retrospective review of data collected from patients with stage III EOC treated with IP platinum and paclitaxel chemotherapy on randomized clinical trials conducted by the GOG: protocols 114 and 172. Data from GOG protocol 104 were not included in the final analysis because data collection forms were substantially different from those used for protocol 114 and 172 and did not provide detailed description of disease sites before and after surgery. Informed consent compliant with federal, state, and local requirements was obtained from all patients prior to receiving treatment on clinical trial. The primary end points for both studies were progression free survival (PFS), OS and disease recurrence. PFS was calculated from the date of enrollment to the date of recurrence, death, or most recent follow-up visit. OS was calculated from date of enrollment to date of death or last contact.
Clinicopathologic and surgical variables were abstracted from patient research charts maintained at the GOG Statistics & Data Center in Buffalo, NY. This included: the type of surgical procedures involved in initial debulking, disease characteristics (location and size of disease) prior to and after surgery using a tumor burden index. Tumor burden was subsequently grouped into intraperitoneal, retroperitoneal (pelvic and para-aortic lymph nodes) and extraperitoneal (brain, lung, inguinal lymph nodes, bone and parenchymal liver/spleen) disease. Finally, sites of recurrence following therapy or sites of persistent disease at second look surgery were recorded. Pearson X2 test was used to examine relationships between categorical variables with the Wilcoxon–Mann– Whitney test for continuous variables. Differences were considered statistically significant at P = 0.05. Kaplan–Meier survival curves were calculated and compared using log-rank test. Cox proportional hazards model was used to assess the association between prognostic variables. Using the approach to model selection suggested by Collett, the models were fit with backward, forward, then stepwise selection to identify significant predictors [10].
Results
Patient characteristics
Data from 845 patients enrolled in the IP and IV arms of GOG-114 and GOG-172 were included in this study. Table 2 summarizes the demographic and clinical characteristics for the 428 patients randomized to IP treatment. The median age was 57 years (range, 49 to 64), 91% were white, and 71% had a performance status of 0. Fifty percent of all patients had poorly differentiated tumors with only 6% of all tumors representing clear cell or mucinous histology. Eighty-three percent of patients had stage IIIC disease with 91% of patients described as having a tumor burden >5 cm prior to surgical cytoreduction. After surgery, 84% of all patients had less than 0.5 cm of residual disease, with 36% having only microscopic disease or no visible residual disease. Bowel resection was completed in 29% of patients as a component of cytoreduction. Pelvic and para-aortic lymph nodes were at least sampled in 52% and 47% of all patients, respectively. Among patient treating with IP chemotherapy, 31% of patients had evidence of nodal metastasis. Forty-one percent of patients had no lymph nodes removed at the time of initial debulking.
Table 2.
Demographic and surgical characteristics of patients randomized to intraperitoneal chemotherapy.
| N | ||
|---|---|---|
| Age | 428 | 57 years (IQR 49–65 years) |
| Race | 428 | |
| White | 91.1% (390) | |
| Other | 8.9% (38) | |
| GOG performance status | 428 | |
| 0 | 70.6% (302) | |
| 1 | 24.1% (103) | |
| 2 | 5.4% (23) | |
| Tumor grade | 428 | |
| 1 | 11.4% (49) | |
| 2 | 38.3% (164) | |
| 3 | 50.2% (215) | |
| Histology | 428 | |
| Not CC/mucinous | 93.7% (401) | |
| CC/mucinous | 6.3% (27) | |
| Recurrence | 428 | |
| Yes | 73.1% (313) | |
| No | 26.9% (115) | |
| Surgical stage | 405 | |
| IIIA | 5.7% (23) | |
| IIIB | 11.4% (46) | |
| IIIC | 83.0% (336) | |
| Bowel resection | 388 | |
| Yes | 28.6% (111) | |
| No | 71.4% (277) | |
| Tumor size before debulking | 229 | |
| Microscopic | 0.4% (1) | |
| ≤0.5 cm | 1.3% (3) | |
| 0.6–1.0 cm | 1.7% (4) | |
| 1.1–2.0 cm | 0.4% (1) | |
| 2.1–5.0 cm | 4.8% (11) | |
| >5.0 cm | 91.3% (209) | |
| Residual disease | 352 | |
| Microscopic | 35.5% (125) | |
| ≤0.5 cm | 48.9% (172) | |
| 0.6–1.0 cm | 15.3% (54) | |
| 1.1–2.0 cm | 0.3% (1) | |
| Pelvic lymph node dissection | 419 | |
| No | 48.4% (203) | |
| Yes | 51.6% (216) | |
| Paraaortic lymph node dissection | 416 | |
| No | 52.6% (219) | |
| Yes | 47.4% (197) | |
| Nodal metastasis | 428 | |
| Negative | 28.3% (121) | |
| Positive | 31.1% (133) | |
| Unknown | 40.7% (174) |
N is the number of non-missing values. Numbers after percents are frequencies.
