Abstract
Men who have sex with men (MSM) with bacterial sexually transmitted diseases (STDs) are at elevated risk for HIV infection, but often do not test for HIV at time of STD diagnosis. We instituted and evaluated a program promoting HIV testing through STD partner services (PS). In May 2012, health departments in Washington State modified STD PS programs with the objective of providing PS to all MSM with early syphilis, gonorrhea, or chlamydial infection and ensuring that those without a prior HIV diagnosis tested for HIV infection. We used chi-square tests and logistic and log-binomial regression to compare the percentage of MSM who received PS, HIV tested, and were newly HIV diagnosed before (January 1, 2010 to April 30, 2012) and during the revised program (May 1, 2012 to August 31, 2014). Among MSM without a prior HIV diagnosis, 2008 (62%) of 3253 preintervention and 3712 (76%) of 4880 during the intervention received PS (p < 0.001). HIV testing among PS recipients increased from 63% to 91% (p < 0.001). PS recipients were more likely to be newly HIV diagnosed than nonrecipients during the preintervention (2.5% vs. 0.93%, p = 0.002) and intervention periods (2.4% vs. 1.4%, p = 0.050). The percentage of MSM with newly diagnosed HIV infection who had a concurrent STD diagnosis increased from 6.6% to 13% statewide (p < 0.0001). Among all MSM with bacterial STDs, 61 (1.9%) preintervention and 104 (2.1%) during the intervention were newly diagnosed with HIV infection (adjusted relative risk = 1.34, p = 0.07). In conclusion, promoting HIV testing through STD PS is feasible and increases HIV testing among MSM. Our findings suggest that integrating HIV testing promotion into STD PS may increase HIV case finding.
Introduction
Case finding and treatment are core components of the US National HIV/AIDS Strategy.1 The US HIV epidemic is concentrated in men who have sex with men (MSM), a group that includes ∼2–3% of the US population, but experiences 68% of all HIV infections in the United States.2 An estimated 15% of HIV-infected MSM in the United States are thought to be undiagnosed3 and therefore unable to change behaviors or receive antiretroviral therapy to reduce morbidity and ongoing transmission.4,5 Identifying these men and ensuring their successful receipt of medical care is a national HIV prevention priority. How best to identify undiagnosed MSM living with HIV is unknown.
Diagnosis with a sexually transmitted disease (STD), particularly syphilis, is perhaps the most consistent risk factor for testing HIV positive and future HIV acquisition.6–8 As a result, the US Centers for Disease Control and Prevention (CDC) recommend that all persons who seek evaluation and treatment for STDs should be screened for HIV infection.9 Despite this, clinicians only HIV test approximately half of persons treated for gonorrhea in the United States, even among MSM,10 and insurance claims data suggest that an even smaller proportion of all persons screened for STDs receive an HIV test.11,12 HIV testing at the time of STD screening or treatment is more common at STD and sexual health clinics10,13,14 and less common in emergent care settings and rural areas, where written informed consent or opt-in testing is required, or when the provider considers the patient to be at low risk or to have tested too recently.10,11,15,16
STD partner services (PS) present an opportunity to ensure that persons diagnosed with STDs receive recommended HIV testing regardless of the practices of the diagnosing or treating provider and, therefore, may be one means for identifying MSM with undiagnosed HIV infection. However, at present, few public health departments provide PS to persons with STDs other than syphilis and HIV,17 and HIV testing is not a specific, consistently measured objective of PS. In May 2012, Washington State initiated a policy to provide STD PS to all MSM with early syphilis, gonorrhea, and chlamydial infection. Part of this effort was a specific focus on ensuring that all MSM diagnosed with these STDs (index cases) and their sex partners tested for HIV infection. In this study, we present an evaluation of this program's effects on delivery of PS, HIV testing, and HIV case finding among index cases.
