Abstract
Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV improves both adherence and depression outcomes relative to enhanced treatment as usual (ETAU). However, in persons with injection drug use (PWIDU) histories, adherence gains seen during treatment have not been maintained postintervention. Therefore, we examined whether heroin or cocaine use at study entry moderated acquisition or maintenance of adherence gains after CBT-AD. HIV-positive adults in treatment for opioid dependence (n = 89) were randomly assigned to CBT-AD or ETAU and completed 3-, 6-, and 12-month assessments. Participants were majority male (61%), white (48%), and heterosexual (79%). Hierarchical linear modeling was used to evaluate whether heroin or cocaine use at baseline interacted with intervention assignment to predict change in adherence during active treatment and follow-up. CBT-AD-related improvement in adherence during the active intervention period did not vary by baseline substance use. However, cocaine use (but not heroin use) at baseline interacted with intervention assignment to predict a significant decline in follow-up adherence (cocaine use × group condition coefficient = −0.77, t = −2.44, p = 0.02) such that by 12 months, adherence among CBT participants was significantly lower among those who used cocaine (45.0%) compared to those who did not (72.3%; t = 2.50, p = 0.018). HIV-positive PWIDU who use heroin or cocaine at baseline can benefit from the CBT-AD intervention to improve adherence to antiretroviral therapy; therefore, providers should not withhold an active psychosocial treatment for HIV-positive PWIDU who are using. Cocaine use at baseline may limit the degree to which gains are maintained postintervention, and therefore, booster sessions may be needed.
Introduction
Adherence to antiretroviral therapy (ART) medications is necessary to maximize the chances of HIV treatment success.1–4 Poor adherence may lead to viral replication, development of medication resistance, and subsequent disease progression.5 Although there is a growing set of evidenced-based interventions to improve adherence to ART,6,7 many of the existing trials found modest effects and did not directly address comorbid psychiatric conditions as part of the intervention. Among people living with HIV/AIDS, psychiatric comorbidities such as clinical depression and substance use are each highly prevalent conditions that place this group at increased risk for poor adherence.8–10
HIV-positive individuals use substances, in general, and cocaine and heroin, in particular, at significantly higher rates than HIV-negative persons.11 For HIV-positive individuals, heroin and cocaine use may be particularly predictive of nonadherence to HIV medication.12 Furthermore, cocaine and heroin use is widespread among persons in treatment (e.g., methadone) for injection drug use (IDU13,14), and cocaine may be a common drug of choice because it can break through the effects of methadone.13,15 Similarly, previous literature has indicated that among individuals with a history of IDU receiving substance abuse treatment, those with HIV were more likely to continue using heroin.16
There are two published trials of cognitive behavioral therapy for adherence and depression (CBT-AD), an intervention to promote adherence to HIV medications and treat depression among HIV-positive adults with a clinical diagnosis of depression.10,17 The initial trial recruited HIV-positive patients in HIV care at a community health center with a large population of men who have sex with men. In that study, those who received CBT-AD had superior adherence and depression outcomes to those who received enhanced treatment as usual (ETAU).17 This was a pilot study, with a relatively small sample size (n = 43) and a crossover design; nonetheless, gains were maintained over follow-up (at 3, 6, and 12 months postbaseline), and those who received CBT had reductions in viral load over time. The second trial was an efficacy study comparing CBT-AD to ETAU in persons with injection drug use (PWIDU) and were receiving drug abuse treatment.18 Findings indicated that in comparison to ETAU, those who received CBT-AD had significant improvement in both adherence and depression at the end of the intervention period.18 However, while the gains for depression were maintained over the 12-month follow-up period (3, 6, and 12 months postbaseline), the gains for adherence were not.18 The current study represents a secondary analysis of this sample data.
The purpose of the current study was to examine whether cocaine and heroin use at baseline (study entry), among PWIDU, moderated either the early adherence gains or the maintenance of adherence gains following CBT-AD in comparison to ETAU. Some providers may be hesitant to start a psychosocial treatment addressing mental health and health behaviors for HIV-positive PWIDU and findings from this study may help providers in the decision-making process.
Methods
Details have been previously published on study participants, procedures, and intervention conditions18; therefore, only brief summaries are provided below.