Survival
The median PFS for the ancillary-study IP patients was 24.9 months (95% CI, 23.0–29.2 months), with median OS at 61.8 months (95% CI, 55.5–69.8 months). In comparison, the median PFS for the 415 patients treated on IV arms of GOG 114 and 172 was 20.2 months (95% CI, 17.8–23.5 months) with median OS at 50.9 months (95% CI, 45.5– 58.6 months). The p-value for differences between the survival distributions of the IP and IV treatment groups for PFS and OS was 0.018 and 0.046, respectively, favoring IP chemotherapy. The adjusted hazard ratio for PFS and OS was 0.84 and 0.85, respectively.
Multivariate analysis
All variables with potential prognostic impact were included in a Cox proportional hazards regression model to identify independent prognostic factors for PFS and OS. Histology, surgical stage, and extent of residual disease were identified as statistically significant variables for PFS in the final multivariate analysis. Patient age, histology, and extent of residual disease were prognostic factors for OS. Table 3 provides a detailed analysis of each of the prognostic factors that were evaluated. Interestingly, surgical stage was predictive of disease progression but not OS. Performance status, race, lymph node status, and tumor grade were not independently associated with clinical outcomes.
Table 3.
Multivariate analysis of prognostic factors for patients randomized to intraperitoneal chemotherapy.
| N | Nevent | Adj. HR (PFS) | P value | Nevent | Adj. HR (OS) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age years | 428 | 347 | 0.99 | 1.00 | 1.02 | 0.464 | 308 | 1.00 | 1.02 | 1.03 | 0.012 |
| Race/ethnicity | |||||||||||
| White | 390 | 321 | Referent | – | 284 | Referent | – | ||||
| Black | 21 | 18 | 0.65 | 1.10 | 1.88 | 0.720 | 17 | 0.74 | 1.29 | 2.24 | 0.369 |
| Other | 17 | 8 | 0.25 | 0.57 | 1.30 | 0.182 | 7 | 0.30 | 0.70 | 1.61 | 0.399 |
| Performance status | |||||||||||
| 0 | 302 | 240 | Referent | – | 211 | Referent | – | ||||
| 1 | 103 | 86 | 0.81 | 1.15 | 1.62 | 0.441 | 78 | 0.76 | 1.10 | 1.59 | 0.610 |
| 2 | 23 | 21 | 0.77 | 1.34 | 2.35 | 0.306 | 19 | 0.88 | 1.61 | 2.92 | 0.121 |
| Histology | |||||||||||
| Serous adenocarcinoma | 298 | 248 | Referent | – | 217 | Referent | – | ||||