Materials and Methods
STD case reporting
Medical providers in Washington State are legally required to complete a case report form for each person they diagnose with syphilis, gonorrhea, or chlamydial infection. This form includes gender of sex partners, allowing health departments to identify MSM for potential intervention. Clinical laboratories are also required to report these infections, and public health staff routinely follow up on laboratory-reported cases that do not have associated provider-initiated case reports. In these cases, public health staff complete case reports using information from medical record reviews or conversations with providers' offices.
PS intervention
In May 2012, health departments in Washington State revised STD PS programs to provide PS to all MSM with early syphilis (primary, secondary, or early latent), gonorrhea, or chlamydial infection. This effort sought to ensure that all MSM and sex partners without a prior HIV diagnosis were tested for HIV infection before closure of the index case. During PS interviews, disease intervention specialists (DIS, the public health workers who provide PS) routinely asked cases whether they had ever had an HIV test, ever tested HIV positive, and whether they had tested for HIV infection at the time of their STD diagnosis or treatment.
If a case reported being HIV negative or not knowing his HIV status and had not tested for HIV infection at the time of his STD diagnosis or treatment, DIS attempted to ensure that he received an HIV test before closing the case. Depending on health department capacity, HIV testing facilities available to the case, and case preference, HIV testing was provided directly by DIS at a public health STD clinic, other health department facility, or through a field visit or indirectly by referral back to the diagnosing provider, to another healthcare provider, or to a local HIV testing site. If testing could not otherwise be arranged, DIS offered to mail cases a home HIV test (OraQuick® In-Home HIV Test or Home Access® HIV-1 Test System). DIS attempted to follow up with cases or providers to confirm that HIV testing occurred and learn case's test results. In addition, as part of this intervention, DIS promoted HIV testing at the time of STD diagnosis or treatment when interacting with staff at diagnosing facilities (e.g., when following up with providers to complete case reports) to increase the likelihood that index cases would be tested for HIV infection before the PS interview.
Before the intervention, all local health jurisdictions prioritized STD cases for PS when diagnosing providers requested assistance with partner management using the case report form.18 Other priorities varied by jurisdiction, and there was no specific effort to promote HIV testing as part of PS.
Data sources
We used matched HIV and STD surveillance/PS data from Washington State from January 1, 2010, through October 20, 2014. STD surveillance and PS data are routinely matched with HIV surveillance data from the Enhanced HIV/AIDS Reporting System (eHARS) through a two-step process. First, an automated probabilistic matching algorithm based on legal and alias names, date of birth, and sex is run weekly. Second, Washington State Department of Health staff conduct a monthly manual review of STD cases not matched to eHARS but with an indication of HIV infection in STD surveillance or PS data (e.g., case reports living with HIV).
Population
MSM with early syphilis, gonorrhea, or chlamydial infection who had not previously been diagnosed with HIV infection were included in this analysis. We defined MSM as men who reported sex with men in the last year during STD PS interviews, whose provider indicated male sex partners on the STD case report, or who were diagnosed with rectal gonorrhea or rectal chlamydial infection. To include only MSM without prior HIV diagnoses in this analysis, we excluded men whose HIV diagnosis date in eHARS preceded the date of their STD diagnosis or who self-reported a prior HIV diagnosis during PS interviews.
HIV testing and diagnosis outcomes
We defined MSM STD cases as having tested for HIV infection at the time of STD diagnosis if they tested within 14 days before the STD diagnosis, at the time of STD diagnosis or treatment, or as a result of PS. Similarly, MSM were determined to have been newly diagnosed with HIV infection at the time of STD diagnosis if they tested newly positive at the time of diagnosis or treatment or as a result of PS. HIV testing and new diagnoses were ascertained by one of the following methods: case self-report during PS interview, medical record review by DIS, or an HIV diagnosis date in eHARS during the relevant time period.
A Public Health—Seattle & King County DIS conducted periodic manual reviews of STD cases whose records suggested that they were newly diagnosed with HIV infection at the time of STD diagnosis to confirm HIV diagnoses and whether the HIV diagnosis occurred before, concurrent with, as a result of, or if it was unrelated to the STD diagnosis. In evaluating the effect of PS on HIV case finding, we included only new HIV diagnoses that HIV tested at the time of STD diagnosis or treatment or as a consequence of receiving STD PS. Persons diagnosed with an STD after their HIV diagnosis were excluded.
