Abstract
OBJECTIVE
The purpose of this study was to evaluate the repeatability of liver mean standardized uptake value normalized to lean body mass (SULmean) in the same patients at different time points within the right lobe of the liver at 18F-FDG PET/CT, in a clinical setting.
MATERIALS AND METHODS
Two PET/CT studies performed on two different dates from each of 130 patients who had normal livers according to structural imaging were included in this reader study. The mean (± SD) length of time between the studies was 235 ± 192 days. SULmean was measured with a 30-mm diameter spherical volume of interest (VOI) placed within the right lobe of the liver (above, below, and at the level of the main portal vein) by two expert readers. ANOVA, intraclass correlation coefficient (ICC), and Bland-Altman analysis were performed.
RESULTS
The ICC for the first and second set of studies varied between 0.487 and 0.535 for reader 1 and between 0.472 and 0.545 for reader 2. The mean percentage variation for SULmean between the two time scans for the VOIs placed above, below, and at the level of the main portal vein were 3.55% ± 23.19%, 4.65% ± 23.87%, and 4.30% ± 23.03%, respectively, for reader 1 and 4.49% ± 23.23%, 4.33% ± 23.74%, and 4.48% ± 23.01%, respectively, for reader 2. Using 95% CI, the reference range for intrapatient variations between the studies in liver SULmean was −0.5 to 0.60.
CONCLUSION
There is only fair repeatability of liver SULmean measured between two time points in the same patient in a clinical setting. Scan-to-scan intrapatient variation in absolute liver SULmean was −0.5 to 0.60.
Keywords: liver mean standardized uptake value (SUV) normalized to lean body mass (SULmean), PET/CT, repeatability, therapy assessment
PET/CT of cancer with combined PET and CT scanners has become a standard component of diagnosis and staging in oncology [1–5]. When evaluating PET/CT for response to therapy, changes may not be visually evident and quantification therefore is important. The most common parameter used to measure tracer accumulation in PET studies is the maximum standardized uptake value (SUV). However, this index is subject to some variability [6–8]. It has been proposed that 18FFDG accumulation in a malignant lesion be compared with the background uptake in the liver parenchyma [9–11].
The liver mean SUV normalized to lean body mass (SULmean) has also been proposed as a quality measure for FDG PET/CT and is used in the PET Response Evaluation Criteria in Solid Tumors (PERCIST) 1.0 [12]. PERCIST defines a 3-cm spherical ROI in the right hepatic lobe as a quality assurance tool to assess the applicability of quantitative comparisons [12]. Liver SULmean has almost perfect inter- and intrareader agreement at a single time point [13, 14]. The repeatability and variability of the liver SULmean in the same patient at different time points and reader reliability of these repeatable measurements have not been established [9, 14–20]. The objective of this study was to evaluate the repeatability and variability of liver SULmean in the same patient performed at different dates measured by different readers and at different locations in the right lobe of the liver.
Materials and Methods
Institutional review board approval was obtained for this HIPAA-compliant retrospective review of PET/CT images and patient records. Informed consent requirements were waived. Two PET/CT studies from each of 130 patients (83 men and 47 women) performed at two different dates in patients who had normal livers according to structural imaging were included in the study. We excluded patients who underwent chemotherapy or radiation therapy involving the abdomen to minimize the impact on the FDG uptake in the liver between the two-time-point PET/CT studies. We reviewed the imaging reports of contrast-enhanced abdominal CT or sonography in the patient records to exclude patients with any liver abnormalities. SULmean was measured with a 30-mm-diameter spherical volume of interest (VOI) manually placed within the right lobe of the liver above, below, and at the level of the main portal vein [13].
