Abstract
The psychosis prodrome, or period of clinical and functional decline leading up to acute psychosis, offers a unique opportunity for identifying mechanisms of psychosis onset and testing early intervention strategies. We summarize major findings and emerging directions in prodromal research and provide recommendations for clinicians working with individuals suspected to be at high risk for psychosis. The past two decades of research have led to three major advances. First, tools and criteria have been developed that can reliably identify imminent risk for a psychotic disorder. Second, longitudinal clinical and psychobiological data from large multisite studies are strengthening individual risk assessment and offering insights into potential mechanisms of illness onset. Third, psychosocial and pharmacological interventions are demonstrating promise for delaying or preventing the onset of psychosis in help-seeking, high-risk individuals. The dynamic psychobiological processes implicated in both risk and onset of psychosis, including altered gene expression, cognitive dysfunction, inflammation, gray and white matter brain changes, and vulnerability-stress interactions suggest a wide range of potential treatment targets and strategies. The expansion of resources devoted to early intervention and prodromal research worldwide raises hope for investigating them. Future directions include identifying psychosis-specific risk and resilience factors in children, adolescents, and non-help-seeking community samples, improving study designs to test hypothesized mechanisms of change, and intervening with strategies that better engage youth, their environmental contexts, and neurodevelopmental targets to improve functional outcomes. Prospective research on putatively prodromal samples has the potential to substantially reshape our understanding of mental illness and our efforts to combat it.
Keywords: clinical high risk, ultra high risk, basic symptoms, early intervention, schizophrenia, dynamic risk
The past two decades have ushered in a new era of research on early intervention and possible prevention of acute psychosis and its associated morbidity. Informed by retrospective research on the precursors to psychosis and prospective longitudinal research on population cohorts and individuals at familial (“genetic”) high-risk (FHR), a “close in” strategy to study individuals likely to develop psychosis within a year or two has been adopted world-wide.1,2 These individuals are identified on the basis of age (typically ages 12-35) and clinical characteristics (primarily new or worsening attenuated psychotic symptoms) suggestive of a psychosis prodrome. As the majority (~65%) will not transition to a diagnosable psychotic disorder, these prospectively identified individuals are typically referred to as at “clinical high risk” (CHR), “ultra high risk” (UHR) or having “at risk mental states” (ARMS). An “Attenuated Psychosis Syndrome” is included in section 3 of DSM-5 to encourage further study and diagnostic consideration.3 Another approach has focused on “Basic Symptoms” (BS) or subtle alterations in mental experiences that are thought to emerge earlier than CHR syndromes.4, 5 We use “psychosis risk” (PR) to refer to the full spectrum of individuals identified as having CHR or BS. The primary goals of PR research are to understand the mechanisms of transition from risk to diagnosable illness and to identify intervention strategies for preventing or mitigating the onset or full expression of psychosis.
DYNAMIC NATURE OF RISK
The PR approach requires accurate identification of individuals at significantly high risk or progressing toward identifiable illness. Perhaps not surprisingly, the early warning signs that are often obvious in hindsight are not as obvious as they emerge. Troubling private experiences may not be disclosed or may be disclosed to people who do not recognize them as symptoms of emerging psychosis. Indeed, many of the earliest signs, such as anhedonia, attention dysfunctions or social difficulties are not specific to emerging psychotic disorders. They may reflect any of a number of causal mechanisms or contributing factors (equifinality), and at the same time, be predictive of outcomes other than psychosis (multifinality).
In the absence of definitive markers of impending illness, prevention and early intervention of psychosis are based on assessment of probabilities. These probabilities are typically based on risk factors and risk indicators. Risk factors such as a family history of psychosis, convey a quantifiably higher likelihood of subsequent illness. They may reflect a direct causal pathway: e.g., brain dysfunction. More often, they reflect an indirect relationship with potential causal mechanisms. For instance, paternal age over 50 does not itself cause psychosis. Rather, it is associated with an increased likelihood of altered genetics believed to play a causal role in psychosis. Similarly, risk indicators, such as increased suspiciousness, do not directly cause psychosis. They signal that psychosis may be emerging.
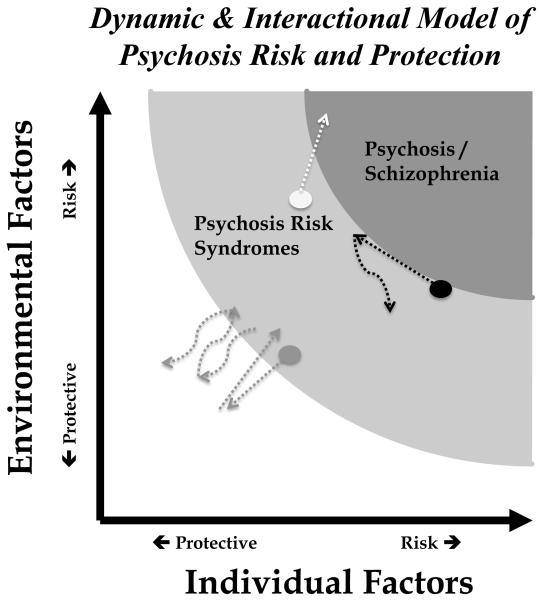
Individuals at PR are typically identified on the basis of risk indicators, clinical signs and symptoms that signal some increased likelihood of developing a psychotic disorder. Subsequent transition to psychosis confirms an individual’s (prior) PR status. A lack of transition is more ambiguous. Many assume that individuals who do not transition are “false positives,” their prior PR identification being incorrect. However, other possibilities exist. Some individuals might transition if followed longer (e.g., Figure 1, white dot). Treatment may prevent the full expression of an otherwise present illness (e.g., Figure 1, black dot). Or PR may have been reduced by a number of individual or environmental factors (e.g., Figure 1, gray dot). PR samples likely contain some of all of these. The majority of PR individuals will not develop a psychotic disorder and a substantial proportion will improve with no treatment at all.6
Figure 1.
This figure illustrates two potential dimensions contributing to risk for psychosis: individual and environmental factors. The dots represent three hypothetical cases assessed to be at different levels of risk based on unique combinations of risk and protective factors. The dotted lines reflect the dynamic nature of the PR concept, its hypothetical change over time in response to changing risk and protective factors. Of note, changes might occur naturalistically or through implementation of effective treatment.
Risk is thus a complex latent construct. Figure 1 provides a model for the dynamic nature of PR, its’ reflection of and responsivity to a multitude of risk and protective factors. The hypothetical trajectories of the three case examples illustrate how risk and protective factors might push someone closer or farther from the psychosis threshold. This dynamic interaction raises complex questions for identification and intervention, particularly at the individual level. Yet, it is this dynamic aspect that raises hope for altering the trajectory of illness.
METHODS
This is not intended as an exhaustive review. We review, within the context of a dynamic diathesis-stress model, selected aspects of science that highlight progress in understanding and intervening in the emergence of psychosis. These include phenomenology, characteristics and correlates of risk, risk assessment, the nature and efficacy of current interventions targeting PR youth, and implications for the benefit/risk ratio of prevention and early intervention. Finally, we offer recommendations to improve this ratio and address ongoing challenges.
PHENOMENOLOGY AND IDENTIFICATION OF EMERGING PSYCHOSIS
Retrospective research on individuals with established illness provided the foundation for the prospective study of emerging psychosis.7-9 Yet prospective studies with repeated symptom assessment over the course of years do not typically capture symptom progression as it occurs.10 Given significant variability in what and how symptoms emerge over time, we offer a description of known trends and symptom examples.
In a majority of cases, psychosis is preceded by a period of subtle changes in experience or functioning, a prodromal period, lasting months to years.9, 11 Often earliest to emerge are heterogeneous symptoms: attention problems, depression, anxiety, avolition, social difficulties, disorganization, and sleep disturbances, so-called “non-specific symptoms”.9, 10, 12, 13 More specific psychotic-like symptoms may emerge closer to the onset of acute psychosis.
To identify prodromal individuals prospectively, two major efforts were initiated. The first capitalized on the prototypical emergence of psychotic-like symptoms and a marked decline in functioning. Syndromes initially posited by Yung and McGorry and later adapted by Miller, McGlashan and colleagues became the standard for identifying PR samples for prospective research.2,13 These syndromes, summarized in Table 1, include the Attenuated Positive Symptoms Syndrome (APSS) of progressive subthreshold positive symptoms; the Brief Intermittent Psychosis Syndrome (BIPS) of brief and spontaneously remitting psychotic-level symptoms; and the Genetic Risk & Deterioration Syndrome (GRDS) reflecting presumed genetic risk (having a first degree family history of psychosis or meeting criteria for schizotypal personality disorder [SPD]) combined with a recent 30% functional deterioration.
Table 1.