Statistical analysis
We examined the effects of promoting HIV testing through STD PS using a pre/postanalysis comparing MSM diagnosed with bacterial STDs before the initiation of the intervention (preintervention period = January 2010–April 2012) and during the initial intervention period (May 2012–August 2014). The preintervention period was selected to be equal in duration to the intervention period.
The numbers of reported STD cases overall and by each STD type (defined by pathogen and anatomic site of infection) were compared between the two periods using incidence rate ratios. The proportion of PS recipients who were tested for HIV infection, the proportion of all cases newly diagnosed with HIV infection, and the proportion of HIV-tested persons who were newly diagnosed, as well as the distribution of cases by county of residence and diagnosing provider type, were compared between the two periods using Pearson's chi-square tests. HIV/STD specialty providers were defined as an STD clinic, HIV/STD testing program, or medical provider specializing in HIV or STD care or MSM health.
Multivariable analyses to adjust for differences in diagnosing provider type, county of residence, and STD type between the two periods were conducted using log-binomial regression for the proportion HIV tested and Poisson regression offset by HIV incidence among MSM in Washington State per quarter for the proportion newly HIV diagnosed. This offset adjusted for temporal trends in HIV incidence.
HIV incidence for each 3-month period was calculated by dividing the number of new HIV cases reported among MSM in eHARS by an estimate of the population of HIV-uninfected MSM in Washington State during the quarter. An annual estimate of the number of HIV-uninfected MSM was calculated as 5.4% (the midpoint between reporting ever having sex with men and reporting sex with men in the past 5 years19) of the intercensal estimate of the number of men aged 15 or older in Washington State in that year minus the number of MSM known to be living with HIV from eHARS. This population estimate was then divided by four for quarterly incidence estimates.
We also compared (1) the proportion of STD cases tested for HIV infection stratified by jurisdiction and provider type and (2) the number of new HIV diagnoses and proportion of STD cases newly HIV diagnosed between the two periods stratified by STD type. During the intervention period, the proportion of cases tested for and newly diagnosed with HIV infection was stratified by whether testing occurred before or after DIS intervention.
Gonorrhea diagnoses among men who have sex with women only
Beginning in May 2012, cases of gonorrhea reported among men who have sex with women only or whose gender of sex partners was unknown (MSW) without a prior HIV diagnosis were also prioritized for PS and HIV testing. We hypothesized that these cases might include MSM who were not being reported as such by the diagnosing provider and therefore might be a source of undiagnosed HIV infection. As part of routine monitoring and evaluation, prioritization of these cases was discontinued in January 2014 after public health officials determined that the effort had been ineffective. We compared receipt of PS and HIV testing at the time of STD diagnosis or treatment between preintervention (September 2010–April 2012) and intervention periods (May 2012–December 2013) of equal duration using Pearson's chi-square tests.
Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and Stata 11.0 (Stata Corp, College Station, TX). Because these analyses were conducted as part of public health program activities, this was not considered human subjects research.
Results
From January 1, 2010 through August 31, 2014, a total of 8133 cases of early syphilis, gonorrhea, or chlamydial infection were diagnosed among MSM without a prior HIV diagnosis in Washington State. Table 1 presents characteristics of these cases stratified by preintervention (n = 3253) or intervention period (n = 4880). The number of reported STD cases increased during the intervention, with particular increases observed among extragenital STDs (rectal or pharyngeal gonorrhea or chlamydial infection; p < 0.001). About two-thirds of cases were diagnosed in King County, which includes Seattle, the urban center of the state, where infections were more likely to be diagnosed by an HIV/STD specialty provider than in other jurisdictions (74% vs. 17%, respectively; p < 0.0001).
Table 1.