PET/CT Protocol
All patients fasted for at least 4 hours before scanning. Serum glucose, FDG dose, patient weight, patient height, and injection-to-scanning time for first and second sets of scans are summarized in Table 1. Patients were scanned either on a Discovery ST or a Discovery LS PET/CT scanner (GE Healthcare). Studies on the first system were performed in 3D acquisition mode with 4.15 minutes per bed position. The images were reconstructed using ordered subset expectation maximization algorithms with 128 × 128 matrix, 21 subsets, two iterations, 3-mm postreconstruction gaussian filter, standard Z filter, 4.7-mm pixel, and 3.27-mm slice thickness. The 2D implementation on the Discovery LS used two iterations, 28 subsets, a 5.5-mm postreconstruction gaussian filter, and 3.9-mm pixels. All PET data were reconstructed with and without CT-based attenuation correction. Unenhanced CT was used for attenuation correction of PET images and for morphologic coregistration. CT parameters included 16-MDCT with 50-cm axial dynamic FOV, weight-based amperage (20–200 mA automated tube current), 120–140 kVp, 3.75-mm reconstructed slice thickness, pitch of 0.984, 0.5-second gantry rotation speed, and 512 × 512 matrix.
TABLE 1.
Patient and Scanning Characteristics for First- and Second-Time-Point PET/CT for Each Patient
| Characteristic | Value |
|---|---|
| Total no. | 130 |
| Sex | |
| Men | 83 |
| Women | 47 |
| Age (y) | 58.6 ± 13.3 (19–86) |
| Plasma glucose (mg/dL) | |
| First scan | 100.7 ± 15.8 (63–158) |
| Second scan | 98.9 ± 16.4 (62–154) |
| FDG dose (mBq) | |
| First scan | 606.8 ± 155.4 (321.9–939.8) |
| Second scan | 595.7 ± 148.0 (284.9–943.5) |
| Weight (kg) | |
| First scan | 74.2 ± 19.7 (41–159) |
| Second scan | 74.9 ± 19.1 (32–159) |
| Height (cm) | 172.1 ± 11.4 (134–198) |
| Uptake time | |
| First scan | 65.7 ± 15.9 (51–182) |
| Second scan | 65.5 ± 13.5 (39–125) |
| Elapsed time between scanning (d) | 235.4 ± 192.1 (33–1105) |
| Patients scanned on Discovery LSa | 48 (37) |
| Patients scanned on Discovery STa | 31 (24) |
| Patients scanned once on each scannera | 51 (39) |
Note—Except where indicated otherwise, data are mean ± SD with range in parentheses. Discovery LS and Discovery ST are manufactured by GE Healthcare.
Data are number with percentage in parentheses.
Liver SULmean Measurements
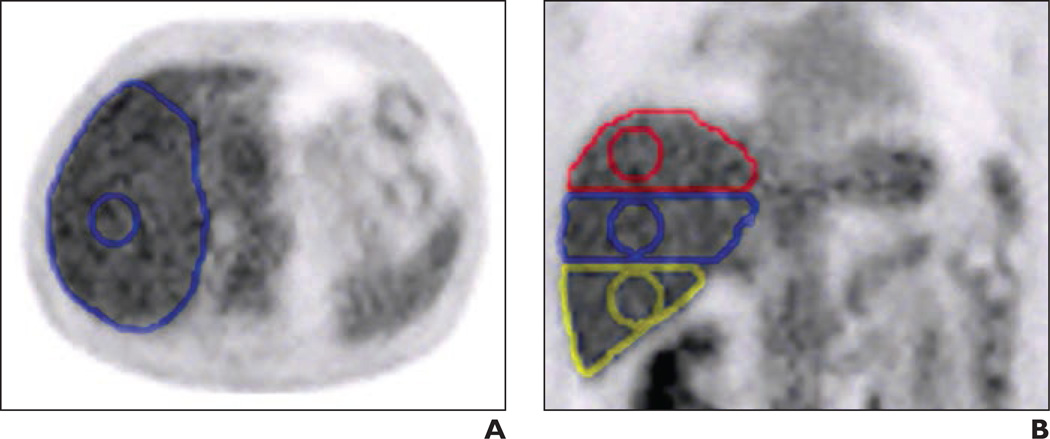
All PET/CT studies were retrieved from the electronic archival system and were then reviewed at a MimVista workstation, version 5.2 (MIM Software). Measurements of SULmean were conducted independently by two board-certified nuclear medicine physicians. Reader 1 completed a 3-year nuclear medicine residency, was board certified in nuclear medicine, and was a T32 research fellow in nuclear medicine. Reader 2 completed a 3-year nuclear medicine residency, a 2-year clinical PET/CT fellowship, and was board certified in nuclear medicine. PET, CT, and fused PET/CT images were reviewed in the axial, coronal, and sagittal planes. Liver SULmean values were measured in the following three separate areas within the right lobe of the liver: the right lobe above the portal vein (segments VII and VIII), below the level of the portal vein in the right lower lobe (segments V and VI), and at the level of the right portal vein (straddling segments V through VIII) (Fig. 1). Both readers independently placed the VOI in these three locations of the liver for the PET/CT studies of all study patients. The VOI was a sphere with a diameter of 30 mm. The right lobe of the liver was used because of its relatively large size and homogeneity. It is the most common site used in clinical practice as a background standard [21–23]. The analyses were done in a random order of scans (between the first set of scans and second set of scans) in multiple reading sessions over a period of 3 months.