| Endometrioid | 49 | 31 | 0.43 | 0.69 | 1.12 | 0.131 | 27 | 0.46 | 0.77 | 1.28 | 0.310 |
| Mixed epithelial | 35 | 27 | 0.59 | 0.94 | 1.48 | 0.788 | 26 | 0.59 | 0.94 | 1.50 | 0.795 |
| Clear-cell carcinoma | 19 | 18 | 1.64 | 2.97 | 5.37 | <0.001 | 18 | 2.27 | 4.20 | 7.74 | <0.001 |
| Other | 27 | 23 | 0.57 | 0.95 | 1.59 | 0.846 | 20 | 0.53 | 0.92 | 1.58 | 0.755 |
| Surgical stage | |||||||||||
| IIIA | 23 | 12 | Referent | – | 11 | Referent | – | ||||
| IIIB | 46 | 38 | 1.07 | 2.28 | 4.88 | 0.033 | 31 | 0.75 | 1.71 | 3.91 | 0.200 |
| IIIC | 336 | 278 | 0.94 | 2.05 | 4.49 | 0.071 | 251 | 0.90 | 2.04 | 4.65 | 0.089 |
| Tumor grade | |||||||||||
| 1 | 49 | 33 | Referent | – | 27 | Referent | – | ||||
| 2 | 164 | 136 | 0.69 | 1.11 | 1.78 | 0.664 | 122 | 0.70 | 1.16 | 1.91 | 0.565 |
| 3 | 215 | 178 | 0.66 | 1.04 | 1.65 | 0.854 | 159 | 0.66 | 1.07 | 1.74 | 0.787 |
| Lymph node status | |||||||||||
| Negative | 121 | 90 | Referent | – | 80 | Referent | – | ||||
| Positive | 133 | 106 | 0.64 | 0.98 | 1.51 | 0.941 | 91 | 0.58 | 0.90 | 1.38 | 0.622 |
| Unknown | 174 | 151 | 0.77 | 1.16 | 1.75 | 0.484 | 137 | 0.64 | 0.97 | 1.48 | 0.900 |
| Residual disease | |||||||||||
| Microscopic | 125 | 81 | Referent | – | 68 | Referent | – | ||||
| ≤0.5 cm | 172 | 153 | 1.19 | 1.64 | 2.26 | 0.002 | 143 | 1.34 | 1.87 | 2.62 | b0.001 |
| >0.5 cm | 55 | 51 | 1.20 | 1.80 | 2.70 | 0.005 | 44 | 1.31 | 2.03 | 3.15 | 0.001 |
a b c represent the lower 95% confidence limit a, the value b, and the upper 95% confidence limit c.
Age
While not a relevant variable in the PFS model for IP patients, age is noted to be significant among predictors for OS with a HR of 1.01 (P = 0.019). Hence, the risk of death increases by 1.01 times for each year a patient is older, after adjustment for the effects of the other variables in the model. Age was also evaluated for linearity, but found not to stray significantly from it within the model.
Histology
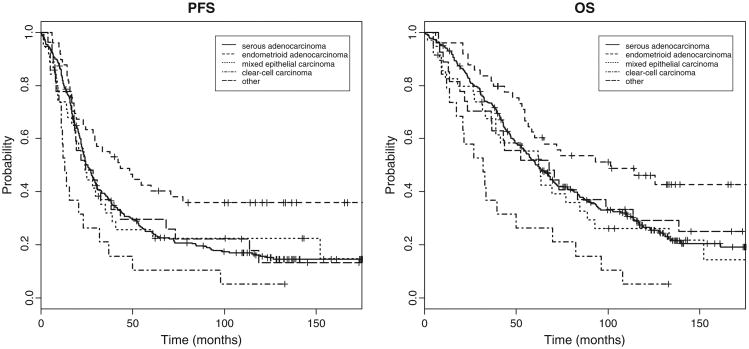
When compared to serous histology, patients with clear cell histology were associated with decreased PFS (HR = 2.66; 95% CI, 1.47–4.82; P = 0.001) and OS (HR = 3.88; 95% CI, 2.11–7.12; P = <0.001) (Fig. 1).
Fig. 1.
Progression free and overall survival curves for patients randomized to intraperitoneal chemotherapy stratified by histology.