Characteristics of 8133 Cases of Bacterial STDs Diagnosed Among MSM Without a Prior HIV Diagnosis in Washington State, January 2010–August 2014
| Preintervention, N (%) | Intervention, N (%) | ||
|---|---|---|---|
| Characteristic | January 2010–April 2012 | May 2012–August 2014 | p |
| Total cases in MSM | 3253 (–) | 4880 (–) | <0.0001 |
| Sexually transmitted disease | |||
| Early syphilis (primary, secondary, early latent) | 347 (11) | 448 (9) | 0.0003 |
| Rectal gonorrhea | 413 (13) | 758 (16) | <0.0001 |
| Urethral gonorrhea | 857 (26) | 1130 (23) | <0.0001 |
| Pharyngeal gonorrhea | 484 (15) | 1047 (21) | <0.0001 |
| Rectal chlamydial infection | 561 (17) | 1265 (26) | <0.0001 |
| Urethral chlamydial infection | 1121 (35) | 1245 (26) | 0.0108 |
| Pharyngeal chlamydial infection | 95 (3) | 282 (6) | <0.0001 |
| King County resident (includes Seattle) | 2201 (68) | 3440 (70) | 0.0067 |
| Diagnosed by HIV/STD specialty providera | 1749 (54) | 2832 (58) | 0.0001 |
HIV/STD specialty provider was defined as an STD clinic, HIV/STD testing program, or medical provider specializing in HIV or STD care or MSM health.
MSM, men who have sex with men; STD, sexually transmitted disease.
Delivery of PS
The proportion of MSM diagnosed with an STD and without a prior HIV diagnosis who received PS increased from 62% in the preintervention period to 76% in the intervention period (p < 0.001, Table 2). Delivery of PS increased concurrent with the intervention for all STD types excluding early syphilis, which has been prioritized for PS historically and had delivery rates near 90% during both periods (Table 2).
Table 2.
Partner Services Delivery, HIV Testing, and HIV Case Finding Among MSM with Bacterial STDs by Intervention Period and STD Type
| Interviewed for PS, % | Tested for HIV (of interviewed), % | Newly HIV diagnosed (of all), % | ||||
|---|---|---|---|---|---|---|
| Preintervention | Intervention | Preintervention | Intervention | Preintervention | Intervention | |
| Overall | 62a | 76a | 63a | 91a | 1.9 | 2.1 |
| Early syphilis | 89 | 91 | 74a | 95a | 6.3 | 6.9 |
| Rectal GC | 70a | 81a | 74a | 93a | 3.0 | 3.9 |
| Urethral GC | 65a | 76a | 45a | 83a | 0.9 | 1.7 |
| Pharyngeal GC | 74b | 81b | 81a | 95a | 1.1 | 1.0 |
| Rectal CT | 64a | 80a | 82a | 96a | 2.0 | 1.1 |
| Urethral CT | 41a | 58a | 47a | 85a | 0.5 | 0.5 |
| Pharyngeal CT | 60b | 78b | 77b | 92b | 4.7 | 2.7 |
An additional 128 cases of GC or CT were reported without anatomic site of infection, of which 3.1% were newly HIV diagnosed at the time of STD diagnosis or treatment.
p < 0.0001; bp < 0.05; otherwise, p > 0.05.
CT, chlamydial infection; GC, gonorrhea; MSM, men who have sex with men; STD, sexually transmitted disease; PS, partner services.