Fig. 1.
Placement of volume of interest (VOI). A and B, Axial (A) and coronal (B) PET images show placement of 30-mm-diameter spherical VOIs within right lobe of liver: upper (red), portal vein (blue), and lower (yellow).
Statistical Analysis
We present central tendencies as mean ± SD. Between-group analysis for two groups was performed with an independent Student t test with a significance level of 0.05. For repeated-measures analyses, adjustment for multiple hypothesis testing in comparing the three locations within the right lobe of the liver was performed with ANOVA with repeated measures, using general linear model, polynomial contrast, and Bonferroni posthoc analysis between all pairs. Reliability of liver SULmean between the readers and between the dual-time-point PET images was measured using the intraclass correlation coefficient (ICC) generated by a two-way random-effects model with an absolute agreement definition and is reported as a point estimate with a 95% CI. The readers are assumed to be randomly selected from a large pool of experts. In this model, the ICC is a measure of absolute agreement and considers systematic differences between the two readers [13]. Results of Bland-Altman analysis with bias and SD of the differences were reported for variability between the readers at each level (the upper, lower, and portal vein levels of the right lobe of the liver). We used MedCalc, version 12.3 (MedCalc Software) and SPSS, version 20 (IBM) statistical packages for all analyses.
Results
Patient Demographics
A total of 130 patients (83 men and 47 women) were included in this study. The length of time between the two studies for each patient averaged 235 ± 192 days. The variation in patient weight between the two studies was 0.62 ± 5.14 kg. The plasma glucose concentrations were within the reference range; the mean difference in plasma glucose concentration was −48 ± 15.7 mg/dL. The mean difference between FDG uptake times was −0.23 ± 16.58 minutes. Patient and scanning characteristics are summarized in Table 1.
Liver SULmean Agreement and Variability: Single-Time-Point Scans
For reader 1, on earlier scans, liver SULmean was 1.53 ± 0.26 for the upper level, 1.51 ± 0.26 for the portal vein level, and 1.54 ± 0.26 for the lower level. On later scans liver SULmean was 1.55 ± 0.26, 1.54 ± 0.25, and 1.57 ± 0.25 for the upper, portal vein, and lower levels, respectively. For reader 2, on earlier scans, mean liver SULmean was 1.52 ± 0.26 for the upper level, 1.52 ± 0.26 for the portal vein level, and 1.53 ± 0.26 for the lower level. On later scans liver SULmean was 1.55 ± 0.26, 1.54 ± 0.25, and 1.56 ± 0.26 for the upper, portal vein, and lower levels, respectively, for reader 2. These results are summarized in Table 2.
TABLE 2.