Instruments and Syndromes for Identifying Psychosis Risk
| Measures | Syndromes | Symptoms | Frequency (Duration) | Onset | |
|---|---|---|---|---|---|
| Clinical High Risk | SIPS2 | Attenuated Positive Symptom Syndrome (APSS) |
1 or more elevated sub-threshold positive symptom |
≥ 1x/week in the past month. | Past year or worsened compared to 1 year ago |
| Brief Intermittent Psychotic Syndrome (BIPS) |
Briefly and recently met psychotic threshold on one or more positive symptom |
1x/month (Present at least several minutes a day) up to 4x/week |
Past 3 months | ||
| Genetic Risk and Decline (GRD) |
Schizotypal Personality Disorder Diagnosis or 1st degree relative with a psychotic disorder and a current Global Assessment of Functioning (GAF) score at least 30% lower than 12 months ago |
||||
| CAARMS19 | Attenuated Psychosis Group (Subthreshold Intensity) (APG) Attenuated Psychosis Group (Subthreshold Frequency) |
Elevated sub-threshold Unusual Thought Content (UTC), Non-Bizarre Ideas (NBI), Disorganized Speech (DS) or Perceptional Abnormalities (PA) and a 30% drop in SOFAS in the past year or a SOFAS ≤ 50 for the past year |
1x/month to 2x/week (> 1 hour per occasion) or 3- 6x/week (<1 hour per occasion) to continuous (for at least a week) |
Past 12 months but no more than 5 years ago |
|
| Psychotic intensity on UTC or NBI or DS or Severe/Psychotic intensity on PA and a 30% drop in SOFAS in the past year or SOFAS ≤ 50 for the past year |
1x/month to 2x/week (> 1 hour per occasion) or 3- 6x/week (<1 hour per occasion) |
Past 12 months but < 5 years |
|||
| Brief Limited Intermittent Psychotic Symptoms (BLIPS) |
Psychotic intensity on UTC or NBI or DS or Severe/Psychotic intensity on PA and a 30% drop in SOFAS in the past year or a SOFAS ≤ 50 for the past year |
3-6x/week (>1 hour per occasion) or daily (<1 hour per occasion) to continuous (for at least a week) |
Occurred during the last 12 months |
||
| Vulnerability Group |
Schizotypal Personality Disorder Diagnosis or 1st degree relative with a psychotic disorder and a 30% drop in SOFAS in the past year or a SOFAS < 50 for the past year |
||||
| Basic Symptoms | SPI-A20 | Cognitive Disturbances (COGDIS) |
At least 2 of the following: (Inability to Divide Attention; Thought Interference; Thought Pressure; Thought Blockages; Disturbance of Receptive Speech; Disturbance of Expressive Speech; Unstable Ideas of Reference; Disturbances of Abstract Thinking; Captivation of Attention by Details of the Visual Field) |
Weekly to daily | Last 3 months and 1st occurrence more than 12 months ago |
Note: SIPS = Structured Interview for Psychosis-Risk Syndromes; CAARMS = Comprehensive Assessment of At-Risk Mental States; SPI-A = Schizophrenia Prediction Instrument for Adults; SOFAS: Social and Occupational Functioning Assessment Scale
The second approach focused on “basic symptoms” (BS), or subjectively experienced changes in self-perception, stress tolerance, thinking, communication, and social function, believed to emerge earlier than the CHR syndromes of the first approach.14 This BS paradigm identified combinations of Cognitive Disturbances (COGDIS) or Cognitive-Perceptive (COPER) disturbances highly predictive of subsequent psychosis in initial prospective research.4, 15 On average, about one third of those identified as at CHR (termed the “late prodrome”) develop a psychotic disorder within three years; comparable but highly variable rates have been found with the less frequently studied BS approach (“early prodrome”). 16-18
Attenuated psychotic symptoms typical of the “late prodrome” include unusually valued or odd ideas, thoughts of reference, magical thinking, a sense that things are odd, or belief that others can read, control, or implant thoughts (sub-threshold delusions). Individuals may be confused about whether experiences are real or imaginary. The sense that others are watching, singling out, or intending harm (sub-threshold paranoia) may begin as a vague uneasiness. Individuals may appear wary or become increasingly withdrawn. Some express a sense of superiority or giftedness or appear expansive (sub-threshold grandiosity). Hallucinations may begin as poorly formed visual or auditory aberrations (though smells, tastes, or physical sensations also occur), “out of the corner of the eye” or as muffled sounds or voices. Speech may become newly vague, metaphorical, stereotyped, concrete, or disorganized. Individuals may increasingly ramble, go off track, or have difficulty maintaining conversations. Experiences of basic symptoms include the subjective disruption of thought process, such as the intrusion of irrelevant, unimportant thoughts, or sense that thoughts are disappearing, blocked, or coming in very rapid succession.
As these symptoms emerge, individuals may find them confusing, frightening, or unusual, but question their veracity (i.e., some insight is present). Behavior may change or become increasingly bizarre. Individuals identified with a PR syndrome tend to be help-seeking, though typically for non-specific or comorbid concerns.21, 22 They have high rates of cognitive complaints (e.g., concentration or memory problems) and negative symptoms (e.g., low motivation or social anhedonia).11, 23 Longstanding or worsening functional difficulties are also common.24, 25 The vast majority has had previous treatment with psychotherapy or psychotropic medications.26 In the second phase of the North American Prodrome Longitudinal Studies (NAPLS 2) sample, treatment had been initiated, on average, three years prior to the onset of a PR syndrome.27 Prior school accommodations and intensive interventions, including hospitalizations, are also common. Even those who do not develop a psychotic disorder have high rates of diagnosable psychopathology and functional difficulties.28, 29
Risk Identification Methods
Despite their phenomenological similarity to psychosis, subthreshold positive symptoms do not portend later psychosis in most individuals.17 In fact, psychotic-like experiences are common, particularly in children and young adolescents, occurring in 5-20 % of the general population.30-32 Thus, screening in the general population with self-report or phone interviews may yield high rates of psychotic-like symptoms, only a very small subset of which correspond to PR criteria on interview.33, 34 The significance of symptom endorsement is dependent on both the screening method and the population screened. Thorough interview assessment of risk by those specially trained is thus important.35
The most commonly used instruments for assessing PR syndromes are semi-structured interviews including the Structured Interview for Psychosis-Risk Syndromes (SIPS), the Comprehensive Assessment of At-Risk Mental States (CAARMS), and the Schizophrenia Prediction Instrument for Adults (SPI-A).2, 19, 20 All elicit time-course, frequency, worsening, and insight and include guidelines for identifying clinical PR syndromes. Symptoms better explained by non-psychotic pathology such as obsessive-compulsive disorder, substance use, or neurological disorder are typically excluded, although this is often difficult to determine. Parent or family interview is helpful to obtain relevant developmental and family history. Finally, assessment of current and past functioning is crucial.36
Risk assessment is complicated by a number of factors. The first of these is age. Although psychotic-like symptoms may first present in childhood, assessment of PR in children remains challenging.11, 18, 37-39 Putatively prodromal symptoms are more common and less predictive of later psychosis.38, 39 PR is typically assessed in adolescence to early adulthood. Yet typical functioning at 13 and 30 differs and normative variability in maturation is broad. Some magical thinking is typical in young teens; embracing of sub-cultural or anti-mainstream trends is not unusual in adolescence. Second, mutually-shared beliefs vary across cultures (e.g., spirits, psychosomaticism). Only symptoms deviating from the person’s cultural norms are considered toward PR. Third, awareness of contextual factors is important for determining the likely veracity of someone’s experience. Sometimes, others are targeting the person for harm. Indeed, many PR have experienced bullying. Finally, since current assessment of PR is based primarily on self-disclosure, the ability of a person to reliably communicate his or her internal experience is critical. This relies, in part, on the assessor’s ability to balance engagement and issues of confidentiality in interactions with youth and family or other informants. Observations and reports of informants vary significantly in quality and accuracy.38, 40
CHARACTERISTICS AND CORRELATES OF PSYCHOSIS RISK
A rich set of data has characterized the PR state using neurocognitive, social function, neuroimaging, hormonal and inflammatory markers, and others not covered here such as electrophysiology. These data are proving useful for: 1. predicting subsequent psychosis transition or function/disability; 2. unravelling possible mechanisms of transition from a PR state to psychosis. The factors discussed here are correlates of risk rather than known causal mechanisms, even those that statistically “predict” later psychosis. Their direct or indirect causal roles are largely unknown. Furthermore, the different domains vary in how often they have been studied (e.g., neurocognition quite a bit, inflammatory measures, infrequently) and the robustness of findings.
Genetic Factors
Evidence for the role of genetic factors in psychotic disorders comes from high concordance rates in the biological relatives of affected individuals, including in those reared apart.41, 42 For example, having a first degree relative with schizophrenia raises one’s risk tenfold, making family history of psychosis one of the strongest risk factors.43 Current evidence from genome-wide association studies suggest that schizophrenia (as other psychotic disorders) does not result from a single gene or genetic abnormality, but rather the complex interaction of hundreds of specific genes.44 Though still in nascent stages, the implication of this work is that individuals could be given a risk score, based on their specific genes. Until this work is complete, family history is still the best proxy for genetic risk.
Neurocognition
Neurocognitive impairments in schizophrenia have been described since the late 19th century.45, 46 The attention, memory, language, motor and executive dysfunctions evident in chronic and first episode schizophrenia, are typically evident well before illness, including in many PR cases.17, 47-54 Genetic and cohort studies evaluating children who later develop schizophrenia, have found impairments in children as young as four years old.50, 51,55-58 In those who develop schizophrenia, there is an increasing developmental lag in fluid intelligence from ages 7 to 13 and in verbal abilities during the teen years.59- 61
Meta-analyses have demonstrated impairments in PR individuals intermediate in severity between healthy controls and first episode psychosis, with significantly greater impairment in those who convert.17, 54 Verbal memory and processing speed deficits are two of the most sensitive measures predicting transition to psychosis.54, 62 Verbal memory has been associated with shorter transition to psychosis.62 Both cognitive functions contribute modestly but independently to the predictive algorithm for transition to psychosis developed in NAPLS 2, with areas under the curve approximately 0.60.63 (Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for psychosis. Submitted manuscript under review.) There is a relatively modest overlap between neurocognition and social functioning, and somewhat stronger relationships between neurocognition and negative symptoms, typically with no more than 10% of the variance shared. 64,65
Surprisingly, the literature, while sparse so far, does not support the idea that neurocognitive impairment progresses from the prodromal phase to the first episode.66, 67 Future work should address heterogeneity of neurocognitive profiles, longitudinal trajectories, and the combined impact of neurocognition and social cognition, the latter a factor of growing importance.68
Social Functioning
Social functioning is a well-known impairment in those at-risk for schizophrenia.69 Difficulties often begin in early childhood and decline further in the period leading up to the first episode. In fact, features like difficulty inferring the intentions of others (theory of mind) have been part of the associated criteria for schizophrenia diagnosis for some time.3 In PR samples, social difficulties have incremental validity in predicting transition to psychosis, even accounting for attenuated positive symptoms, and are a central aspect of persistent functional difficulties, regardless of transition.15, 28, 35, 36, 70-72 The modest overlap among neurocognition, negative symptoms, and social functioning suggest that these domains make substantially separate contributions to the course and outcome of CHR individuals.64,65
Neuroimaging
There is substantial evidence of gray matter (GM) abnormalities prior to the onset of psychosis in individuals at FHR and at PR.73,74 FHR youth ages 8 to 30 have substantial GM volume abnormalities compared to controls, with an accelerated volume reduction over time in association with psychotic-like symptoms and cognitive deficits. Prefrontal cortex (PFC) alterations are the most consistently reported, followed by smaller hippocampal volume.74
Brain structural alterations in PR samples are generally neuroanatomically similar to, but less severe than reported in established schizophrenia.73 Compared with controls, PR groups show both smaller GM volume and cortical thinning in PFC, lateral temporal cortex (particularly superior temporal gyrus [STG]), and, to a lesser extent, parietal cortex. Less PFC GM has been associated with impaired executive function and greater severity of symptoms in CHR whereas smaller STG GM has been linked with deficits involving semantic fluency.75-77
Evidence of progressive loss of gray matter in PR subjects who convert to psychosis implicates disturbances in developmental neuromaturational processes in the onset of psychosis. 78-84 This was observed most definitively in the NAPLS-2 multisite study.85 Thirty-five PR subjects who transitioned to psychosis showed a steeper rate of gray matter loss in the right superior frontal, middle frontal, and medial orbitofrontal cortical regions as well as a greater rate of expansion of the third ventricle than 135 healthy controls or 239 PR subjects who did not. These findings were associated with baseline levels of an aggregate measure of proinflammatory cytokines in plasma (see below) and were comparably observed in those on or not on antipsychotic medications.