HIV testing at time of STD diagnosis or treatment
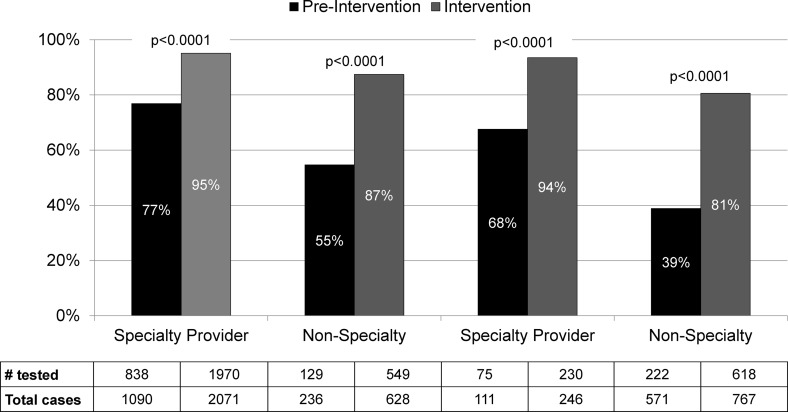
Implementation of the intervention was associated with an increase in HIV testing among MSM without a prior HIV diagnosis who received PS (63–91%, p < 0.001; Table 2). Figure 1 depicts the percent of HIV-negative/unknown MSM diagnosed with bacterial STDs who were tested for HIV infection at the time of STD diagnosis or treatment in the preintervention versus intervention periods, stratified by jurisdiction (King County vs. other Washington) and provider type (HIV/STD specialty provider vs. other). Significant increases in HIV testing were observed across all strata (p < 0.0001 for all); however, specialty providers were more likely to test MSM for HIV infection at the time of STD diagnosis or treatment before and during the intervention periods (p < 0.001). Similarly, the intervention was associated with significant increases in HIV testing within each STD type, although HIV testing during the preintervention period occurred more commonly with early syphilis and extragenital infections, which are more likely to be diagnosed by specialty providers (Table 2).
FIG. 1.
Effect of partner services intervention on the percentage of MSM with bacterial STDs tested for HIV infection at time of STD diagnosis or treatment, by provider type and county of residence, among those receiving partner services. HIV/STD. Specialty providers were defined as STD clinics, HIV/STD testing programs, or medical providers specializing in HIV or STD care or MSM health. MSM, men who have sex with men; STD, sexually transmitted disease.
In multivariable analyses, the increase in HIV testing among PS recipients associated with the intervention remained significant when adjusting for provider type, county of residence, and STD type (p < 0.001). During the intervention period, 91% of PS recipients who tested for HIV infection tested before the initial PS interview and 9% tested after. These proportions remained consistent throughout the intervention period (data not shown). MSM diagnosed with STDs by specialty providers were significantly more likely to have tested before PS interview than those diagnosed by other providers (97% vs. 79%, p < 0.0001).
HIV case finding
The number of MSM with bacterial STDs newly diagnosed with HIV infection increased from 61 during the preintervention period to 104 during the intervention period; however, the proportion of all STD cases diagnosed with HIV infection did not increase significantly [1.9% preintervention to 2.1% during; relative risk (RR) = 1.14, 95% confidence interval (CI) 0.83–1.56; p = 0.42; Table 2]. In a multivariable analysis adjusting for provider type, county of residence, STD type, and temporal trends in HIV incidence, the intervention was associated with a 1.34-fold increase in the proportion of STD cases newly diagnosed with HIV infection (adjusted RR = 1.34, 95% CI 0.97–1.83; p = 0.07). In addition, the proportion of new HIV diagnoses among MSM in Washington State concurrently diagnosed with an STD increased from 6.6% (61/930) preintervention to 13% (104/797) in the intervention period (RR = 1.99, 95% CI 1.47–2.69; p < 0.0001).