Summary of Mean Liver Standardized Uptake Value Normalized to Lean Body Mass at Different Levels of Liver as Measured by Readers 1 and 2 on Two-Time-Point PET/CT
| Value | Upper Liver Level | Portal Vein Level | Lower Liver Level |
|---|---|---|---|
| Reader 1 | |||
| First scan | 1.53 ± 0.26 | 1.51 ± 0.26 | 1.54 ± 0.26 |
| Second scan | 1.55 ± 0.26 | 1.54 ± 0.25 | 1.57 ± 0.25 |
| Reader 2 | |||
| First scan | 1.52 ± 0.26 | 1.52 ± 0.26 | 1.53 ± 0.26 |
| Second scan | 1.55 ± 0.26 | 1.54 ± 0.25 | 1.56 ± 0.26 |
Note—Data are mean ± SD.
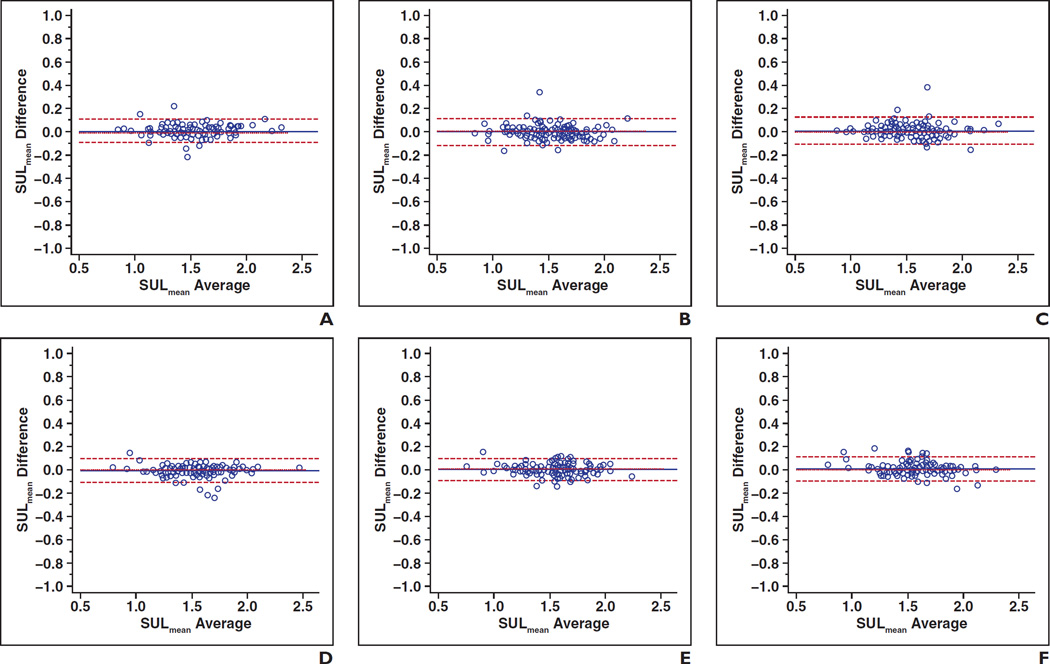
There was high agreement between the two readers for each of the three right liver lobe levels in both first and second scans. In earlier images, interreader ICC for the SULmean, controlling for location, was 0.990 (95% CI, 0.985–0.993) for the upper level, 0.987 (0.981–0.991) for the portal vein level, and 0.986 (0.981–0.990) for the lower level. In later images, interreader ICCs for the SULmean were 0.990 (0.986–0.993) for the upper level, 0.991 (0.987–0.994) for the portal vein level, and 0.989 (0.984–0.992) for the lower level. Figure 2 shows Bland-Altman plots of interreader agreement of the liver SULmean controlling for time point and liver location.
Fig. 2.
Bland-Altman plots show interreader agreement of liver mean standardized uptake value normalized to lean body mass (SULmean) controlling for time point and liver location.
A–C, Plots show first time point: upper liver (A) (intraclass coefficient [ICC] = 0.990), portal vein (B) (ICC = 0.987), and lower liver (C) (ICC = 0.986).