Study subjects and setting
Enrollment occurred between July 2005 and October 2008 and included (89 randomized) individuals between the ages of 18 and 65 who were HIV seropositive, prescribed ART for HIV, endorsed a history of IDU and were currently enrolled in opioid treatment for at least 1 month, and met criteria for a diagnosis of current mood disorder or subsyndromal depressive mood disorder (defined as past history of major depression, with a current level of residual symptoms that did not meet diagnostic threshold). Treatment for opioid dependence varied, with the majority of participants receiving methadone (n = 63, 70%) and the remainder receiving buprenorphine therapy, group or individual substance abuse drug-free counseling, and/or actively participating in Narcotics Anonymous.
Excluded were those with any active untreated, unstable, major mental illness that would interfere with study participation (e.g., active mania or psychosis), inability or unwillingness to provide informed consent, or current participation in CBT for depression.
Participants were recruited at four methadone clinics in the greater Boston area, HIV clinics at Massachusetts General Hospital (MGH) and Rhode Island Hospital, and through community outreach and recruitment flyers posted in other HIV care or substance abuse settings. Visits were conducted at a hospital-based research clinic and informed consent was obtained from all participants. All study procedures were approved by the Institutional Review Boards at MGH (Boston, MA) and Rhode Island Hospital (Providence, RI).
Study design and procedures
Study visits
After an initial evaluation spread across two baseline visits (included a diagnostic evaluation of DSM-IV diagnoses) to determine study eligibility and a 2-week period where participants started using the Medication Event Monitoring System (MEMS) caps, there were three major follow-up assessment visits for both experimental and control arms: T1—acute/post-treatment outcome ∼3 months after baseline, T2—3-month follow-up occurring 6 months after baseline, and T3—9-month follow-up occurring 12 months after baseline. These assessments included an MEMS cap evaluation for adherence (i.e., MEMS19) and evaluation for depression (self-report and interview by an independent assessor blinded to study condition), as well as HIV plasma RNA and CD4+ lymphocyte counts either drawn for the study or abstracted from participants' medical records if collected in the month before the assessment. Samples acquired during the baseline assessment that had a viral load of over 1000 copies per milliliter were tested for genotypic resistance.
Randomization
Study coordinators randomly assigned participants at their first visit after the baseline in blocks of two, stratified by biological sex, depression severity (current major depression or residual symptoms only), and adherence (baseline MEMS adherence above or below 80%). Assignment to treatment or control conditions was concealed from study therapists and participants until the conclusion of the Life-Steps (a single-session intervention on HIV medication adherence) visit.
Safren et al.18 described the flow of participants in the study from baseline assessment through randomization and follow-up.18 In summary, 154 participants were assessed for eligibility, 65 were excluded (did not meet criteria or declined participation), 89 were randomized (CBT-AD n = 44; ETAU n = 45), and 23 were lost by the 12-month follow-up (CBT-AD n = 8; ETAU n = 15; CBT vs. ETAU not statistically different). Only two participants in the intervention arm did not receive the intervention/modules as assigned.
Adherence and substance use measures
Adherence assessment
MEMS caps recorded each instance of bottle opening, monitoring the antiretroviral medication considered the most difficult to remember to take by the participant. If the medications were equally difficult to remember, participants used the cap for the pill that they took most frequently. To account for doses that participants may have taken without opening the pill cap (e.g., took out doses for the entire day in the morning), we counted a dose as taken if participants could recall specific instances when they took their medications but did not use the cap.18,20,21 A dose was considered missed if it was not taken within a 2-h window of the time designated by the participant's medical provider. For the acute outcome (baseline to post-treatment), adherence was operationalized as the percentage of MEMS adherence using the time since the last visit, as intervention visits were scheduled weekly or, at most, every 2 weeks. For the follow-up longitudinal analyses, we used adherence in the past 2 weeks.
Substance use
The Addictions Severity Index (ASI)-Lite measures the severity of problems in seven areas of functioning that are frequently affected in participants with substance use disorders.22 This clinician-administered instrument required participants to report their history and current (past 30 day) use of heroin, cocaine, and other illicit substances. Dichotomous baseline variables indicating whether participants had used heroin or cocaine over the past 30 days were used in these analyses.