Residual disease
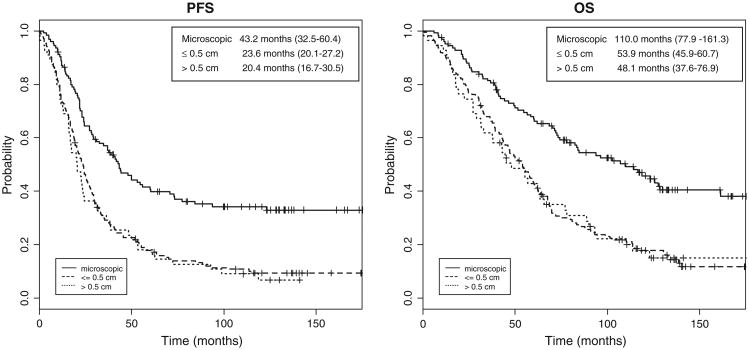
Using microscopic residual disease as the referent, both PFS (HR = 1.71; 95% CI, 1.26–2.32; P < 0.001) and OS (HR = 1.89; 95% CI, 1.37–2.62; P < 0.001) were decreased for patients with ≤0.5 cm. Likewise, PFS (HR = 1.79; 95% CI, 1.20–2.68; P = 0.004) and OS (HR = 1.92; 95% CI, 1.25–2.96; P = 0.003) were decreased for patients with residual disease from 0.6 to 1.0 cm in size (Fig. 2). When survival data were evaluated for each study, patients with no residual disease treating with IP chemotherapy on GOG-114 had median PFS of 41.1 months (95% CI; 24.2–54.6) and OS of 83.8 months (95% CI; 60.1–161.3). IP patients on GOG 172 with no residual disease had median PFS of 60.4 months (95% CI 36.9–N/A) and OS of 127.6 months (95% CI; 84.7–N/A). Not surprisingly, patients with microscopic disease also had the best survival outcomes of all patients randomized to receive IV chemotherapy with a median PFS of 33.4 months (95% CI; 26.1–44.2) and median OS of 82.4 months (65.9–111). Patients with microscopic disease treated with IP chemotherapy had median PFS and OS of 43 and 110 months, respectively, compared to 33 and 82 months for patients receiving IV chemotherapy with no gross residual. Despite this marked differences in median PFS and OS, these measures were not statistically significant for PFS (P = 0.09) or for OS (P = 0.23).
Fig. 2.
Progression free (PFS) and overall survival (OS) curves for patients randomized to intraperitoneal chemotherapy stratified by residual disease following primary cytoreductive surgery. Median PFS and OS for patients with microscopic residual disease were 43 months and 110 months, respectively.
Lymph node status
For patients receiving IP chemotherapy, lymph node status was not an independent predictor of PFS or OS. However, when patients from both treatment arms were evaluated, patients receiving IV chemotherapy with unknown lymph node status had a significantly poorer outcome with a PFS of 16.3 months (95% CI, 15.4–19.9, p = 0.005) and OS of 39.8 months (95% CI, 34.6–49.5, P = 0.013) compared to patients with negative lymph nodes that received systemic therapy with a PFS of 28.1 months (95% CI, 22.6–33.1) and OS of 72.6 months (95% CI 53.6–91.4) (Table 4).
Table 4.
Progression free and overall survival for patients randomized to intravenous and intraperitoneal chemotherapy stratified by lymph nodes negative (surgically excised and without disease), lymph nodes positive (surgically excised and with disease), and lymph nodes unknown (not surgically excised).
| N | Median PFS (months) | HR | P value | Median OS (months) | HR | P value | |
| IV lymph node | |||||||
| Negative | 118 | 28.07 | Referent | – | 72.64 | Referent | – |
| Positive | 125 | 22.18 | 1.30 | 0.09 | 56.25 | 1.28 | 0.14 |
| Unknown | 172 | 16.33 | 1.51 | 0.005 | 39.79 | 1.47 | 0.01 |
| IP lymph node | |||||||
| Negative | 121 | 30.72 | 0.92 | 0.59 | 75.33 | 0.96 | 0.84 |
| Positive | 133 | 27.01 | 1.04 | 0.82 | 63.18 | 1.06 | 0.71 |
| Unknown | 174 | 21.63 | 1.13 | 0.43 | 54.44 | 1.11 | 0.49 |
Relapse sites
Relapse of disease in the peritoneal cavity was significantly lower for patients treated with IP chemotherapy than for patients on IV treatment arms (43% versus 56%, P < 0.001). Though not statistically significant, there were more recurrences noted in extraperitoneal locations such as brain, chest, inguinal lymph nodes, and bone.