D–F, Plots show second time point: upper liver (D) (ICC = 0.990), portal vein (E) (ICC = 0.991), and lower liver (F) (ICC = 0.989).
Liver SULmean Agreement and Variability: Two-Time-Point Scans and Same VOI Location
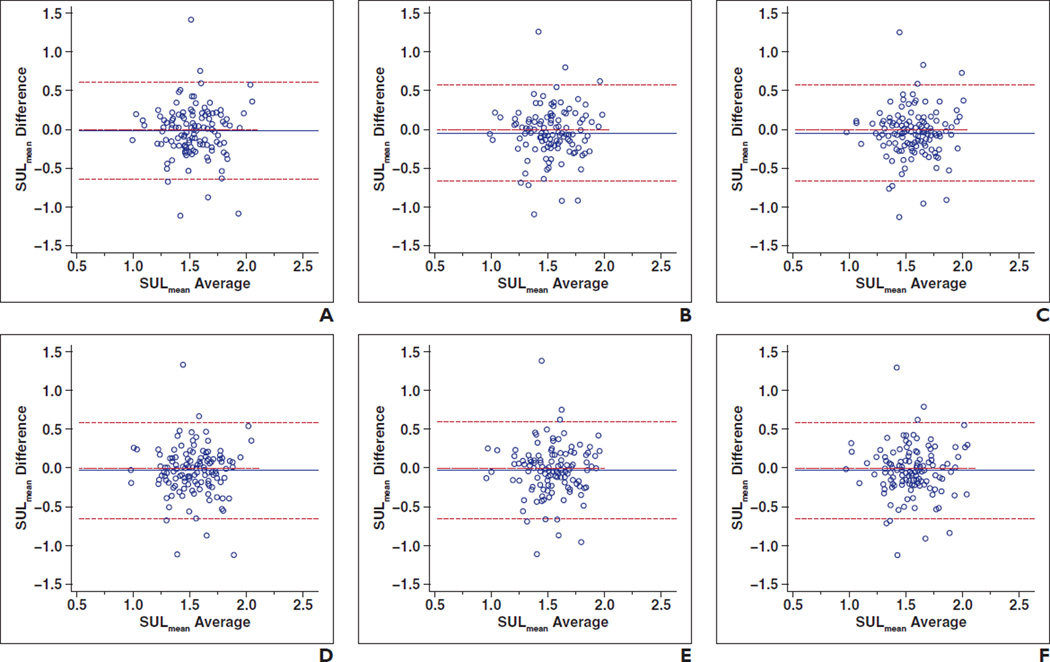
ICCs for the SULmean controlling for reader and location were 0.345 (0.071–0.538) for the upper level, 0.366 (0.101–0.552) for the portal vein level, and 0.383 (0.125–0.564) for the lower level for reader 1. ICCs for the liver SULmean measured by reader 2 controlling for location were 0.412 (0.166–0.585) for the upper level, 0.377 (0.117–0.560) for the portal vein level, and 0.438 (0.204–0.604) for the lower level. These results are summarized in Table 3. Figure 3 shows Bland-Altman plots of intrareader agreement of liver SULmean between two-time-point scans.
TABLE 3.
Absolute Change and Intraclass Coefficient (ICC) for Mean Liver Standardized Uptake Value Normalized to Lean Body Mass (SULmean) at Two-Time-Point PET/CT Controlling for Reader and Location
| Value | Upper Liver Level | Portal Vein Level | Lower Liver Level |
|---|---|---|---|
| Reader 1 | |||
| Change in SULmean | −0.02 (−0.65 to 0.61) | −0.02 (−0.65 to 0.59) | −0.02 (−0.65 to 0.59) |
| ICC | 0.345 (0.071–0.538) | 0.366 (0.101–0.552) | 0.383 (0.125–0.564) |
| Reader 2 | |||
| Change in SULmean | −0.02 (−0.65 to 0.58) | −0.02 (−0.65 to 0.60) | −0.02 (−0.65 to 0.58) |
| ICC | 0.412 (0.166–0.585) | 0.377 (0.117–0.560) | 0.438 (0.204–0.604) |
Note—Data in parentheses are 95% CI.