Intervention conditions
Study interventionists included clinical psychologists, psychology pre- and postdoctoral fellows, and one master's level psychologist, who all participated in weekly group supervision (i.e., verbal and audio review of sessions). Participants in both the treatment (CBT-AD) and comparison (ETAU) condition received a single-session intervention on HIV medication adherence (Life-Steps), which involves 11 informational, problem-solving, and cognitive-behavioral steps.23 In each step, participants and the clinician define the problem, generate alternative solutions, make decisions about the solutions, and develop a plan for implementing them. Participants also received adherence tools such as a cue-dosing watch that could sound two alarms per day and assistance with setting reminders for their schedule. Participants also had a letter mailed to their medical and, as appropriate, mental health providers documenting study participation, depression or other psychiatric disorders and recommending that these conditions should continue to be assessed or treated.
Those assigned to the experimental condition also received eight sessions of CBT-AD.18 Consisting of five modules, this approach integrated adherence counseling with traditional CBT techniques, including psychoeducation, motivational interviewing, behavioral activation/activity scheduling, cognitive restructuring, problem solving, and relaxation training for the treatment of depression. Sessions were ∼50-min long and occurred weekly, with the goal of completing them within ∼3 months. A more detailed description of the intervention can be found in our published manuals.18
Statistical analyses
Hierarchical linear models (HLM) with HLM 6.06 software were used to evaluate the interaction of random assignment with substance use on both the acquisition and maintenance of medication adherence (MEMS) gains in separate models. The structure of the HLM is provided by the following equations:
Level 1 model
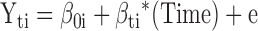 |
Level 2 model
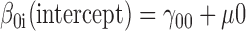 |
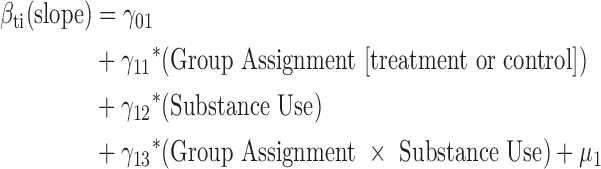 |
The Level 1 model included the Time variable (weeks since baseline), which provided the structure of the model. The evidence of moderation is estimated from the significance of the slope (γ coefficient) associated with the interaction term (γ13). For the HLM evaluating maintenance of adherence, baseline adherence was included as an additional covariate in the Level 2 model to control for prerandomization levels. For the HLM, all continuous measures in the Level 2 model were centered about their group means and all dichotomous variables were coded 1/0. Model parameters were estimated using full-maximum likelihood estimation with robust standard errors. In all analyses that used HLM, unconstrained models were run to confirm significant individual variation about the slope and intercept before accounting for random assignment.
Results
Participant characteristics
Detailed information on demographic and clinical characteristics for participants was previously presented in Safren et al.18 In brief, participants' mean age (standard deviation) was 46.9 (7.2) and they were primarily male (61%), white (48%), and heterosexual (79%) with a low education level (26%—partial high school, 29%—high school graduation/General Educational Development). Furthermore, 67% of participants were on disability, 62% met DSM-IV criteria for one additional diagnosis beyond depression, and 19% had a CD4+ T-cell count <200. There were no differences on baseline demographic or clinical variables between the two randomized conditions, except for CD4 levels being higher among the CBT-AD group compared to the ETAU group.18 At baseline, 22.73% (n = 10) of the CBT-AD participants and 28.89% of the ETAU participants reported cocaine use, while 18.18% (n = 8) of the CBT-AD participants and 33.33% (n = 15) of the ETAU participants reported heroin use. In the entire study sample, heroin use (25.8%) and cocaine use (25.8%) were the most commonly used substances.
Moderation of CBT-AD-related adherence improvement during active treatment
In HLM analyses testing whether cocaine or heroin use at baseline interacted with intervention assignment (CBT-AD vs. ETAU) in predicting medication adherence improvement during active treatment, CBT-AD-related adherence improvement during active treatment did not vary by heroin (coeff. = 1.08, SE = 0.84 t = 1.28, p = 0.20) or cocaine use (coeff. = 0.44, SE = 0.88 t = 0.51, p = 0.62). Irrespective of whether they used or did not use heroin or cocaine at baseline, participants' medication adherence improved during the CBT-AD treatment in comparison to ETAU. In addition, these results held while controlling for ongoing cocaine and heroin use in post hoc analyses.