Inflammation
In recent years, there has been increased recognition of the role of inflammatory processes in neuropsychiatric disorders, including psychosis.86-90 Patients with schizophrenia have altered levels of inflammation, oxidative stress and metabolism.91 In the absence of direct in vivo measures of neuroinflammation, diffusion tensor imaging (DTI) provides indirect evidence, at least in first episode schizophrenia, of excess “free water”.92 The absence of this finding in preliminary studies of people with chronic schizophrenia has led to the hypothesis that an acute inflammatory process may occur during the onset of psychosis, that wanes over time in response to antipsychotic medications (known to be partially anti-inflammatory) or as a result of the natural evolution of the illness. During the PR phase, an index of plasma analytes reflecting inflammation, oxidative stress, hormones and metabolism, has differentiated PR individuals who developed psychosis from controls and PR individuals who did not develop psychosis.93 Inflammation, oxidative stress, and dysregulation of the hypothalamic-pituitary axis (HPA) are promising markers, but replication and further research are needed.
Stress, Stress Sensitivity and Cortisol
The generally accepted “diathesis/vulnerability-stress” model suggests that individuals at risk for or suffering from a psychotic disorder may be more vulnerable to stress and that stressors precipitate psychotic episodes. Reviews of the literature have generally revealed inconsistent evidence for higher rates of stressful life events in samples at PR or with established psychotic disorders than in comparison groups.94 While there is increasing evidence that childhood adversity, including physical and sexual abuse, or bullying, are associated with PR, these stressors often occur years before the onset of psychotic symptoms and may or may not play a direct causal role.95-98 However, PR individuals tend to experience events as more subjectively stressful than non-PR individuals, suggesting that the prodromal period may be a phase of heightened subjective stress and stress sensitivity.94, 99, 100
Cortisol, a hormone produced in response to stress via activity of the HPA axis, has been implicated in both the vulnerability to and maintenance of psychosis. As cortisol is both elevated and dysregulated in psychotic disorders such as schizophrenia, there is interest in understanding whether the HPA axis plays a role in triggering psychotic symptoms and disorders. Indeed, PR subjects tend to manifest higher baseline cortisol that correlates with psychotic symptom severity.101-103 In the NAPLS-2 study, significantly higher baseline cortisol was found in PR subjects who later transitioned to psychosis than in controls or those whose clinical PR symptoms remitted over a two year follow-up.103
Environmental Risk Factors
Environmental risk factors have long been implicated in the development of psychosis. Perhaps the strongest evidence comes from twin studies that find a roughly 50% schizophrenia concordance rate among monozygotic twins, meaning that factors other than genes contribute to illness. 104 In fact a number of environmental factors are associated with higher than normal psychosis incidence. Broadly, these fall into three categories: those thought to affect neural and other physical system development, those that contribute to early or chronic adversity, and factors that exert effects later in life.
Among those likely to affect neuromaturation, increased incidence of schizophrenia has been associated with: complications of pregnancy like maternal bleeding, diabetes, preeclampsia, or rH incompatability; abnormal fetal development (including malnutrition or exposure to virus); and delivery complications like asphyxia, hypoxia, emergency C-section, and forceps delivery.51 Maternal exposure to a number of viruses and stressors and advanced paternal age also increase risk for psychosis, the latter believed to be related to increased risk of genetic aberrations.105-107
Other risk factors likely exert their effect by increasing the adversity of the environment (presumably increasing individual stress and/or decreasing access to resources or protective mechanisms for normative development). Higher incidence of psychosis has been found in developing countries, cohorts raised in urban areas, and minority groups that either migrated or live amongst majority groups.108-112 Family factors such as communication deviance, hostility, and criticism have also been linked to increased risk for later psychosis.113, 114 Specific mechanisms reflected by these environmental PR factors continue to be debated, though they likely interact with biological factors.105,115, 116
Finally, a few environmental factors contribute to risk more proximally to the onset of psychosis, as noted in the discussion of stressful life events. In addition, substance abuse, particularly stimulants and cannabis, may be precipitants of first or subsequent psychotic episodes.117, 118 However, their causal role is debated.119In fact, a positive link between substance use and later conversion to psychosis is infrequently found.120 In the first phase of NAPLS (N =370), PR subjects with cannabis abuse or dependence had higher rates of conversion and converted sooner than non-disordered users and non-users.121 However, this finding did not hold when comorbid alcohol use was considered.121 Outcome and transition to psychosis may be more strongly associated with early-onset use, frequent use and continued use rather than overall lifetime use.122 More data from targeted intervention trials and usage over the time to transition or remission will be valuable in determining whether cannabis use is a potentially modifiable risk factor.123
Protective Factors
Whereas the opposites of factors that increase risk might be considered protective (e.g., normal pregnancy and delivery, low communication deviance), demonstration of buffering against risk requires a reduction of incidence in the context of risk. 124 A few factors with some evidence for such a protective effect include family environment, estrogen, and psychosocial and psychopharmacological treatments. For instance, Tienari and colleagues’ longitudinal study of familial high and low risk adoptees found that particularly benign adoptive family environments normalized the rates of schizophrenia spectrum outcomes in familial high-risk adoptees.113 Preliminary support for this in PR samples comes from the association of caregiver warmth and moderate parent involvement with symptom and functional improvements over time.125,126
Estrogen is widely believed to have neuroprotective effects in schizophrenia, including protection against oxidative stress and inflammation.127 Evidence comes from its interaction with the major neurotransmitter systems implicated in schizophrenia, animal research suggesting it may enhance cognition and reverse deficits reflective of the symptom and cognitive deficits of schizophrenia, and from clinical trials demonstrating additive effects when combined with antipsychotics. Its potential in PR samples is unknown. However, other treatments, including psychosocial therapies and psychopharmacological agents, to the degree that they reduce known risk, serve a protective role in PR individuals. Many of them explicitly target individual, environmental, and neuroprotective factors. These are discussed in more detail later on.
IMPROVING RISK DETECTION AND ASSESSMENT
As outlined above, but in contrast to the common dialogue, PR is not dichotomous. Rather, individuals vary along a somewhat fluid continuum in which individual and environmental risk factors interact over time (see Figure 1).128, 129 A challenge for the field is to draw on group data to estimate risk in individuals. The goal is to maximize sensitivity, the accurate identification of PR when present, and specificity, the avoidance of PR identification when it is not present. In assessing the value of an assessment tool or risk algorithm, Positive Predictive Value (PPV) is often used, here defined as the percentage of individuals identified as PR who subsequently develop psychosis. A PPV of 75% indicates that three-fourths of those identified as PR develop psychosis within the given follow-up. Percentages depend on the prevalence of PR and psychosis in the population sampled. Thus, the PPV of a given interview in help-seekers of a specialized PR center will differ from the PPV of that same interview in a public school.
PR is a probabilistic designation given to people along varied developmental trajectories. Identifying individuals as at PR or treating them within a specialized psychosis clinic may, in itself, negatively impact those trajectories.130 Given this and some evidence that PR syndromes have become less predictive of transition over time, there is increased interest in targeting prevention strategies to those most at-risk.131 As evidence accumulates, risk stratification algorithms are being developed to estimate risk based on individual combinations of predictive biomarkers and clinical factors.129 Treatment could then be staged, with more sensitive, broader risk indicators (like PR status) identifying larger groups for clinical attention and more specific and higher risk indicators prompting interventions of increasing intensity or risk.132 Thus, in the future, identification of a PR syndrome might be followed by neuropsychological testing, EEG, biosampling, and MRI assessment, with indicators from each contributing to an actuarially derived estimation of risk.85, 133
A small number of studies have investigated clinical factors that add to prediction of psychosis over and above CHR or COGDIS criteria (which improve prediction when combined).36,134 These multi-step algorithms yield PPVs around 80%, but have rarely been replicated.36, 135, 136 For example, in NAPLS 1, three to five risk indicators (GRDS, see Table 1, unusual thought content, suspiciousness/paranoia, social impairment, substance abuse history) increased the PPV from 35% (PR status alone) to 68-80%.36 Similarly, in the Orygen Youth Health program in Australia, the presence of at least one of the following in addition to PR increased PPV to 80.8%: 1) being in both the vulnerability and attenuated psychosis groups (see Table 1) 2) symptoms present over 5 years, 3) GAF score less than 40, or 4) significant inattention.137 In the European Prediction of Psychosis Study, a PPV of 83.3% was reached when overall subthreshold positive symptoms, bizarre thinking, sleep disturbance, schizotypal personality disorder, lower level of recent functioning, and/or lower education were added.135 Other studies have presented comparable results.138 Thus, specific positive symptoms and poor and/or decreasing social and role function may be indicators of greater risk in those manifesting PR syndromes.
A smaller number of studies have investigated how biomarkers might improve risk algorithms. The FePsy study, for example, found that neuroanatomical patterns generated from a PR sample improved prediction of psychosis in two novel samples, again correctly identifying roughly 80% of converters.139 Neurocognition also appears to add to risk algorithms, with verbal learning/memory and executive functioning most consistently adding incremental predictive power to CHR indicators—again with PPVs hovering around 80%.138,140 Nieman and colleagues found that adding parietal P300 amplitude, an event related potential measure, and premorbid adjustment to PR status increased PPV to 70%.141 Markers of neuroinflammation, oxidative stress, and dysregulation of the biological stress response system also appear to be promising biological markers of increased risk.103, 142
The future of this work is in the development of “risk calculators” that generate a probabilistic estimation of risk for individuals.143, 144 A few attempts to use retrospectively generated algorithms to generate prospective prognostic classification systems have generally been encouraging, but systems of mathematical risk stratification are intimately tied to the statistical parameters and individual characteristics of the samples on which they were generated.15,141 In the NAPLS study, information learned from NAPLS 1 and the literature at large has been applied a priori to NAPLS 2 with promising results.143 This next step will require replication of results, more work combining biological and clinical risk indicators, and a better understanding of the role that protective factors and treatment play in reducing risk.136,140 Given what we already know about equifinality and multifinality in development and psychopathology, the future of PR detection will likely be painted in dynamic and pluralistic strokes, weighing clusters of clinical, biological, and environmental factors, rather than the dichotomous presence or absence of syndromes.