Conclusion
This study represents a retrospective analysis of a large group of stage III EOC patients who received primary surgical cytoreduction to <1 cm followed by IP platinum and paclitaxel chemotherapy during participation in cooperative group trials. A review of the similarities and differences between the experimental (IP) and control arms (IV) of these three trials will support that it is difficult to compare studies using different cytotoxic agents and dosing schedules. Experimental and control arms for patients treated on GOG 104 were the most similar with route of administration as the only difference; however, this study was completed prior to the use of paclitaxel as a standard agent in the treatment of advanced ovarian cancer, and as such paclitaxel was not included in this study. Patients receiving IP chemotherapy in GOG 114 were given an additional 2 cycles of IV carboplatin at an AUC of 9 prior to starting IP treatment which may have provided a survival advantage regardless of the IP treatment. Finally, patients receiving IP chemotherapy on GOG 172 were given a higher dose of cisplatin as well as an additional dose of IP paclitaxel on day 8 which may have influenced survival. While we acknowledge these differences in the treatment arms, in the final outcome, patients treated with IP chemotherapy have improved OS compared to patients treated with IV chemotherapy alone. Yet, despite these marked improvements in survival, there have been challenges with toxicity as well. In GOG-172, only 42% of patients in the IP treatment arm completed six cycles of the assigned therapy. Reasons for discontinuation included port complications, chemotherapy-related side effects, and complaints related to IP infusion such as grade 3–4 abdominal pain [11]. Because of the technical challenges and toxicities noted in GOG-172, widespread use of IP chemotherapy has been slow to be embraced. The most recent phase III trial, GOG 252, decreased the dose of IP cisplatin to 75 mg/m2 in an effort to reduce toxicities in this arm. Once these data are mature we will learn more about whether dose reduction improves toxicity profiles for these patients and whether modifications to improve treatment tolerability compromise survival. Our objective for the current ancillary data analysis was to identify subsets of patients treated with IP chemotherapy that may maximally benefit from this modality of treatment.
We were particularly interested in evaluating the value of IP chemotherapy for patients with retroperitoneal disease. As we began to review surgical data it became apparent that our ability to answer this question would be hindered by a surprising number of patients that did not have any surgical evaluation of pelvic or para-aortic lymph node basins. Despite the fact that all patients underwent cytoreductive surgery with <1 cm of residual tumor, only 59% of patients had lymph nodes sampled or completely excised at the time of primary surgery. Of the 254 patients that underwent lymph node evaluation, metastatic disease was identified in 52%. There were no statistically significant differences in PFS or OS for IP patients that were of node positive, node negative or node unknown status which suggests that IP chemotherapy is equally effective for patients with both intraperitoneal and retroperitoneal disease. We had hoped to further stratify patients with stage IIIC disease into those who met classification by retroperitoneal disease only, in the absence of large volume intraperitoneal disease. Unfortunately, only three patients were identified and so we were unable to adequately power survival in stage IIIC patients with peritoneal verses retroperitoneal disease. Of note, patients with unknown lymph node status that randomized to IV chemotherapy had significantly poorer median PFS (16 months) and OS (40 months) than IV patients with negative lymph nodes (28 and 72 months), respectively.
Another question that we sought to answer was whether the administration of IP chemotherapy would alter the pattern of recurrence sites for patients compared to those treated with systemic chemotherapy. The primary site for relapse of disease for patients with EOC after a period of clinical remission is in the abdomen or pelvis [12]. Interestingly, significantly fewer recurrences were noted in the intraperitoneal cavity for patients that received IP chemotherapy than for patients that received systemic therapy. The clinical significance of this is not clear at this time, but provides more information for investigators designing clinical trials in the future. This should also serve to heighten the awareness of physicians that follow this population of patients in surveillance that recurrence patterns may be altered. The most recent cooperative group trial for patients with advanced EOC may further elucidate a role for dose-dense paclitaxel in combination with IP chemotherapy. GOG 252 which has recently completed accrual included an experimental arm with IP carboplatin (AUC 6) and IV paclitaxel (80 mg/m2) on days 1, 8, and 15. Perhaps additional systemic chemotherapy given in combination with IP chemotherapy may prove to have benefit in reducing extraperitoneal recurrences.
A number of factors have been identified as prognostic for survival in patients with EOC. In the early 1990s, studies by the GOG identified age, performance status, the presence of ascites, residual disease and administration of a platinum-based agent as significant predictors of survival in patients with advanced EOC [13–17]. As contemporary follow-up to these studies, Winter and colleagues reviewed data collected from 1895 patients with stage III EOC who had undergone primary surgical cytoreduction followed by six cycles of IV platinum and paclitaxel chemotherapy on GOG trials [18]. Age, performance status, tumor histology, and residual tumor were identified as independent predictors of prognosis in patients with stage III EOC. Grade of tumor and race were not associated with clinical outcomes.