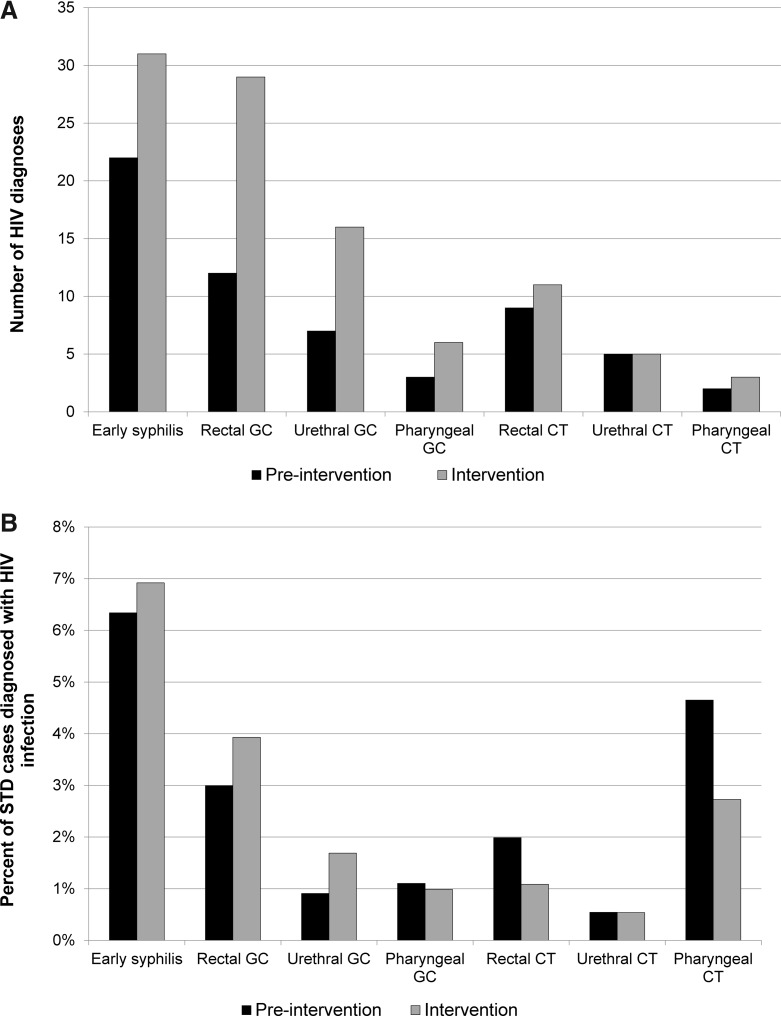
Stratified by STD type, the proportion of STD cases newly diagnosed with HIV infection increased slightly among men diagnosed with early syphilis, rectal gonorrhea, or urethral gonorrhea; was stable among men diagnosed with pharyngeal gonorrhea or urethral chlamydial infection; and decreased among men diagnosed with rectal chlamydial infection or pharyngeal chlamydial infection. However, none of these differences was statistically significant (Table 2 and Fig. 2). Only six STD cases, who HIV tested after the initial PS interview, were newly diagnosed with HIV infection during the intervention period. Of these six cases, five had been diagnosed with urethral gonorrhea and one with early syphilis, and five were diagnosed by nonspecialty providers.
FIG. 2.
Effects of partner services intervention on new HIV diagnoses among MSM with bacterial STDs by pathogen and anatomic site of infection. (A) Number of new HIV diagnoses. (B) Percentage of STD cases newly diagnosed with HIV infection. MSM, men who have sex with men; STD, sexually transmitted disease.
Intervention effects among MSW with gonorrhea
From September 1, 2010 through December 31, 2013, a total of 3057 cases of gonorrhea were reported among MSW without a prior HIV diagnosis in Washington State. The proportion of MSW gonorrhea cases who received PS increased from 48% (594/1250) preintervention to 58% (1048/1807) during the intervention (p < 0.0001). Among PS recipients, HIV testing at the time of STD diagnosis or treatment increased from 27% (160/594) to 71% (740/1048) (p < 0.0001). During the intervention period, 60% of PS recipients who tested for HIV infection tested before the initial PS interview and 40% tested after. However, no cases were newly diagnosed with HIV infection among all MSW diagnosed with gonorrhea during either the preintervention or intervention period. Receipt of PS and HIV testing among PS recipients fell to 48% and 57%, respectively, in the eight months following discontinuation of the intervention.
Discussion
We found that it was feasible to use case reports to focus STD PS on MSM and that making HIV testing an explicit objective of STD PS was associated with an increase in HIV testing among MSM diagnosed with STDs. This increase was particularly pronounced among men diagnosed outside of medical practices that focus specifically on HIV/STD or MSM health. We also observed an increase in new HIV diagnoses among MSM with bacterial STDs during our intervention compared to a historical control period and a trend toward an increase in the proportion of all STD cases diagnosed with HIV infection. Overall, these findings demonstrate that STD PS can be used to promote HIV testing in a population at high risk for HIV infection and suggest that this intervention may increase HIV case finding.