INTERVENING IN PSYCHOSIS RISK
The argument for intervention prior to diagnosable psychotic disorder is based not only on the potential for preventing or minimizing incipient psychotic illness, but also on the distress, impairment, and often treatable comorbid conditions that accompany PR.21 Intervention efforts have included a range of targets and strategies, pharmacological and psychosocial, commensurate with the diversity of risk factors and presentations. Given the implications of successful early interventions, there has been a drive to secure evidence for clinical efficacy and cost effectiveness.145-148 This eagerness, however well intentioned, has been countered by calls for caution and careful consideration of potential risks.149, 150 In this section, we review the overall evidence for intervention with PR samples, the core treatments and strategies tested to date, innovations currently under investigation, and recommendations for ethical practice and future research.
There have been 13 randomized controlled trials (RCT) testing experimental interventions with PR samples (see Table 2). Two tested pharmacological agents alone.151, 152 Nine tested psychosocial interventions alone.153-161 Two tested antipsychotic medications in conjunction with psychosocial treatments.155, 162
Table 2.
Treatments Tested in Randomized Controlled Trials (RCT) of PR Samples
| Treatment | Treatment Targets | Specific Studies: Evidence, Methodological Limitations, Comments |
|---|---|---|
|
Risperidone 0.5-2 mg/day 6-12 months |
APS/Transition |
McGorry et al. (2002)162: Risperidone + CBT + Needs Based Intervention (NBI) vs. NBI; Mean dose = 1.3 mg/day; RR = 0.54; Both groups improved on symptoms and functioning; Effects of medication and CBT are confoundedŧ; Assessors not blinded |
|
McGorry et al. (2013)155: Risperidone + CBT vs Placebo + CBT; Dose up to 2mg/d ; RR= 0.76; All groups improved similarly on symptoms & functioning; Large sample; ⅓ dropped out of study; Poor study medication adherence; High rates of antidepressant use all groups | ||
|
Olanzapine 5-15mg/day 12 months |
APS/Transition |
McGlashan et al. (2006)152: RR= 0.43; EG showed nearly significant greater improvement in APS than CG; Both groups improved significantly on functioning; Only 1/5 completed study; EG reported higher rates of fatigue and weight gain; Participants had high rates of family mental illness so results may not generalize to other groups |
|
Omega 3 Fatty Acids 1.2 g/day, 12 weeks |
APS/Transition; Negative Sxs; Health/Wellbeing |
Amminger et al. (2010)151: RR= 0.18*; EG showed superior improvement on both symptomatic and functional outcomes; Good adherence in both groups Note: A second unpublished RCT by the same group has failed to replicate this finding148 |
|
Cognitive Behavioral Therapy (CBT) 20- 26 sessions 6-12 months (includes models that are primarily cognitive therapy, CT) |
APS/Transition; Distress; Negative Sx; Health/Wellbeing |
McGorry et al. (2002)162: Risperidone + CBT + NBI vs. NBI (See above) RR = 0.54; Fidelity not reported. Contribution of CBT unknownŧ |
|
Morrison et al. (2004)157: Comparison: monthly monitoring; RR= 0.21* - 0.25; EG had reduced likelihood of transition, reduced APS, and being prescribed anti-psychotics, but not functioning; Mostly men; Treatment fidelity not reported; Assessments not fully blinded | ||
|
Addington et al. (2011)153: Comparison: ST; RR= 0.13; Both groups improved in APS, depression, anxiety, but EG improved more rapidly in APS; No group improved in negative symptoms or functioning. Fidelity to CBT adequate. Raters blinded. ⅓ dropped out | ||
|
Morrison et al. (2012)158: Comparison: TAU + monitoring; RR= 0.70; EG had significantly lower APS; No group differences in transition rates, distress, functioning, depression, social anxiety or quality of life. Large sample; Rater blinding fair; Generally adherent CBT | ||
|
Van der Gaag et al. (2012)161: CBT + TAU vs. TAU; RR= 0.47*; Fewer EG than CG transitioned; EG lower in distress and feeling entrapped; Groups did not differ on depression, anxiety, quality of life or social functioning; Therapists generally competent | ||
|
Bechdolf et al. (2012)154: CBT part of Intensive Psychological Intervention (IPI), other components are mentioned below; RR= 0.05* for entire IPI; Contribution of CBT unknownŧ | ||
|
McGorry et al. (2013)155: CBT + placebo vs. ST + placebo; RR= 0.74 ; No significant group differences in transition rates, symptoms or functioning, although all improved (see above) | ||
|
Family Focused Therapy 18 sessions 6 months |
APS/Transition; Family Functioning; Negative Sxs; Health/Wellbeing |
Miklowitz et al. (2014)156: CG received 3 sessions psychoeducation; EG had significantly greater improvements in APS than CG and more role improvement if participant age ≥ 19; CG showed more role improvement in participants between ages 16 and 19; O’ Brien et al. (2014): (same trial as above) EG had greater improvement in constructive communication and greater reduction in conflict; ½ did not complete 6 months |
|
Integrated Treatment Assertive Community Treatmentŧ, Multifamily Psychoeducationŧ & Social Skills Trainingŧ |
APS/Transition; Distress; Family Functioning; Negative Sxs; Health/Wellbeing |
Nordentoft et al. (2006)159: 2 years of treatment; compared with community care; RR= 0.26*; Integrated significantly better than standard care at reducing negative symptoms; Relative contribution of components and medication unknown; Schizotypal personality disorder sample, so atypical PR; Treatment fidelity not reported; Assessors not blinded |
|
Bechdolf et al. (2012)154: 12 month intervention; IPI also included individual CBT and CR noted elsewhere; comparison was supportive counseling; RR= 0.05* for entire IPI, relative contributions of components unknown; Treatment fidelity not assessed; Assessors not blind | ||
|
Cognitive Remediation (CR)/Enhancement |
Cognitive Difficulties |
Bechdolf et al. (2012)154: 12 sessions Cogpack; RR= 0.05* for entire IPI (see above); Contribution of CR unknownŧ |
|
Piskulic et al. (2015)160: 40 hours Brain Fitness over 10-12 weeks vs. computer games; No significant EG vs CG differences in cognition; Significant improvement in social functioning in EG and in working memory in CG during follow-up; ½ of EG, ⅓ CG dropped out |
Notes: APS/Transition = Attenuated Positive Symptoms/ Transition to Psychosis
EG = Experimental Group
CG = Control Group
IPI = Integrated Psychological Intervention
Negative Sxs = Negative Symptoms
RR = 12 month relative risk, risk for transition to psychotic disorder of EG relative to CG (1 = equal risk in both groups) reported by van der Gaag et al., 2013 and Schmidt et al., 2015
ST = Supportive Therapy
TAU = treatment as usual
RR significantly different from 1.00, p < 0.05
Treatments or strategies without specific evidence because they are components of experimental treatment packages
The most recent meta-analyses have found a significant pooled impact on reducing the risk for transition to psychosis.146-148 This effect is strongest in the short-term but remains significant over 2 years and longer. The mean relative reduction in risk (RR) was 64% at 6 months, 54-56% at one year, and 35-42% at 2-4 year follow-ups (only 5 studies).146-148 These equate to the prevention or delay of one transition to psychosis for every 9-15 individuals treated (number needed to treat, NNT).
At this point, no individual treatment stands out as more efficacious than another, meaning there is no gold standard treatment for PR.145-148 Similarly, pooled effects of both psychopharmacological and psychosocial treatments show a reduction of risk for both, with the NNT = 7, 13 respectively, which is very promising.146 Results have been less robust for samples with a mean age under 18.148 Unfortunately, results for functional outcomes have been not as strong. Although all groups improved functionally over time, there were no significant differences between experimental and control or between psychosocial and pharmacological treatment effects at the meta-analytic level.146, 148
The Nature of Current Interventions
Pharmacological (see Table 2)
In the United States, a substantial minority of PR youth is prescribed antipsychotic medications; more receive a wide range of other psychotropic agents.163 This is true in specialized clinics as well as the community. Four published RCTs examined pharmacological agents, including a nutritional supplement, in the treatment of PR youth (Risperidone, 0.5-2mg; Olanzapine, 5-15mg; Omega 3 Fatty Acids, 1.2 g). Active treatment ranged from 2 to 12 months with a mean follow-up of 15 months. Drop-out rates ranged between 13 and 55% and adjunctive psychosocial treatments were only partially controlled.148
Psychosocial (summarized in Tables 2 and 3)
Table 3.
Psychosocial Treatment Strategies for Individuals at High Psychosis Risk
| Treatment | Common Strategies Described in Manuals and Manuscripts |
|---|---|
| Cognitive Behavioral Therapy (CBT, including cognitive therapy, CT) |
|
| Family Focused Therapy (FFT) |
|
| Case Management |
|
| Assertive Community Treatment (ACT, typically modified from standard model for chronic mental illness) |
|
| Multifamily Group Psychoeducation (MFGPE) |
|
| Cognitive Remediation/ Enhancement (CR) |
|
| Social Skills Training (SST) |
|
| Supported Employment/ Education |
|
| Supportive Therapy (ST) |
|
| Needs Based Intervention (NBI) |
|
| Crisis Intervention |
|
Note: Neither treatments nor strategies are generally exclusive. There is both overlap in the strategies across different treatments and inclusion of some treatments within other treatments. Exceptions occur in the case of specific comparisons, e.g., of CBT and supportive therapy in which fidelity to supportive therapy prohibits use of specific CBT strategies. PR = Psychosis Risk
Across trials, individuals received, on average, seven months of psychosocial therapy (range: 2-12 months), and were followed up for another 17 months (range 2-48 months).148 Drop-out rates were modest (15 to 45%).148 Some limitations are noted. First, studies varied widely in the degree of quality controls such as fidelity assessments and blinding of assessors, critical to interpreting their findings (see Table 2 and meta-analyses for details). Second, in many cases, individuals were allowed to receive supplemental therapies (including evidence-based care for comorbid disorders). Thus, there has been very limited control for a number of potentially therapeutic factors other than the experimental treatments.
Cognitive and cognitive behavioral strategies (collectively referred to as CBT) have been studied more than any other psychosocial intervention. Although there are meaningful differences between models, all involve some common elements (see Table 3). They differ in the degree to which they focus specifically on positive symptoms versus mood, stress, or functioning and the degree to which positive symptoms are normalized. CBT has established efficacy for common comorbidities such as anxiety and depression, whose symptoms are reported to be most distressing. 21, 164 CBT, therefore, has potential benefits for addressing symptoms and functioning both specific and non-specific to PR.