In a similar fashion, we have attempted to identify prognostic factors for survival in stage III EOC patients who underwent surgical cytoreduction followed by IP chemotherapy with cisplatin and paclitaxel. Our study revealed patient age, histology, and residual disease to be predictors for OS in stage III EOC patients treated with IP chemotherapy. Performance status, race, surgical stage and tumor grade were not independent predictors of survival.
Age at the time of diagnosis for patients with EOC has been consistently recognized as an independent predictor of clinical outcome [16,18–21]. Part of this survival difference is due to the frequency of young patients with early stage disease and tumors of low grade or low malignant potential. However, when early stage EOC and low malignant potential tumors are extracted, younger women will still have significantly better survival rates than older women with stage III and IV disease [20]. Another reason for the discrepancy in clinical outcome may be related to performance status and co-existing medical co-morbidities in an older patient population. This undoubtedly influences the decision making process of a surgeon regarding whether to undertake an aggressive cytoreductive surgery as the primary step in treatment. While these ideas represent plausible reasons for the age-related differences, it must be emphasized that patient age stands as a prognostic factor for survival independent of residual disease, performance status, grade, and stage. A better understanding of the changes in tumor biology or immune response in older patients may lead to new insights into the best treatment methods for this rapidly increasing population.
Most studies describing the role of histology in prognosis have focused on mucinous and clear cell tumors [22–24]. Because these tumor types are less common, the studies have been retrospective and comprised of small numbers of patients. In the current study, patients with endometrioid histology had the best prognosis compared to serous histology, with clear cell carcinoma histology conferring the poorest outcome. This is consistent with the large data analysis published by Winter et al. in which patients with mucinous and clear cell histology were associated with poorer PFS and OS compared to serous histology [18]. Our findings also correlate with those collected from a large retrospective review of 455 patients with stage III EOC in Norway in which patients with endometrioid tumors had the best prognosis, with relative hazard of 1.5–1.9 for patients with mixed, serous and unclassified tumors and 5.4–7 for patients with mucinous and clear cell tumors [25].
There is overwhelming, retrospective evidence that residual tumor following surgical cytoreduction is an independent predictor of OS for patients with EOC [14,17,25,26]. This ancillary data study provides further evidence that even within a population of patients with <1 cm of residual disease, less is more. Our analysis has shown that extent of residual disease is an independent predictor of survival whereas stage of disease is not. This suggests that efforts to reduce the tumor burden to no gross residual disease may mitigate the impact of stage. This is supported by survival data from the patients with no gross residual regardless of treatment arm. For example, patients that randomized to IV chemotherapy with no gross residual also had superior survival outcomes than counterparts with small volume residual disease. It should be noted that while IP patients with no gross residual demonstrated a 28 month advantage in median OS compared to IV patients with microscopic disease (110 vs. 82 months, respectively), there were no statistically significant differences in this analysis (P = 0.23). It is likely that once we had stratified by both treatment regimen and residual disease we were not powered to definitely answer this question.
To further highlight the value of complete surgical cytoreduction, patients treated on the IP arm of GOG-172 with no gross residual disease had survival measures that are unprecedented, with a median PFS of 60 months and OS of 128 months. Regardless of difficulties in achieving no gross residual disease through aggressive surgical cytoreduction or the challenge of managing toxicity associated with IP chemotherapy, these outcome measures exceed any previously reported in a population with advanced ovarian cancer.
In summary, the current study indicates that age at the time of diagnosis, histology, and residual disease remaining following surgical cytoreduction are independent predictors of prognosis in stage III EOC patients treated with IP chemotherapy. Despite concerns regarding the effectiveness of using IP chemotherapy for patients with retroperitoneal disease, no significant differences in PFS or OS were noted in patients with positive, negative, or unknown lymph node status. This study further validates the data from previous studies that the goal of surgical cytoreduction should be that of no remaining visible disease as patients with microscopic disease fared significantly better than those with <1 cm. The survival measures for patients with no gross residual disease treated with IP chemotherapy are unprecedented with median OS of 110 months. These are very encouraging data and should promote further investigation of novel strategies for implementing IP chemotherapy for patients with no residual disease. We should also continue to educate patients and treating physicians in an effort to promote the use of IP chemotherapy. Finally, patterns of recurrence are altered in patients treated with IP chemotherapy in that fewer intraperitoneal relapses were identified compared to patients treating with systemic therapy.