While our findings suggest a potential role for STD PS in increasing HIV case finding, the ability of other areas to institute a similar program and the generalizability of our findings are uncertain. Our intervention required a significant pre-existing public health STD infrastructure, including STD case report forms that include information on the gender of case's sex partners, routine follow-up by public health staff regarding laboratory-reported cases without case reports from providers, and a web-based statewide database that DIS use for case investigation. These resources, which are not routinely available throughout the United States, allowed health departments to identify MSM for PS. Instituting the intervention we describe in the absence of this infrastructure would require significant, up-front public health investments.
The characteristics of the HIV epidemic in Washington State and our area's clinical infrastructure may have affected our findings and are important in interpreting our results. We estimate that 94% of all MSM living with HIV in King County, the urban center of Washington State, are already diagnosed,20 new diagnoses are declining in MSM,21 and a large proportion of STD cases diagnosed in MSM are made in clinical settings designed to serve these men.22 These facts, which represent successes in HIV/STD prevention and care, make it difficult for new interventions to identify large numbers of new HIV cases. In areas where a smaller proportion of MSM living with HIV are aware of their status, new diagnoses are stable or increasing, or fewer MSM STD diagnoses occur in specialty clinical settings, integrating HIV testing into STD PS could be associated with substantially greater case finding and cost-effectiveness. This issue requires additional investigation in other parts of the United States.
We found that STD PS could significantly increase HIV testing among MSW with bacterial STDs, but that this increase did not identify new HIV cases. While this experience led us to discontinue our effort to promote HIV testing among MSW through STD PS, these findings may be less informative for other parts of the United States. The HIV epidemic in Washington State is highly concentrated, with approximately three-quarters of new diagnoses occurring among MSM.21 Efforts to use STD PS to promote HIV case finding among MSW could be more effective in areas with larger heterosexual HIV epidemics or where fewer MSM are open about their sexuality.
This program evaluation has some limitations. First, most HIV testing and diagnosis occurred before PS interviews, and it is therefore difficult to distinguish between the effect of changes in provider or MSM testing behavior (also targeted by public health intervention) and direct PS delivery. However, the proportion of MSM receiving HIV testing before PS did not change over time during the intervention, suggesting that DIS promoting HIV testing at the time of STD diagnosis or treatment when interacting with staff at diagnosing facilities did not affect provider behavior. Second, HIV incidence decreased and STD diagnoses increased during the study period, possibly affecting the ability to detect an increase in HIV case finding. In particular, increases in extragenital testing for gonorrhea and chlamydial infection led to increases in diagnosis of extragenital infections, potentially increasing the likelihood of concurrent HIV and STD diagnosis. Adjusting for temporal trends in HIV incidence and changes in the distribution of STD types (among other confounders) did strengthen the association observed between the intervention and the proportion of STD cases newly diagnosed with HIV infection, but we were unable to adjust for all potential confounding in this observational analysis using programmatic data. Finally, ascertainment of testing HIV negative at the time of STD diagnosis and the timing of HIV testing with respect to PS delivery (before vs. after PS interview) improved concurrent with the intervention. It is unlikely that ascertainment of testing newly HIV positive was affected because we matched STD and HIV surveillance.
In conclusion, despite a high risk for having undiagnosed HIV infection, MSM with bacterial STDs often do not receive HIV testing at the time of their STD diagnosis or treatment. Promoting HIV testing through STD PS is feasible, increases HIV testing among MSM, and may increase HIV case finding in this population.
Acknowledgments
This program and its evaluation were supported by the US Centers for Disease Control and Prevention (CDC PS12-1201 and 3H25PS004364), the Washington State Department of Health, and Public Health—Seattle & King County. The evaluation was also supported by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK, of the National Institutes of Health under award number P30AI027757. R.P.K. was supported by NIAID (K01 AI095060). The authors thank Jason Carr at the Washington State Department of Health for providing estimates from HIV surveillance; Dr. James Hughes at the University of Washington for statistical support; and the disease intervention specialists of Washington State for their work conducting partner services and promoting HIV testing in persons with STDs.