Common adjunctive treatments include Case management, assertive community treatment (ACT), and Crisis management or intervention. These include intensive multidisciplinary team-based approaches and assertive efforts to enhance engagement, provide in vivo treatment, and maximize independent living skills, treatment compliance, and client satisfaction (e.g., Early Psychosis Prevention and Intervention Centre [EPPIC], Modified ACT and Family-Aided Community Treatment [FACT]).159,165
Family therapies are another logical treatment choice for PR youth due to their robust efficacy with established psychotic disorders, high rates of help-seeking by families of PR youth, the legal and financial dependence of many PR youth on their families, and the potential for families to buffer the impact of environmental stressors.113, 114 With PR, both single and multiple family group formats have been employed. Family focused therapy (FFT) is a six-month treatment and the only family treatment specifically tested in a published RCT. It focuses on psychoeducation, communication skill-building, and problem-solving.156 Integrated treatment models have included multifamily psychoeducational groups, either 3 sessions in conjunction with other treatments, or more comprehensive models, including intensive psychoeducation and bi-weekly group meetings for 1-2 years.165
Cognitive remediation/enhancement (CR) has been investigated in a few pilot studies with PR youth, given the relationship of cognitive difficulties to functional outcomes. 154, 160, 166-169 Building on demonstrated efficacy with established illness, CR with PR has used established computerized cognitive remediation programs like COGPACK, Lumosity, or PositScience.170,171 These programs provide training in cognitive domains such as attention, speed of processing, executive function, learning and memory, and social perception. Individuals typically complete repeated training sessions multiple times/week. The Brain Fitness Program (BFP), and trials of Lumosity and SocialVille are examples. social skills training, either individual or via groups, also targets cognition and functioning, including social perception, social skills, and skills for living and well-being (e.g., Integrated Psychological Intervention).139, 154, 167 Supported Employment and Education directly assist individuals in finding and maintaining appropriate work or school participation. Both are included in FACT.165
Supportive therapy, although typically provided to those in the comparison groups of randomized trials, has been widely offered to PR youth, with sometimes strikingly similar outcomes as the experimental treatments.172 It is usually distinguished by the absence of cognitive behavioral techniques and sometimes by a lower frequency of sessions (e.g. monthly or as needed rather than weekly). Similarly, needs-based intervention (NBI) specifically focuses on presenting symptoms and pertinent social, family, and vocational issues. In several trials, these therapies provide support and education to families in addition to crisis and case management.151
Not surprisingly, given the range of ages, functional difficulties, and contextual factors relevant to treating PR youth, there have been a number of efforts to provide integrated treatment. The earliest model was developed by EPPIC in Australia. This model integrated CBT, low-dose risperidone, needs-based case management, and pharmacological treatment of comorbid disorders.162 Other examples include the OPUS program in Denmark (assertive community treatment, social skills treatment, and multi-family group psychoeducation), and an integrated intervention for the “early initial prodromal state” in Germany (individual and group CBT, cognitive remediation, and family psychoeducation).133 A widely disseminated American model of integrated treatment tested in a quasi-experimental trial is FACT (multifamily group psychoeducation, modified assertive community treatment, supported employment and education, and pharmacological treatment by protocol).165 Important questions remain. Is more psychosocial treatment better than less? And does personalizing treatment components to specific needs enhance outcomes?
Innovations Under Investigation
A number of countries have initiated substantial reform of youth mental health services to enhance early intervention or have redirected funding to new interventions.173-175 Preliminary trials have examined pharmacological agents including amisulpride and aripiprazole, more traditional medications (antidepressants, lithium) as well as more benign agents (glycine, aspirin, D-serine).176-182 Innovative psychosocial interventions being developed and tested include mobile technologies, social networking, exercise-based cognitive remediation, and multiuser biofeedback videogames.183-185 Additional targets of these new interventions include inflammation, healthy brain development, engagement and motivation, generalization and durability of effects, and enhanced effects for younger cohorts. Given their strong evidence-base for addressing functional difficulties associated with established psychosis, adaptations of supported employment and education, social skill-building, and cognitive remediation are also of particular interest. 186
Implications for Benefit-Risk Estimation
The arguments for prevention and early intervention in psychosis are compelling. But, consideration must be given to risks for harm, particularly to individuals identified and treated who would not transition to psychosis, even without intervention. The most serious risks identified have been for antipsychotic medications. Weight gain, sexual dysfunction, and extra-pyramidal side effects can be substantial, pose serious risk to health and well-being, and may be particularly intolerable for adolescents and young adults.187 More challenging to measure is the impact of receiving treatment or being identified and “labeled” as at PR, including the potential for altered self-perception, behavioral choices, and life trajectories, internalized stigma, and anxiety.130 These may vary widely by individual and contextual factors but clinicians and community members may have significant potential to shape their impact.164, 188
Recommendations for PR Assessment and Intervention
A number of international organizations offer guidelines based on current evidence and expert opinion.148, 189-193 Based on these and our review of the literature, we offer the following recommendations to clinicians:
1) Do not ignore early signs and symptoms of PR. They warrant proper assessment and monitoring over time as early detection can make a meaningful difference in a person’s clinical trajectory.146, 148 It is increasingly accepted that psychosocial, in particular CBT, and pharmacological interventions can at least delay the onset of acute psychosis in PR.
2) PR status should be assessed by trained clinicians according to recommended guidelines prior to intervention with a specific psychosis-prevention focus.18, 194
3) Particularly in cases of children and younger adolescents, rapidly progressing, impairing, or distressing symptoms, atypical presentations, developmental disorders, and individuals with significant substance use or possible medical/neurological complexity, we recommend a comprehensive multidisciplinary and multidimensional assessment that carefully considers not only current risk and protective factors, but their trajectories over time.
4) Comorbid conditions such as anxiety, depression, and substance abuse should be treated first, based on available evidence and guidelines for those conditions. Comorbidities may cause or contribute to attenuated psychotic symptoms and PR. Appropriate caution should be followed in providing any treatments with potential to increase PR such as stimulants.
5) Psychosocial and low risk pharmacological agents (CBT, family therapy, omega 3 fatty acids, case management and crisis intervention) should be offered prior to riskier and more intensive treatments (in particular antipsychotic medication).
6) Antipsychotics are recommended only when initial treatments have proved ineffective, in the case of severe and rapid worsening of positive symptoms, when these symptoms are associated with significant deterioration of functioning or risk to self or others, or when symptomatic stabilization is needed for psychosocial treatments to be effective.148, 194
7) Clinicians should be thoughtful and use good judgment in providing feedback and education and in negotiating a treatment plan appropriate for each individual and family’s cultural context, values, and unique set of risk and protective factors.
8) Cognitive, social, and occupational functioning should be primary treatment targets, not just symptoms. Until evidence is available to support specific strategies, clinicians are encouraged to draw upon evidence-based strategies for functional difficulties in the context of established disorders such as supported employment or education, social skills training, CBT, and cognitive remediation.
9) Diet and lifestyle are also important treatment targets, particularly when there is substance misuse, including nicotine, and when individuals are on medications with risks for metabolic side effects. Diet, sleep, exercise, regular schedule, and general good health habits can directly impact, not only symptoms, but overall wellbeing.195
10) As with all good clinical care, clinicians should monitor symptoms, functioning, and safety regularly and seek consultation when treatments are ineffective.
CONCLUSIONS AND IMPLICATIONS
Although the vision of preventing psychosis is not new, the potential to realize this vision has taken form in recent decades.196 Progress can be marked in three major advances. The first is development of tools and criteria for identifying young people at imminent risk for a major psychotic disorder. The second is the longitudinal examination of symptomatic, psychobiological, and functional changes over time, particularly in individuals who transition to diagnosable illness, offering insights into possible causal pathways and mechanisms. The third is the piloting of interventions to prevent or delay this transition, reduce distress and suffering, and improve long term social and occupational functioning for those at PR.
Currently, specialized structured interviews identify individuals with roughly a 30-35% risk of transitioning to a psychotic disorder over 3 years or more (Table 1).17 Consideration of additional factors, such as social functioning and family history of psychosis can aid in identifying those with the highest risk. Risk calculators incorporating psychobiological markers are being developed to improve assessment of PR at the individual level. We expect that, within the next decade, these tools will be able to incorporate measures of change over time (e.g. gray matter volume loss) to further improve accuracy and detect more heterogeneous pathways to illness.
Improved risk assessment could also benefit from further research in three areas: 1) PR in individuals under age 18, particularly in the context of comorbid developmental disorders, 2) resilience and protective factors and 3) improved PR screening in community settings. 18, 34, 195 Although a significant proportion of individuals with schizophrenia experience the onset of this disease before age 18, current assessment tools have unknown or reduced predictive value in this age group.37, 197 Expansion of population-based research in this age group is needed to better distinguish PR indicators from normative experience at different ages.34 Careful prospective and longitudinal assessment of resilience and protective factors, such as is being conducted in the Philadelphia Neurodevelopmental Cohort, are also needed to understand how these moderate PR and different outcomes.38 Finally, our capacity to detect PR in non-help-seeking individuals will rely on improved screening tools for use in general population or less-specialized treatment settings. Longitudinal population-based screening studies will be essential to developing and refining these tools.10, 34, 38, 39
One of the most important advances possible with PR identification has been in the capacity to measure psychobiological, clinical, and environmental changes leading up to the full expression of a psychotic disorder. The first step has been the identification of clinical and biological indicators associated with later transition to psychosis. Currently, the most promising of these are social deficits and a decline in social functioning, deficits in verbal memory and processing speed, excessive cortical thinning in the right superior frontal, middle frontal and medial orbitofrontal regions, elevated cortisol, and plasma proinflammatory cytokines.26, 103, 134, 136-142 Additional steps are now being taken to understand the impact of both individual and environmental risk factors on neuromaturation and symptoms. For instance, both animal studies and longitudinal studies of child development are beginning to converge on potential mechanisms by which adversity may have its impact.198, 199 Although much of this work may relate to risk for broad psychopathology, insights into psychosis-specific mechanisms are emerging.
One of the most exciting prospects of intervention trials is that they may both alter outcomes and reveal modifiable risk factors and mechanisms of illness progression. For instance, if CBT can significantly reduce rates of transition to psychosis, conceptualizations of psychotic disorders need to better integrate the impact of cognitive and behavioral factors on biology. Of course, mechanisms that reduce symptoms may not be the same as those that produce them. But part of the impact of environmental factors may be in their shaping of a person’s subjective response to both internal and external events. The diversity of clinical presentations, trajectories, and risk factors implicates a complex cascade of causal mechanisms, a puzzle whose pieces will come together only with the large collaborative work now being established.