Fig. 3.
Bland-Altman plots show intrareader agreement of liver mean standardized uptake value normalized to lean body mass (SULmean) between two-time-point scans controlling for reader and location.
A–C, Plots for reader 1: upper liver (A) (intraclass coefficient [ICC] = 0.345), portal vein (B) (ICC = 0.366), and lower liver (C) (ICC = 0.383).
D–F, Plots for reader 2: upper liver (D) (ICC = 0.412), portal vein (ICC = 0.377) (E), and lower liver (F) (ICC = 0.438).
Mean absolute variation in liver SULmean at the two-time-point scans was 0.018 ± 0.322, 0.032 ± 0.315, and 0.030 ± 0.316 between the first and second scans for reader 1 at the upper, portal, and lower levels, respectively, and 0.033 ± 0.313, 0.028 ± 0.318, and 0.033 ± 0.314, respectively, for reader 2 (Fig. 3). The mean percentage bias for SULmean between the two-time-point scans was 3.55% ± 23.19%, 4.65% ± 23.87%, and 4.30% ± 23.03% between the first and second scans for reader 1 at the upper, portal, and lower levels, respectively, and 4.49% ± 23.23%, 4.33% ± 23.74%, and 4.48% ± 23.01%, respectively, for reader 2.
Variability of Liver SULmean: Influence of Scanners
Of the 130 patients, 58 and 72 PET/CT studies were performed on the Discovery LS and Discovery ST systems, respectively, for the first time point. For the second time point, 55 and 75 were performed on the Discovery LS and Discovery ST systems, respectively. A total of 48 patients underwent PET/CT on the Discovery LS scanner at both time points, 31 were performed on the Discovery ST, and 51 were mixed (done on the Discovery LS at one time point and the Discovery ST at the other time point).
We performed an ANOVA to look for the influence of scanner change on the bias between the two-time-point liver SULmean values, fixing reader and location. No statistically significant results were found between the Discovery LS–Discovery LS, Discovery ST–Discovery ST, and mixed scanner groups. These results are summarized in Table 4.
TABLE 4.
Influence of Scanner Change on Bias Between Two-Time-Point Liver Standardized Uptake Value Normalized to Lean Body Mass Values for Each Reader and Location
| Value | Discovery LS (n = 48) |
Discovery ST (n = 31) |
Mixed (n = 51) |
p | Interscan Time > 6 Mo (n = 65) |
Interscan Time < 6 Mo (n = 65) |
p |
|---|---|---|---|---|---|---|---|
| Reader 1 | |||||||
| Upper liver | 0.01 | −0.01 | 0.04 | 0.8 | 0.04 | 0.00 | 0.5 |
| Portal vein | 0.02 | −0.02 | 0.08 | 0.4 | 0.05 | 0.01 | 0.5 |
| Lower liver | 0.04 | −0.04 | 0.07 | 0.3 | 0.04 | 0.01 | 0.6 |
| Reader 2 | |||||||
| Upper liver | 0.03 | 0.00 | 0.06 | 0.7 | 0.05 | 0.02 | 0.5 |
| Portal vein | 0.03 | −0.03 | 0.06 | 0.5 | 0.05 | 0.01 | 0.5 |
| Lower liver | 0.05 | −0.04 | 0.07 | 0.3 | 0.05 | 0.01 | 0.5 |
Note—The bias differences were statistically insignificant. Both early and later PET/CT studies were performed on Discovery LS scanner, both studies were performed on Discovery ST, and one mixed study was performed using Discovery LS and one was performed using Discovery ST. Both scanners manufactured by GE Healthcare.
Variability of Liver SULmean: Influence of Time Interval
We also performed an independent Student t test to investigate the influence of the time elapsed between the two PET/CT studies on the change of liver SULmean. We used a cut-point of 6 months to separate the patients who underwent PET/CT within 6 months (n = 65) and patients who underwent PET/CT at greater than 6 months (n = 65). No statistically significant difference was noted between the two groups, and the results are included in Table 4.