Moderation of CBT-AD-related adherence improvement maintenance at follow-ups
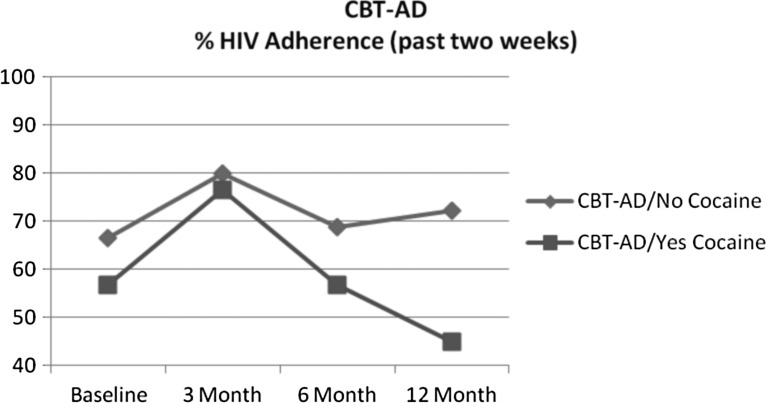
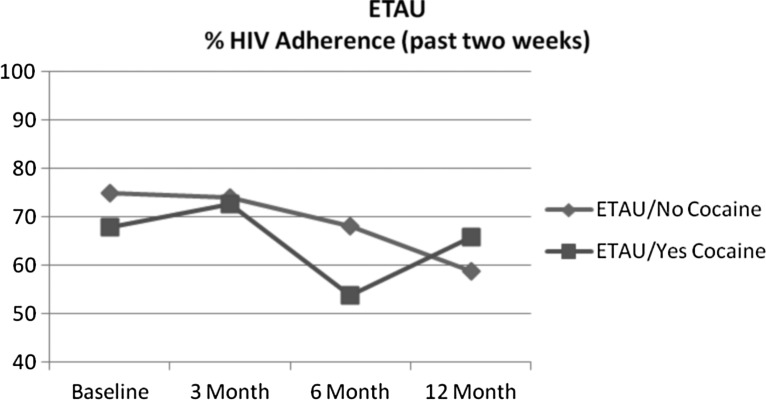
For cocaine use at baseline, intervention assignment significantly interacted with cocaine to predict decline in medication adherence during follow-up (coeff. = −0.77, SE = 0.32 t = −2.44, p = 0.02). This significant result also remained while controlling for ongoing cocaine and heroin use in post hoc analyses. Accordingly, those who used cocaine at baseline had greater adherence declines after the intervention ended than those who did not use cocaine at baseline. Follow-up t-test analyses indicated that at the12-month follow-up visit, adherence of those assigned to the CBT-AD arm was significantly lower among cocaine users (45.0%) relative to nonusers (72.3%; t = 2.50, p = 0.018) (Fig. 1). However, for the ETAU condition, adherence at the 12-month follow-up did not significantly differ between cocaine users (66%) and nonusers (59%; t = −0.49, p = 0.63) (Fig. 2).
FIG. 1.
CBT-AD percentage HIV medication adherence (past 2 weeks). CBT-AD, cognitive behavioral therapy for adherence and depression.
FIG. 2.
ETAU percentage HIV medication adherence (past 2 weeks). ETAU, enhanced treatment as usual.
For heroin use at baseline, however, intervention assignment did not significantly interact with heroin in predicting adherence decline (coeff. = −0.13, SE = 0.37, t = −0.36, p = 0.72) even when controlling for ongoing heroin and cocaine use in post hoc analyses. Table 1 presents the mean descriptive scores for ART adherence across conditions by cocaine and heroin use, at baseline and follow-up visits (Table 1).
Table 1.