Can we prevent psychosis?
Current evidence suggests that we can reduce risk for transition to psychosis in the short-term.146-148 Longer follow-ups of large samples are needed to truly test prevention. Risk reduction with psychosocial treatment compares quite favorably to that of pharmacological treatment (e.g., 57% vs. 55% at one year, 48% vs. 34% at two years).148 Given concerns about side effects and intolerability of antipsychotics, particularly in young people whose brains and identities are still developing, this is quite encouraging. It reminds us of the very real potential of developing and enhancing interventions with families, schools, and communities.193
With increased attention to the development and testing of early interventions world-wide, there is opportunity to advance intervention capacity in several ways. First, we need to test specific theories of change. Intervention studies should be designed to assess hypothesized mechanisms of change, including three or more assessment time points and assessment of temporal and dose-response relationships to outcomes. Second, we need to better understand moderators of treatment engagement and outcomes to move toward personalized medicine. These should include demographic, psychological, biological, and psychosocial factors. Third, we need to better address age and stage. Needs, meaning, resources, and treatment seeking vary significantly from childhood to early adulthood. New developments are testing youth-friendly messages, settings, technology, and social networking. Staging and personalized care may be particularly important for the heterogeneity found in PR groups. Low stigma, wellness-oriented, and need-based care may be sufficient for those with fewer and milder symptoms, with more intensive, psychosis-specific treatments reserved for those with more severe, impairing, and distressing symptoms. Fourth, evidence for the role of the environment in the onset and recurrence of psychotic symptoms suggests that this may be an underutilized intervention target. The predominant focus continues to be on the individual. Discrepancies in risk identification and help-seeking by race, income, education, and culture support broad reform efforts to combat stigma, raise public mental health literacy, and create more protective educational, occupational, community, and living situations across entire populations. 26, 193, 200
Prevention of acute psychosis and psychotic disorder, although worthy goals, may be less important than the prevention of disability. Many PR individuals have significant disability even in the absence of a transition to psychosis.28 With the increase in attention to functional outcomes and the often harder to treat factors, such as cognition, that predict them, it will be important to work toward smarter treatment, not just more treatment. This will mean providing the right treatment to the right person at the right time. We have a lot to learn to approach this goal, but there is more hope for an individual at PR today than ever before.
Acknowledgments
Source of Funding:
This work was supported by funding from NIH: NIMH K23 MH102358-01A1 (KAW); U01 MH 081928-06 (LJS & DIS), NIMH R01 MH096027 (LJS, DIS, and CB), NIMH R01 MH101052 (LJS & DIS), NIMH RO1 MH 105246 (LJS & DIS).
Footnotes
Conflicts of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Contributor Information
Kristen A Woodberry, Beth Israel Deaconess Medical Center Harvard Medical School
Daniel I Shapiro, Beth Israel Deaconess Medical Center Harvard Medical School
Caitlin Bryant, Beth Israel Deaconess Medical Center
Larry J. Seidman, Beth Israel Deaconess Medical Center Harvard Medical School
REFERENCES
- 1.McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull. 2003;29:771–90. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–87. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- 4.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 5.Schultze-Lutter F, Ruhrmann S, Hoyer C, Klosterkötter J, Leweke FM. The initial prodrome of schizophrenia: different duration, different underlying deficits? Compr Psychiatry. 2007;48(5):479–88. doi: 10.1016/j.comppsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Simon AE, Velthorst E, Nieman DH, Linszen D, Umbricht D, de Haan L. Ultra high-risk state for psychosis and non-transition: A systematic review. Schizophr Res. 2011;132(1):8–17. doi: 10.1016/j.schres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Freedman B, Chapman LJ. Early subjective experience in schizophrenic episodes. J Abnorm Psychol. 1973;82:46–54. doi: 10.1037/h0034952. [DOI] [PubMed] [Google Scholar]
- 8.Gross G, Huber G, Klosterkötter J, Linz M. BSABS: Bonn Scale for the Assessment of Basic Symptoms. Springer; Berlin: 1987. [Google Scholar]
- 9.Häfner H, Maurer K, Löffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29(2):325–40. doi: 10.1093/oxfordjournals.schbul.a007008. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez MD, Saka MC, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry. 2010;167(9):1075–82. doi: 10.1176/appi.ajp.2010.09060883. [DOI] [PubMed] [Google Scholar]
- 11.Woodberry KA, Serur RA, Hallinan SB, et al. Frequency and pattern of childhood symptom onset reported by first episode schizophrenia and clinical high risk youth. Schizophr Res. 2014;158(1-3):45–51. doi: 10.1016/j.schres.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodromal phase in schizophrenia. Schizophr Bull. 2002;28(3):415–29. doi: 10.1093/oxfordjournals.schbul.a006950. [DOI] [PubMed] [Google Scholar]
- 13.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–70. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 14.Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35(1):5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67:241–51. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 16.Schultze-Lutter F, Schimmelmann BG, Ruhrmann S, Michel C. 'A rose is a rose is a rose', but at-risk criteria differ. Psychopathology. 2013;46(2):75–87. doi: 10.1159/000339208. [DOI] [PubMed] [Google Scholar]
- 17.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):1–10. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 18.Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30:405–16. doi: 10.1016/j.eurpsy.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 20.Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia Proneness Instrument, Adult Version (SPI-A) Giovanni Fioriti; Rome: 2007. [Google Scholar]
- 21.Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105(1–3):10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68(1):37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 24.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. Risk factors for transition to first episode psychosis among individuals with “at-risk mental states. Schizophr Res. 2004;71(2–3):227–37. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99(1–3):119–24. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadenhead KS, Addington J, Cannon T, et al. Treatment history in the psychosis prodrome: characteristics of the North American Prodrome Longitudinal Study Cohort. Early Interv Psychiatry. 2010;4(3):220–6. doi: 10.1111/j.1751-7893.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodberry KA, Addington J, Bearden CE, et al. Psychosocial treatment prior to identification of clinical high risk status: characteristics of the North American prodrome longitudinal study (NAPLS)-2 cohort. Schizophr Bull. 2015;41(Supp. 1):S340. [Google Scholar]
- 28.Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–5. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172(3):249–58. doi: 10.1176/appi.ajp.2014.13030418. [DOI] [PubMed] [Google Scholar]
- 30.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42(09):1857–63. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 31.Morgan C, Fisher H, Hutchinson G, et al. Ethnicity, social disadvantage and psychotic-like experiences in a healthy population based sample. Acta Psychiatr Scand. 2009;119(3):226–35. doi: 10.1111/j.1600-0447.2008.01301.x. [DOI] [PubMed] [Google Scholar]
- 32.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 33.Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(2):181–91. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- 34.Kaymaz N, Drukker M, Lieb R, et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–53. doi: 10.1017/S0033291711002911. [DOI] [PubMed] [Google Scholar]
- 35.Nelson B, Yuen HP, Wood SJ, et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- 36.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schimmelmann BG, Walger P, Schultze-Lutter F. The significance of at-risk symptoms for psychosis in children and adolescents. Can J Psychiatry. 2013;58:32–40. doi: 10.1177/070674371305800107. [DOI] [PubMed] [Google Scholar]
- 38.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schimmelmann BG, Michel C, Martz-Irngartinger A, Linder C, Schultze-Lutter F. Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: Findings from the BEAR and BEARS-kid studies. World Psychiatry. 2015;14(2):189–197. doi: 10.1002/wps.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crlenjak C, Ratheesh A, Blaikie S, Dodd S, Hughes A, Simpson R. ‘Let me understand…’ assessment in early psychosis. Orygen Youth Health Research Center; Melbourne, Australia: 2014. [Google Scholar]
- 41.Tsuang MT, Stone WS, Faraone SV. Genes, environment and schizophrenia. Br J Psychiatry Suppl. 2001;40:s18–24. doi: 10.1192/bjp.178.40.s18. [DOI] [PubMed] [Google Scholar]
- 42.Tienari P, Wynne LC, Moring J, et al. The Finnish adoptive family study of schizophrenia: implications for family research. Br J Psychiatry. 1994;164(23):20–26. [PubMed] [Google Scholar]
- 43.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19(2):261–85. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 44.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraepelin E. Dementia Praecox and Paraphrenia. E & S Livingston; Edinburgh, Scotland: 1919. [Google Scholar]
- 46.Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; New York: 1950. [Google Scholar]
- 47.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 48.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 49.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–51. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 50.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–93. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 51.Cannon M, Caspi A, Moffit TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder. Arch Gen Psychiatry. 2002;59:449–56. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 52.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 and younger who subsequently developed schizophrenia. Psychol Med. 2011;42:743–55. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 53.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–87. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 54.Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: A quantitative and qualitative review. Curr Pharm Des. 2012;18(4):399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- 55.Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV. Neuropsychological risk indicators for schizophrenia: A review of family studies. Schizophr Bull. 1994;20:96–108. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- 57.Agnew-Blais JC, Buka SL, Fitzmaurice GM, Smoller JW, Goldstein JM, Seidman LJ. Early childhood IQ trajectories of adults with schizophrenia and affective psychoses in the New England Family Studies. Schizophr Bull. 2015;41:817–23. doi: 10.1093/schbul/sbv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England High-Risk Family Study. Psychol Med. 2013;43:119–31. doi: 10.1017/S0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–9. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized test performance. Am J Psychiatry. 2002;159(7):1183–9. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 61.MacCabe JH, Wicks S, Lofving S, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: A Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70:261–70. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- 62.Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67(6):578–88. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cornblatt BA, Carrión RE, Auther A, et al. Psychosis Prevention: A Modified Clinical High Risk Perspective From the Recognition and Prevention (RAP) Program. Am J Psychiatry. 2015;172(10):986–94. doi: 10.1176/appi.ajp.2015.13121686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168:806–13. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer EC, Carrion RE, Cornblatt BA, et al. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in NAPLS-1. Schizophr Bull. 2014;40(6):1452–61. doi: 10.1093/schbul/sbt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becker HE, Nieman DH, Wiltink S, et al. Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psychol Med. 2010;40(10):1599–1606. doi: 10.1017/S0033291710000048. [DOI] [PubMed] [Google Scholar]