Highlights.
Survival for IP chemotherapy patients with no residual disease is unprecedented.
Prognostic factors include age, histology, and extent of residual disease.
More recurrences occur extraperitoneal for patients treated with IP chemotherapy.
Footnotes
Presented: Plenary presentation at Society of Gynecologic Oncologists Annual Meeting on March 27, 2012; Austin, TX. Invited presentation at Semi-Annual Gynecology Oncology Group Meeting on July 24, 2013; Boston, MA. This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), as well as the Ovarian Cancer Research Fund as a Young Investigator Award from the Gynecologic Oncology Group. The following institutions participated in this study: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, Eastern Virginia Medical School, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Southwestern Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona Health Science Center, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, Gynecologic Oncology Network, Ellis Fischel Cancer Center, and Fletcher Allen Health Care.
Conflict of interest statement: The authors declare that there are no conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 8.Dedrick RL, Flessner MF. Pharmacokinetic problems inperitoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89:480–7. doi: 10.1093/jnci/89.7.480. [DOI] [PubMed] [Google Scholar]
- 9.Los G, Mutsaers PH, Ruevekamp M, McVie JG. The use of oxaliplatin versus cisplatin in intraperitoneal chemotherapy in cancers restricted to the peritoneal cavity in the rat. Cancer Lett. 1990;51:109–17. doi: 10.1016/0304-3835(90)90045-y. [DOI] [PubMed] [Google Scholar]
- 10.Collett D. Modeling survival data in medical research. 2003 [Google Scholar]
- 11.Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100:27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Cannistra SA. Intraperitoneal chemotherapy comes of age. N Engl J Med. 2006;354:77–9. doi: 10.1056/NEJMe058308. [DOI] [PubMed] [Google Scholar]
- 13.Hoskins WJ. The influence of cytoreductive surgery on progression-free interval and survival in epithelial ovarian cancer. Baillieres Clin Obstet Gynaecol. 1989;3:59–71. doi: 10.1016/s0950-3552(89)80042-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–9. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 15.Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol. 1991;9:1138–50. doi: 10.1200/JCO.1991.9.7.1138. [DOI] [PubMed] [Google Scholar]
- 16.Thigpen T, Brady MF, Omura GA, Creasman WT, McGuire WP, Hoskins WJ, et al. Age as a prognostic factor in ovarian carcinoma. The Gynecologic Oncology Group experience. Cancer. 1993;71:606–14. doi: 10.1002/cncr.2820710218. [DOI] [PubMed] [Google Scholar]
- 17.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–66. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 18.Winter IIIWE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 19.Duska LR, Chang YC, Flynn CE, Chen AH, Goodman A, Fuller AF, et al. Epithelial ovarian carcinoma in the reproductive age group. Cancer. 1999;85:2623–9. doi: 10.1002/(sici)1097-0142(19990615)85:12<2623::aid-cncr19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Loizzi V, Lin YG, Brewster WR, Osann K, DiSaia PJ. Stages III and IV invasive epithelial ovarian carcinoma in younger versus older women: what prognostic factors are important? Obstet Gynecol. 2003;102:156–61. doi: 10.1016/s0029-7844(03)00399-5. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK, Urban R, Cheung MK, Osann K, Shin JY, Husain A, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006;95:1314–20. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60:412–7. doi: 10.1006/gyno.1996.0065. [DOI] [PubMed] [Google Scholar]
- 23.Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 24.Akahira JI, Yoshikawa H, Shimizu Y, Tsunematsu R, Hirakawa T, Kuramoto H, et al. Prognostic factors of stage IV epithelial ovarian cancer: a multicenter retrospective study. Gynecol Oncol. 2001;81:398–403. doi: 10.1006/gyno.2001.6172. [DOI] [PubMed] [Google Scholar]
- 25.Makar AP, Baekelandt M, Trope CG, Kristensen GB. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56:175–80. doi: 10.1006/gyno.1995.1027. [DOI] [PubMed] [Google Scholar]
- 26.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]