Unadjusted Mean Descriptive Scores for Adherence Across Conditions by Cocaine and Heroin Use, at Baseline and Follow-Up Visits
| Baseline (since last visit weeks) | 3 Months (past 2 weeks) | 6 Months (past 2 weeks) | 12 Months (past 2 weeks) | |
|---|---|---|---|---|
| CBT-AD adherence | ||||
| Cocaine | ||||
| Yes | 56.79 (34.89) | 76.43 (28.78) | 56.75 (33.71) | 45.00 (27.21) |
| No | 66.58 (24.72) | 79.88 (21.58) | 68.86 (32.23) | 72.29 (29.87) |
| Heroin | ||||
| Yes | 45.74 (26.47) | 70.09 (32.00) | 38.69 (35.31) | 47.96 (34.12) |
| No | 68.49 (25.95) | 81.25 (20.55) | 71.43 (29.48) | 68.62 (29.82) |
| ETAU adherence | ||||
| Cocaine | ||||
| Yes | 67.81 (26.89) | 72.73 (24.03) | 53.90 (32.63) | 65.87 (32.25) |
| No | 74.90 (26.20) | 74.01 (25.96) | 68.13 (29.64) | 58.73 (36.88) |
| Heroin | ||||
| Yes | 60.89 (26.80) | 70.54 (19.98) | 51.19 (28.87) | 54.64 (36.50) |
| No | 78.97 (24.24) | 75.00 (27.29) | 70.00 (30.37) | 64.92 (34.54) |
CBT-AD, cognitive behavioral therapy for adherence and depression; ETAU, enhanced treatment as usual.
Discussion
Our previous study found that a time-limited intervention (CBT-AD vs. ETAU) showed efficacy in decreasing depressive symptoms and improving HIV medication adherence in a sample of HIV-positive adults with depression and an IDU history18; however, improvements in HIV medication adherence were not maintained. The present study explored whether or not cocaine or heroin use at baseline moderated treatment-related (CBT-AD vs. ETAU) adherence gains over the follow-up period. While neither cocaine nor heroin use moderated adherence improvement during active treatment, cocaine use at study entry predicted a significantly steeper decline in adherence during follow-up in those who received the CBT-AD treatment. To our knowledge, this is the first study to show that while participants are in active treatment, their drug use status at baseline does not relate to improvement in ART adherence for HIV treatment; however, during follow-up, participants who reported illicit substance use at baseline, in this case cocaine, had significantly worse decline in adherence even when ongoing substance use was accounted for.
Clinically, the present study may help providers in decision-making regarding whether or not to start a psychosocial treatment addressing mental health and health behaviors for HIV-positive PWIDU histories, as some may be reluctant to do so if the patient is actively using. Our results suggest that providers should not withhold an active psychosocial treatment for HIV-positive PWIDU who are actively using, because there is at least short-term benefit during treatment; addressing adherence beyond the period of time-limited treatment, however, may demand novel approaches for maintaining gains.
During active treatment, participants received weekly problem solving and CBT focused on both adherence to medications and treatment of their depressed mood. However, after treatment ended, participants did not attend additional sessions to review the use of CBT skills or receive reinforcements such as social support from their clinician,24 which may have helped even those with active cocaine and heroin use. Ongoing cocaine use may worsen adherence when no longer mitigated by ongoing psychosocial treatment; in fact, there is evidence suggesting that cocaine use is more predictive of HIV medication nonadherence than heroin.9,25 Furthermore, the lives of HIV-positive individuals who use cocaine may also be impacted by structural factors such as unstable housing or other unanticipated (at the time of treatment discontinuation) life stress26 that complicate medication adherence.27–30 There is also research suggesting that cocaine users have increased difficulty with focus and concentration, which may negatively impact medication adherence.31,32
Our findings also suggest that for individuals who report using cocaine at the time that they start an adherence intervention, ongoing adherence counseling after treatment discontinuation may be needed to maintain intervention-related gains. Given that HIV is a chronic illness and medication adherence is necessary for long-term effective disease management,2,33 ongoing adherence counseling could be integrated into standard of care in settings that HIV-positive cocaine users frequent (e.g., substance abuse clinics). This is in line with recent research suggesting the significant value of integrated care, particularly within a primary care setting, for HIV risk reduction and substance use treatment.34 Because CBT-AD showed efficacy in significantly improving adherence during active treatment among HIV-positive individuals with depression and a history of IDU irrespective of heroin or cocaine, this suggests that the key to sustaining adherence gains after active treatment may be booster sessions of CBT-AD. Adherence counseling via CBT-AD18 can also be a useful augment to standard treatment when HIV-positive patients experience lapses in cocaine use. Future research should also identify specific CBT-AD skills or support that may require reinforcement during booster sessions, to maintain adherence gains. It is unclear why cocaine use, but not heroin use, moderated the adherence maintenance. It is possible that because many participants were on methadone, effects of heroin were mitigated.