- 67.Woodberry KA, McFarlane WR, Giuliano AJ, et al. Change in neuropsychological functioning over one year in youth at clinical high risk for psychosis. Schizophr Res. 2013;146(1-3):87–94. doi: 10.1016/j.schres.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Addington J, Barbato M. Social Cognition in those at high risk of psychosis. In: Schutt RK, Seidman LJ, Keshavan MS, editors. Social neuroscience: Brain, mind, and society. Harvard University Press; Cambridge, MA: 2015. [Google Scholar]
- 69.Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychol Bull. 2008;134(4):561–83. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- 70.Cornblatt BA, Carrión RE, Addington J, et al. Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 2012;38:1247–57. doi: 10.1093/schbul/sbr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin A, Wood SJ, Nelson B, et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res. 2011;132:1–7. doi: 10.1016/j.schres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Salokangas RK, Nieman DH, Heinimaa M, et al. Psychosocial outcome in patients at clinical high risk of psychosis: a prospective follow-up. Soc Psychiatry Psychiatr Epidemiol. 2013;48:303–11. doi: 10.1007/s00127-012-0545-2. [DOI] [PubMed] [Google Scholar]
- 73.Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: A review of structural MRI findings. Child Adolesc Psychiatr Clin N Am. 2013;22(4):689–714. doi: 10.1016/j.chc.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thermenos HW, Keshavan MS, Juelich RJ, et al. A review of neuroimaging studies of young relatives of persons with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162:604–35. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- 75.Dazzan P, Soulsby B, Mechelli A, et al. Volumetric Abnormalities Predating the Onset of Schizophrenia and Affective Psychoses: An MRI Study in Subjects at Ultrahigh Risk of Psychosis. Schizophr Bull. 2012;38(5):1083–91. doi: 10.1093/schbul/sbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koutsouleris N, Gaser C, Bottlender R, et al. Use of neuroanatomical pattern regression to predict the structural brain dynamics of vulnerability and transition to psychosis. Schizophr Res. 2010;123(2-3):175–87. doi: 10.1016/j.schres.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 77.Iwashiro N, Suga M, Takano Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137(1-3):124–31. doi: 10.1016/j.schres.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 78.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: A cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 79.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra- high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–76. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 82.Ziermans TB, Schothorst PF, Schnack HG, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–30. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–14. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Walter A, Studerus E, Smieskova R, et al. Hippocampal volume in subjects at high risk of psychosis: A longitudinal MRI study. Schizophr Res. 2012;142:217–22. doi: 10.1016/j.schres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson G, Berk M, Dodd S, et al. Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:1–4. doi: 10.1016/j.pnpbp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–9. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarz E, Guest PC, Rahmoune H, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2012;17:494–502. doi: 10.1038/mp.2011.42. [DOI] [PubMed] [Google Scholar]
- 89.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–9. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res. 2014 Jul 4; doi: 10.1016/j.schres.2014.06.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–72. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perkins DO, Jeffries CD, Addington J, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 2015;41(2):419–28. doi: 10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trotman HD, Holtzman CW, Walker EF, et al. Stress exposure and sensitivity in the clinical high-risk syndrome: Initial findings from the North American Prodrome Longitudinal Study (NAPLS) Schizophr Res. 2014;160:104–9. doi: 10.1016/j.schres.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shevlin M, Houston JE, Dorahy MJ, Adamson G. Cumulative traumas and psychosis: an analysis of the national comorbidity survey and the British Psychiatric Morbidity Survey. Schizophr Bull. 2008;34(1):193–9. doi: 10.1093/schbul/sbm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galletly C, Van Hooff M, McFarlane A. Psychotic symptoms in young adults exposed to childhood trauma--a 20 year follow-up study. Schizophr Res. 2011;127(1-3):76–82. doi: 10.1016/j.schres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 97.Holtzman CW, Trotman HD, Goulding SM, et al. Stress and Neurodevelopmental Processes in the Emergence of Psychosis. Neuroscience. 2013;249:172–91. doi: 10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schlosser DA, Pearson R, Perez VB, Loewy RL. Environmental risk and protective factors and their influence on the emergence of psychosis. Adolesc Psychiatry (Hilversum) 2012;2(2):163–71. doi: 10.2174/2210676611202020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2012;37(10):1600–13. doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Thompson KN, Berger G, Phillips LJ, Komesaroff P, Purcell R, McGorry PD. HPA axis functioning associated with transition to psychosis: Combined DEX/CRH test. J Psychiatr Res. 2007;41:446–50. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 101.Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119(2):401–8. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mizrahi R, Addington J, Rusjan PM, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71(6):561–7. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Walker E, Trotman H, Pearce B, et al. Cortisol Levels and Risk for Psychosis: Initial Findings from the North American Longitudinal Study. Biol Psychiatry. 2013;74:410–7. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56(2):162–8. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 105.Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torrey EF, Miller J, Rawlings R, Yolken RH. Sesonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 107.Torrey EF, Burka S, Cannon TD, et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res. 2009;111(1-3):1–5. doi: 10.1016/j.schres.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 108.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.March D, Hatch SL, Morgan C, et al. Psychosis and place. Epidemiol Rev. 2008;30:84–100. doi: 10.1093/epirev/mxn006. [DOI] [PubMed] [Google Scholar]
- 110.Pedersen CB, Mortensen PB. Evidence of a dose–response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58(11):1039–46. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 111.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 112.Boydell J, Van Os J, McKenzie K, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323:1336–8. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tienari P, Wynne LC, Sorri A, et al. Genotype-environment interaction in schizophrenia-spectrum disorder Long-term follow-up study of Finnish adoptees. Br J Psychiatry. 2004;184(3):216–22. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- 114.Hooley JM, Woodberry KA, Ferriter C. Family factors in schizophrenia and bipolar disorder. In: Hudson JL, Rapee RM, editors. Psychopathology and the family. Elsevier; Boston: 2005. [Google Scholar]
- 115.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence--conditional on genetic risk. Schizophr Bull. 2005;31(4):795–9. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 116.Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47(3):210–20. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 117.Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483–6. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 118.Gururajan A, Manning EE, Klug M, van den Buuse M. Drugs of abuse and increased risk of psychosis development. Aust N Z J Psychiatry. 2012;46(12):1120–35. doi: 10.1177/0004867412455232. [DOI] [PubMed] [Google Scholar]
- 119.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–7. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 120.Addington J, Case N, Saleem MM. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry. 2014;8(2):104–12. doi: 10.1111/eip.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Auther AM, Cadenhead KS, Carrion RE, et al. Alcohol confounds relationship between cannabis misuse and psychosis conversion in a high-risk sample. Acta Psychiatr Scand. 2015;132:60–8. doi: 10.1111/acps.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valmaggia LR, Day FL, Jones C, et al. Cannabis use and transition to psychosis in people at ultra-high risk. Psychol Med. 2014;44:2503–12. doi: 10.1017/S0033291714000117. [DOI] [PubMed] [Google Scholar]
- 123.Schvarcz A, Bearden CE. Early detection of psychosis: recent updates from clinical high-risk research. Curr Behav Neurosci Rep. 2015;2:90–101. doi: 10.1007/s40473-015-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mäki P, Veijola J, Jones PB, et al. Predictors of schizophrenia—a review. Br Med Bull. 2005;73(1):1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- 125.Schlosser DA, Zinberg JL, Loewy RL, et al. Predicting the longitudinal effects of the family environment on prodromal symptoms and functioning in patients at-risk for psychosis. Schizophr Res. 2010;118(1-3):69–75. doi: 10.1016/j.schres.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O'Brien MP, Gordon JL, Bearden CE, Lopez SR, Kopelowicz A, Cannon TD. Positive family environment predicts improvement in symptoms and social functioning among adolescents at imminent risk for onset of psychosis. Schizophr Res. 2006;81:269–75. doi: 10.1016/j.schres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B. A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int J Endocrinol. 2015:615356. doi: 10.1155/2015/615356. 16 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bell RQ. Multiple-risk cohorts and segmenting risk as solutions to the problem of false positives in risk for the major psychoses. Psychiatry. 1992;55:370–81. doi: 10.1080/00332747.1992.11024610. [DOI] [PubMed] [Google Scholar]
- 129.Ruhrmann S, Schultze-Lutter F, Schmidt SJ, Kaiser N, Klosterkötter J. Prediction and prevention of psychosis: current progress and future tasks. Eur Arch Psychiatry Clinl Neurosci. 2014;264(Suppl 1):S9–16. doi: 10.1007/s00406-014-0541-5. [DOI] [PubMed] [Google Scholar]
- 130.Yang LH, Wonpat-Borja AJ, Opler MG, Corcoran CM. Potential stigma associated with inclusion of psychosis risk syndrome in the DSM-V: An empirical question. Schizophr Res. 2010;120(1-3):42–8. doi: 10.1016/j.schres.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yung AR, Yuen HP, Berger G, et al. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull. 2007;33:673–81. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55(8):486–97. doi: 10.1177/070674371005500803. [DOI] [PubMed] [Google Scholar]