Discussion
The purpose of our study was to evaluate the agreement and variability in measuring liver SULmean between PET/CT performed at two time points in the same patient when the parameter was measured by different readers at three different locations within the right lobe of the liver in a clinical setting. In this study, we have shown that liver SULmean has excellent interreader agreement for same-time scanning regardless of the site of VOI placement within the right lobe of the liver, similar to our prior study [13]. However, there was only weak agreement between the liver SULmean between the studies performed at two different time points in the same patient. The significance of our results for response monitoring PET/CT is the determination of the expected normal variation of liver SULmean. Differences in normal liver SULmean up to 1.1 between baseline and follow-up studies are to be expected in the healthy population. A difference greater than these limits can indicate technical error and preclude quantitative comparison of the studies in a clinical setting. When the difference is greater than this range, interpreting clinicians need to investigate and be vigilant about any technical errors contributing to the effects and may need to take this account in the interpretation of the PET/CT study.
The previous study by Paquet et al. [9] investigated the intrapatient variability of FDG uptake in normal tissue in 70 patients who underwent two FDG PET studies. The studies were performed 271 ± 118 days apart. The ROI differed from ours in that it was defined in a single slice in the middle of the right lobe of the liver with a mean size of 19.2 cm3. The authors cite an absolute difference of 0.05 ± 0.2 between the two studies for liver SULmean and an ICC of 0.57. This is indicative of a moderate degree of agreement at best and is similar to our results. The authors further concluded that SUVs measured in the liver of patients who were cancer free were stable over time. In our study, we found an average absolute difference of 0.03 ± 0.27.
In terms of absolute variation in liver SULmean, using 95th percentiles, the reference range in our patient population for intrapatient variation was −0.5 to 0.6. This is in comparison with intrapatient variation of −0.9 to 1.1 for liver SUV in the recent study by Boktor et al. [24]. The likely explanation for the smaller variation in liver SULmean in our patient population is that we selected patients who had normal liver by structural imaging and we excluded patients who underwent systemic chemotherapy or radiation therapy involving the abdomen between the two studies. The study by Boktor et al. did not exclude such patients, which may explain the differences between the studies.
The determination of SUV is susceptible to many technical confounders in addition to intrapatient physiologic variability [25]. We used two different PET/CT machines during the period when our studies were performed. As shown in the Results section, no statistically significant difference in the bias in the liver SULmean was noted among the two-time-point scans, whether the same scanner was used or the scanner was switched between the pair of studies. There was also no statistically significant difference in the bias between studies performed at an interval of l6 months or less and those performed at greater than 6 months. Hence, the variation in repeatability of liver SULmean observed in this study is applicable in a clinical setting as the normal reference range of SULmean in the same patient.
There are several limitations to our study. This is a repeatability study performed in a clinical context with wide variation between scanning times, with the longest interval of 1105 days. However, this simulates the PET/CT studies performed in real-world clinical practice and has the highest applicability. We did not investigate the change in liver SULmean because of systemic therapy response because our intention was to establish the normal variability. Because this was a retrospective study, we did not strictly control the FDG dose or uptake time, which may have contributed to some of the variability. In addition other factors, such as the patients’ underlying conditions and medications, may have affected the liver FDG uptake between the two time points.
Conclusion
Liver SULmean on FDG PET/CT has excellent interreader agreement, with similar values and variance whether measured at the upper, lower, or portal vein levels on the same scan. However, there was only a fair intraclass correlation between liver SULmean measured at two different time points in the same patient. Scan-to-scan intrapatient variation in absolute liver SULmean was −0.5 to 0.60. A difference greater than these limits may indicate technical error and could preclude quantitative comparison of the studies.