Findings should be interpreted in the context of several limitations. We enrolled a convenience sample of participants in Massachusetts and Rhode Island, geographical areas with many medical and social service resources; therefore, our findings may not be representative among all HIV-positive adults with depression and history of IDU in the United States. For instance, there is evidence suggesting that combined substance abuse, violence, and HIV/AIDS lead to a disproportionate level of disease and treatment outcome among people of color in the United States.30 Yet, close to half of our sample identified as white, suggesting that further research is needed to confirm generalizability in diverse populations. In addition, at study entry, participants were in active treatment for opioid dependence, and thus, results cannot be generalized to those not currently in active treatment for opioid dependence. However, concurrent substance treatment was not a requirement for continued study participation, so it is possible that participants were not receiving substance use treatment across the study period. Heroin and cocaine use was assessed via retrospective self-report measures, which may have been impacted by social desirability bias such as underreporting.35 Adherence may have been underestimated if the MEMS cap was not used; therefore, at each assessment, participants were asked if they recalled taking pills without the use of the MEMS cap, and adherence scores were adjusted accordingly.
Despite these limitations, our finding that cocaine use at baseline moderated follow-up adherence maintenance among HIV-positive adults with depression and a history of IDU highlights the importance of taking illicit substance use into consideration when developing interventions to promote adherence on a long-term basis beyond active treatment. Furthermore, our findings suggest that additional research is needed to establish strategies for promoting maintenance of adherence skills following effective brief adherence interventions such as CBT-AD, particularly among individuals who struggle with illicit substance use. Offering booster sessions of CBT-AD following participants' engagement in the full intervention may maintain adherence gains overtime, and interventions that aim to integrate CBT-AD strategies into standard care for HIV-positive illicit drug users may establish feasibility and promote cost efficiency.
Acknowledgments
This research was supported by the National Institute of Health grants R01 DA018603 (PI Dr. Steven Safren), K24MH094214 (PI Dr. Steven Safren), and 2P30AI060354-11 (PI Dr. Bruce Walker). Some of Dr. Sannisha Dale's effort was supported by 1K23MH108439-01 (PI Dr. Sannisha Dale).
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroval agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (Last accessed April25, 2016) [Google Scholar]
- 2.Yeni PG, Hammer SM, Carpenter CCJ, et al. . Antiretroviral treatment for adult HIV infection in 2002: Updated recommendations of the International AIDS Society-USA Panel. JAMA 2002;288:222–235 [DOI] [PubMed] [Google Scholar]
- 3.Thompson MA, Aberg JA, Cahn P, et al. . Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010;304:321–333 [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Service Panel on Antiretroviral Agents in HIV-Infected Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2009. Available at: http://aidsinfo.nih.gov/content-files/adultadolescentGL.pdf (Last accessed November19, 2015)
- 5.Thomas F, Aggleton P, Anderson J. “If I cannot access services, then there is no reason for me to test”: The impacts of health service charges on HIV testing and treatment amongst migrants in England. AIDS Care 2010;22:526–531 [DOI] [PubMed] [Google Scholar]
- 6.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr 2006;43(Suppl 1):S23–S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010;376:367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabkin JG, McElhiney MC, Ferrando SJ. Mood and substance use disorders in older adults with HIV/AIDS: Methodological issues and preliminary evidence. AIDS 2004;18(Suppl 1):S43–S48 [PubMed] [Google Scholar]
- 9.Baum MK, Rafie C, Lai S, et al. . Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr 2009;50:93–99 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Batchelder AW, Psaros C, Safren S. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011;58:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn J, Laranjeira RR. HIV-risk behaviour among non-heroin using cocaine injectors and non-injectors in São Paulo, Brazil. AIDS Care 2000;12:471–481 [DOI] [PubMed] [Google Scholar]