Author Disclosure Statement
M.R.G. has received research support from Cempra Pharmaceuticals and Melinta Therapeutics. The remaining authors have no potential conflicts of interest to declare.
References
- 1.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Available at: www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf (Last accessed November9, 2015)
- 2.Centers for Disease Control and Prevention. HIV Surveillance Report, 2013; Volume 25 Available at: www.cdc.gov/hiv/library/reports/surveillance (Last accessed November9, 2015) [Google Scholar]
- 3.Hall HI, An Q, Tang T, et al. Prevalence of diagnosed and undiagnosed HIV infection—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2015;64:657–662 [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005;39:446–453 [DOI] [PubMed] [Google Scholar]
- 6.Piot P, Laga M. Genital ulcers, other sexually transmitted diseases, and the sexual transmission of HIV. BMJ 1989;298:623–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 2010;5:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workowski KA. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015;61:S759–S762 [DOI] [PubMed] [Google Scholar]
- 10.Bradley H, Asbel L, Bernstein K, et al. HIV testing among patients infected with Neisseria gonorrhoeae: STD Surveillance Network, United States, 2009–2010. AIDS Behav 2013;17:1205–1210 [DOI] [PubMed] [Google Scholar]
- 11.Chen JY, Ma Q, Everhard F, Yermilov I, Tian H, Mayer KH. HIV screening in commercially insured patients screened or diagnosed with sexually transmitted diseases or blood-borne pathogens. Sex Transm Dis 2011;38:522–527 [DOI] [PubMed] [Google Scholar]
- 12.Tao G, Zhang CX. HIV testing of commercially insured patients diagnosed with sexually transmitted diseases. Sex Transm Dis 2008;35:43–46 [DOI] [PubMed] [Google Scholar]
- 13.Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med 2004;19:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Balbuena S, Hoyos J, Rosales-Statkus ME, et al. Low HIV testing uptake following diagnosis of a sexually transmitted infection in Spain: Implications for the implementation of efficient strategies to reduce the undiagnosed HIV epidemic. AIDS Care. DOI: 10.1080/09540121.2015.1123808 [DOI] [PubMed] [Google Scholar]
- 15.Joore IK, Reukers DF, Donker GA, et al. Missed opportunities to offer HIV tests to high-risk groups during general practitioners' STI-related consultations: An observational study. BMJ Open 2016;6:e009194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munro HL, Lowndes CM, Daniels DG, Sullivan AK, Robinson AJ. National study of HIV testing in men who have sex with men attending genitourinary clinics in the United Kingdom. Sex Transm Infect 2008;84:265–270 [DOI] [PubMed] [Google Scholar]
- 17.Golden MR, Hogben M, Handsfield HH, St Lawrence JS, Potterat JJ, Holmes KK. Partner notification for HIV and STD in the United States: Low coverage for gonorrhea, chlamydial infection, and HIV. Sex Transm Dis 2003;30:490–496 [DOI] [PubMed] [Google Scholar]
- 18.Golden MR, Kerani RP, Stenger M, et al. Uptake and population-level impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: The Washington State community-level randomized trial of EPT. PLoS Med 2015;12:e1001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J 2012;6:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellows IE, Morris M, Birnbaum JK, et al. A new method for estimating the number of undiagnosed HIV infected based on HIV testing history, with an application to men who have sex with men in Seattle/King County, WA. PLoS One 2015;10:e0129551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Public Health–Seattle & King County HIV/AIDS Epidemiology Unit, Washington State Department of Health Infectious Disease Assessment Unit. 2014. HIV/AIDS Epidemiology Report, Volume 83 [Google Scholar]
- 22.Katz DA, Dombrowski JC, Bennett A, Buskin S, Thibault C, Golden MR. Earlier diagnosis of HIV infection among men who have sex with men (MSM) in HIV/STD-focused healthcare services [Abstract TP27]. In: 2014 STD Prevention Conference Abstracts (Atlanta, GA). Sex Transm Dis 2014;41:S46–S47 [Google Scholar]