- 133.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The Psychosis High-Risk State. A comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–20. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schultze-Lutter F, Klosterkötter J, Ruhrmann S. Improving the clinical prediction of psychosis by combining ultra-high risk criteria and cognitive basic symptoms. Schizophr Res. 2014;154(1-3):100–6. doi: 10.1016/j.schres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 135.Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Probably at-risk, but certainly ill - Advocating the introduction of a psychosis spectrum disorder in DSM-V. Schizophr Res. 2010;120:23–37. doi: 10.1016/j.schres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 136.Thompson A, Nelson B, Yung A. Predictive validity of clinical variables in the “at risk” for psychosis population: international comparison with results from the North American Prodrome Longitudinal Study. Schizophr Res. 2011;126(1-3):51–7. doi: 10.1016/j.schres.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 137.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: Psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131–42. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 138.Riecher-Rössler A, Pflueger MO, Aston J, et al. Efficacy of Using Cognitive Status in Predicting Psychosis: A 7-Year Follow-Up. Biol Psychiatry. 2009;66(11):1023–30. doi: 10.1016/j.biopsych.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 139.Koutsouleris N, Borgwardt S, Meisenzahl EM, Bottlender R, Möller HJ, Riecher-Rössler A. Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: Results from the fepsy study. Schizophr Bull. 2012;38(6):1234–46. doi: 10.1093/schbul/sbr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koutsouleris N, Davatzikos C, Bottlender R, et al. Early recognition and disease prediction in the at-risk mental states for psychosis using neurocognitive pattern classification. Schizophr Bull. 2012;38(6):1200–15. doi: 10.1093/schbul/sbr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nieman DH, Ruhrmann S, Dragt S, et al. Psychosis Prediction: Stratification of Risk Estimation With Information-Processing and Premorbid Functioning Variables. Schizophr Bull. 2013;40(6):1482–90. doi: 10.1093/schbul/sbt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perkins DO, Jeffries CD, Addington J, et al. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results From the NAPLS Project. Schizophr Bull. 2015;41(2):419–28. doi: 10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cannon TD. Biomarkers of Vulnerability and Progression in the Psychosis Prodrome. Oral session conducted at the 15th annual International Congress on Schizophrenia Research; Colorado Springs, CO. March, 2015. [Google Scholar]
- 144.Clark SR, Schubert KO, Baune B. Towards indicated prevention of psychosis: using probabilistic assessments of transition risk in psychosis prodrome. J Neural Transm. 2015;122(1):155–69. doi: 10.1007/s00702-014-1325-9. [DOI] [PubMed] [Google Scholar]
- 145.Preti A, Cella M. Randomized-controlled trials in people at ultra-high risk of psychosis: a review of treatment effectiveness. Schizophr Res. 2010;123(1):30–6. doi: 10.1016/j.schres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 146.van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 months and longer-term follow-ups. Schizophr Res. 2013;149(1–3):56–62. doi: 10.1016/j.schres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. doi: 10.1136/bmj.f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):388–404. doi: 10.1016/j.eurpsy.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 149.Marshall M, Rathbone J. Early intervention for psychosis. Schizophr Bull. 2011;37(6):1111–4. doi: 10.1093/schbul/sbr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heinssen RK, Insel TR. Preventing the Onset of Psychosis: Not Quite There Yet. Schizophr Bull. 2015;41(1):28–9. doi: 10.1093/schbul/sbu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–54. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 152.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790–9. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 153.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive-behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125(1):54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 154.Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200(1):22–9. doi: 10.1192/bjp.bp.109.066357. [DOI] [PubMed] [Google Scholar]
- 155.McGorry PD, Nelson B, Phillips LJ, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2013;74(4):349–56. doi: 10.4088/JCP.12m07785. [DOI] [PubMed] [Google Scholar]
- 156.Miklowitz DJ, O’Brien MP, Schlosser DA, et al. Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53(8):848–58. doi: 10.1016/j.jaac.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–7. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 158.Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;5(344):e2233. doi: 10.1136/bmj.e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nordentoft M, Thorup A, Petersen L, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophr Res. 2006;83(1):29–40. doi: 10.1016/j.schres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 160.Piskulic D, Barbato M, Liu L, Addington J. Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res. 2015;225(1-2):93–8. doi: 10.1016/j.psychres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 161.van der Gaag M, Nieman DH, Rietdijk J, et al. Cognitive-behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull. 2012;38(6):1180–8. doi: 10.1093/schbul/sbs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–8. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 163.Woods SW, Addington J, Bearden CE, et al. Psychotropic medication use in youth at high risk for psychosis: comparison of baseline data from two research cohorts 1998–2005 and 2008–2011. Schizophr Res. 2013;148(1):99–104. doi: 10.1016/j.schres.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bryant C, Woodberry KA, Seidman LJ, et al. Disclosing a developing disorder: sharing symptoms vs. diagnoses by youth at high risk for psychosis. Poster presented at the Harvard Medical School Department of Psychiatry Research Day; Boston, MA. March, 2015. [Google Scholar]
- 165.McFarlane WR, Levin B, Travis L, et al. Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophr Bull. 2015;41(1):30–43. doi: 10.1093/schbul/sbu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Holzer L, Urben S, Passini CM, et al. A Randomized Controlled Trial of the Effectiveness of Computer-Assisted Cognitive Remediation (CACR) in Adolescents with Psychosis or at High Risk of Psychosis. Behav Cogn Psychother. 2014;42(4):421–34. doi: 10.1017/S1352465813000313. [DOI] [PubMed] [Google Scholar]
- 167.Hooker CI, Carol EE, Eisenstein TJ, et al. A pilot study of cognitive training in clinical high risk for psychosis: initial evidence of cognitive benefit. Schizophr Res. 2014;157(1–3):314–6. doi: 10.1016/j.schres.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rauchensteiner S, Kawohl W, Ozgurdal S, et al. Test-performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiatry Res. 2011;185(3):334–9. doi: 10.1016/j.psychres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 169.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia. Schizophr Bull. 2000;26(1):119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 170.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 171.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171(5):510–22. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McGorry P, Markulev C, Nelson B, et al. The NEURAPRO-E study: a multicenter RCT of Omega-3 fatty acids and cognitive-behavioral case management for patients at ultra high risk of schizophrenia and other psychotic disorders. Schizophr Bull. 2015;41(Supp. 1):S322–3. [Google Scholar]
- 173.Commonwealth for Australia [accessed May 14, 2015];A ten year roadmap for national mental health. 2012 [Online] http://www.health.gov.au/internet/main/publishing.nsf/Content/mental-roadmap.
- 174.Birchwood M, Connor C, Leste H, et al. Reducing duration of untreated psychosis: care pathways to early intervention in psychosis services. Br J Psychiatry. 2013;203:58–64. doi: 10.1192/bjp.bp.112.125500. [DOI] [PubMed] [Google Scholar]
- 175.Substance Abuse and Mental Health Services Administration (SAMHSA) [accessed May 14, 2015];Now is the Time – Prevention and Early Intervention, Healthy Transitions Program. 2015 [Online] http://www.samhsa.gov/priorities/now-is-the-time.
- 176.Ruhrmann S, Bechdolf A, Kuhn KU, et al. Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. Br J Psychiatry Suppl. 2007;51:s96–101. doi: 10.1192/bjp.191.51.s88. [DOI] [PubMed] [Google Scholar]
- 177.Woods SW, Tully EM, Walsh BC, et al. Aripiprazole in the treatment of the psychosis prodrome: an open-label pilot study. Br J Psychiatry Suppl. 2007;51:s96–101. doi: 10.1192/bjp.191.51.s96. [DOI] [PubMed] [Google Scholar]
- 178.Cornblatt BA, Lencz T, Smith CW, et al. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68(4):546–57. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- 179.Woods SJ, Berger GE, Dell’Olio M, et al. Effects of low dose lithium on hippocampal neuropathology in people at ultra-high risk for psychosis. Biol Psychiatry. 2007;61:259s–60s. [Google Scholar]
- 180.Woods SW, Walsh BC, Hawkins KA, et al. Glycine treatment of the risk syndrome for psychosis: report of two pilot studies. Eur Neuropsychopharmacol. 2013;23(8):931–40. doi: 10.1016/j.euroneuro.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yale University . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): [2014 August 27]. 2000. Randomized controlled trial of aspirin vs placebo in the treatment of pre-psychosis. NLM identifier: NCT02047539] https://www.clinicaltrials.gov/ct2/show/NCT02047539?term=NCT02047539&rank=1. [Google Scholar]
- 182.Kantrowitz JT, Woods SW, Petkova E, et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry. 2015;2(5):403–12. doi: 10.1016/S2215-0366(15)00098-X. [DOI] [PubMed] [Google Scholar]
- 183.Schlosser D, Kim D, Campellone T, Ward C, Vinogradov S. PRIME: A neuroscience-informed mobile app intervention to treat reward processing impairments and improve quality of life in recent onset schizophrenia. Schizophr Bull. 2015;41(Supp. 1):S332. [Google Scholar]
- 184.Mittal VA. Exercise-based cognitive remediation for youth at ultra high-risk for psychosis. Schizophr Bull. 2015;41(Supp. 1):S324–5. [Google Scholar]
- 185.Woodberry KA, Serur RA, Hallinan S, et al. Integrating biofeedback games and family therapy to reduce risk for psychosis. Poster presented at the Harvard Medical School Department of Psychiatry Research Day; Boston, MA. March 2014. [Google Scholar]
- 186.Dixon LB, Dickerson F, Bellack AS, et al. The 2009 Schizophrenia PORT treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Francey SM, Nelson B, Thompson A, et al. Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurobiology and ethics in the era of early intervention. Schizophr Res. 2010;119(1):1–10. doi: 10.1016/j.schres.2010.02.1071. [DOI] [PubMed] [Google Scholar]
- 188.Yang LH, Link BC, Ben-David S, et al. Stigma related to labels and symptoms to individuals at clinical high-risk for psychosis. Schizophr Res. 2015;168:9–15. doi: 10.1016/j.schres.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database of Systematic Reviews. 2011;(Issue 6) doi: 10.1002/14651858.CD004718.pub3. Art. No.: CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cochrane [accessed: May 14 2015];Early intervention for Psychosis. 2015 [Online] http://www.cochrane.org/CD004718/SCHIZ_early-intervention-for-psychosis.
- 191.National Institute for Health and Clinical Excellence (NICE) [accessed: May 14 2015];Psychosis and schizophrenia. 2015 [Online] https://www.nice.org.uk/guidance/conditions-and-diseases/mental-health-and-behavioural-conditions/psychosis-and-schizophrenia.
- 192.Deutsche Geselleschaft fur Psychiatrie, Psychotherapie und Nervenheilkunde (DGPPN) Behandlungsleitlinie Schizophrenie. Steinkopff Vertag; Darmstadt: 2006. [Google Scholar]
- 193.Kohler C, Borgmann-Winter KE, Huford I, Neustadter E, Yi J, Calkins ME. Is prevention a realistic goal for schizophrenia? Curr Psychiatry Rep. 2014;16(4):439. doi: 10.1007/s11920-014-0439-y. [DOI] [PubMed] [Google Scholar]
- 194.Nelson B, Hughes A, Leicester S, et al. A stitch in time: interventions for young people at ultra high risk of psychosis. Orygen Youth Health Research Centre; Melbourne, Australia: 2014. [Google Scholar]
- 195.Singh F, DeJoseph M, Cadenhead KS. Therapeutic considerations in individuals at clinical risk for developing psychosis. Fortschr Neurol Psychiatr. 2012;80(10):570–9. [Google Scholar]
- 196.Sullivan HS. The onset of schizophrenia. Am J Psychiatry. 1927;7:105–134. doi: 10.1176/ajp.151.6.134. [DOI] [PubMed] [Google Scholar]
- 197.Okkels N, Vernal DL, Jensen SOW, McGrath JJ, Nielsen RE. Changes in the diagnosed incidence of early onset schizophrenia over four decades. Acta Psychiatr Scand. 2013;127(1):62–8. doi: 10.1111/j.1600-0447.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- 198.Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 200.McGorry PD. Evidence based reform of mental health care. BMJ. 2005;331:586–7. doi: 10.1136/bmj.331.7517.586. [DOI] [PMC free article] [PubMed] [Google Scholar]