- 12.Rosen MI, Black AC, Arnsten JH, et al. . Association between use of specific drugs and antiretroviral adherence: Findings from MACH 14. AIDS Behav 2013;17:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condelli WS, Fairbank JA, Dennis ML, Rachal JV. Cocaine use by clients in methadone programs: Significance, scope, and behavioral interventions. J Subst Abuse Treat 1991;8:203–212 [DOI] [PubMed] [Google Scholar]
- 14.Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Depend 1995;37:29–35 [DOI] [PubMed] [Google Scholar]
- 15.Kolar AF, Brown BS, Weddington WW, Ball JC. A treatment crisis: Cocaine use by clients in methadone maintenance programs. J Subst Abuse Treat 1990;7:101–107 [DOI] [PubMed] [Google Scholar]
- 16.Applebaum AJ, Bullis JR, Traeger LN, et al. . Rates of mood and anxiety disorders and contributors to continued heroin use in methadone maintenance patients: A comparison by HIV status. Neurobehav HIV Med 2010;2010:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safren SA, O'Cleirigh C, Tan JY, et al. . A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 2009;28:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safren SA, O'Cleirigh CM, Bullis JR, et al. . Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. J Consult Clin Psychol 2012;80:404–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber E. Compliance: A new monitoring method with the Medication Event Monitoring System. Med Monatsschr Pharm 1988;11:308–309 [in German]. [PubMed] [Google Scholar]
- 20.Liu H, Miller LG, Hays RD, et al. . Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr 2006;41:315–322 [DOI] [PubMed] [Google Scholar]
- 21.Llabre MM, Weaver KE, Durán RE, et al. . A measurement model of medication adherence to highly active antiretroviral therapy and its relation to viral load in HIV-positive adults. AIDS Patient Care STDS 2006;20:701–711 [DOI] [PubMed] [Google Scholar]
- 22.McLellan AT, Kushner H, Metzger D, et al. . The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9:199–213 [DOI] [PubMed] [Google Scholar]
- 23.Safren SA, Otto MW, Worth JL. Life-steps: Applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract 1999;6:332–341 [Google Scholar]
- 24.Leserman J, Jackson ED, Petitto JM, et al. . Progression to AIDS: The effects of stress, depressive symptoms, and social support. Psychosom Med 1999;61:397–406 [DOI] [PubMed] [Google Scholar]
- 25.Arnsten JH, Demas PA, Grant RW, et al. . Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 2002;17:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayaki J, Stein MD, Lassor JA, Herman DS, Anderson BJ. Adversity among drug users: Relationship to impulsivity. Drug Alcohol Depend 2005;78:65–71 [DOI] [PubMed] [Google Scholar]
- 27.Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS 1997;11:1665–1670 [DOI] [PubMed] [Google Scholar]
- 28.Kidder DP, Wolitski RJ, Campsmith ML, Nakamura GV. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health 2007;97:2238–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N, Squier C, Sivek C, et al. . Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: Prospective assessment with implications for enhancing compliance. AIDS Care 1996;8:261–269 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan KA, Messer LC, Quinlivan EB. Substance abuse, violence, HIV/AIDS (SAVA) syndemic effects on viral suppression among HIV positive women of color. AIDS Patient Care STDS 2015;29:S42–S48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlmeier MD, Peters K, Wildt BTT, et al. . Comorbidity of alcohol and substance dependence with attention-deficit/hyperactivity disorder (ADHD). Alcohol Alcohol 2008;43:300–304 [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Wang G-J, Ma Y, et al. . Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci 2003;23:11461–11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan M, Golin CE, Jones CD, et al. . Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Ann Intern Med 2012;157:785–795 [DOI] [PubMed] [Google Scholar]
- 34.Drainoni M-L, Farrell C, Sorensen-Alawad A, et al. . Patient perspectives of an integrated program of medica care and substance use treatment. AIDS Patient Care STDS 2014;28:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wish ED, Hoffman JA, Nemes S. The validity of self-reports of drug use at treatment admission and at followup: Comparisons with urinalysis and hair assays. NIDA Res Monogr 1997;167:200–226 [PubMed] [Google Scholar]