Acknowledgments
A. K. Tahari was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number T32EB006351.
References
- 1.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46:983–995. [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non–small cell lung carcinoma. AJR. 2013;200:635–640. doi: 10.2214/AJR.12.9138. [DOI] [PubMed] [Google Scholar]
- 5.Malladi A, Viner M, Jackson T, Mercier G, Subramaniam RM. PET/CT mediastinal and liver FDG uptake: effects of biological and procedural factors. J Med Imaging Radiat Oncol. 2013;57:169–175. doi: 10.1111/1754-9485.12015. [DOI] [PubMed] [Google Scholar]
- 6.Keyes JW., Jr SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]
- 7.Huang SC. Anatomy of SUV: standardized uptake value. Nucl Med Biol. 2000;27:643–646. doi: 10.1016/s0969-8051(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee JR, Madsen MT, Bushnel D, Menda Y. A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun. 2000;21:685–690. doi: 10.1097/00006231-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of 18F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–788. [PubMed] [Google Scholar]
- 10.Benz MR, Evilevitch V, Allen-Auerbach MS, et al. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–1046. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imsande HM, Davison JM, Truong MT, et al. Use of 18F-FDG PET/CT as a predictive biomarker of outcome in patients with head-and-neck non–squamous cell carcinoma. AJR. 2011;197:976–980. doi: 10.2214/AJR.10.4884. [DOI] [PubMed] [Google Scholar]
- 12.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET Response Criteria in Solid Tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viner M, Mercier G, Hao F, Malladi A, Subramaniam RM. Liver SULmean at FDG PET/CT: interreader agreement and impact of placement of volume of interest. Radiology. 2013;267:596–601. doi: 10.1148/radiol.12121385. [DOI] [PubMed] [Google Scholar]
- 14.Laffon E, Adhoute X, de Clermont H, Marthan R. Is liver SUV stable over time in 18F-FDG PET imaging? J Nucl Med Technol. 2011;39:258–263. doi: 10.2967/jnmt.111.090027. [DOI] [PubMed] [Google Scholar]
- 15.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 16.Nakamoto Y, Zasadny KR, Minn H, Wahl RL. Reproducibility of common semi-quantitative parameters for evaluating lung cancer glucose metabolism with positron emission tomography using 2-deoxy-2-[18] fluoro-D-glucose. Mol Imaging Biol. 2002;4:171–178. doi: 10.1016/s1536-1632(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 17.Heijmen L, de Geus-Oei LF, de Wilt JH, et al. Reproducibility of functional volume and activity concentration in 18F-FDG PET/CT of liver metastases in colorectal cancer. Eur J Nucl Med Mol Imaging. 2012;39:1858–1867. doi: 10.1007/s00259-012-2233-6. [DOI] [PubMed] [Google Scholar]
- 18.de Langen AJ, Vincent A, Velasquez LM, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–708. doi: 10.2967/jnumed.111.095299. [DOI] [PubMed] [Google Scholar]
- 19.Velasquez LM, Boellaard R, Kollia G, et al. Repeatability of 18F-FDG PET in a multicenter phase I study of patients with advanced gastrointestinal malignancies. J Nucl Med. 2009;50:1646–1654. doi: 10.2967/jnumed.109.063347. [DOI] [PubMed] [Google Scholar]
- 20.Weber WA, Ziegler SI, Thödtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–1777. [PubMed] [Google Scholar]
- 21.Minn H, Zasadny KR, Quint LE, Wahl RL. Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–173. doi: 10.1148/radiology.196.1.7784562. [DOI] [PubMed] [Google Scholar]
- 22.Wieder HA, Ott K, Lordick F, et al. Prediction of tumor response by FDG-PET: comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging. 2007;34:1925–1932. doi: 10.1007/s00259-007-0521-3. [DOI] [PubMed] [Google Scholar]
- 23.Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–4610. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- 24.Boktor RR, Walker G, Stacey R, Gledhill S, Pitman AG. Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J Nucl Med. 2013;54:677–682. doi: 10.2967/jnumed.112.108530. [DOI] [PubMed] [Google Scholar]
- 25.Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–1527. [PubMed] [Google Scholar]