Abstract
We examined the longitudinal association between alcohol use and liver fibrosis, measured by FIB-4 Score, among HIV-infected individuals by (1) antiretroviral therapy (ART) class, and (2) the presence of hepatitis C (HCV) co-infection. This was a prospective cohort study of 550 individuals in the Johns Hopkins HIV Clinical Cohort initiating ART between 2000 and 2012. The relationship between alcohol consumption (defined using NIAAA categories of non-, moderate, and hazardous drinkers) and liver fibrosis (FIB-4 score) by ART class was assessed using linear mixed effects models. Additionally, we examined whether the presence of HCV modified and whether viral load mediated the relationship between alcohol use and liver fibrosis. Overall, FIB-4 levels were 15.6% higher in hazardous drinkers compared to moderate drinkers (p = 0.025) after adjusting by age, sex, and race. Hazardous drinkers on PI-based regimens had FIB-4 scores 26.9% higher than moderate drinkers (p = 0.015). However, there was no difference in FIB-4 levels between hazardous drinkers on non-PI-based regimens compared to moderate drinkers (1.83% versus moderate drinkers, p = 0.848). There was no significant difference in FIB-4 between nondrinkers and moderate drinkers, irrespective of ART regimen. These associations were not modified by HCV status or mediated by viral load changes. Individuals with hazardous alcohol consumption and on PI-based regimens had significantly increased liver fibrosis, as measured by the FIB-4. These data suggest that providers should consider level of alcohol consumption when choosing an ART regimen to minimize detrimental effects on the liver.
Introduction
Hazardous alcohol use is a potent risk factor for liver disease.1 In people living with HIV, hazardous alcohol use may be especially harmful, as it is associated with decreased adherence to antiretroviral therapy (ART),2 increased ART discontinuation,3 and decreased viral suppression.4 Hazardous alcohol use is prevalent among individuals with HIV,5,6 and can indirectly affect the liver through its impact on ART adherence and consequent HIV viremia, which has been independently associated with liver disease progression.7 With the improved survival among persons living with HIV (PLWH), liver related morbidity and mortality has increased,8,9 possibly due to aging, long-term liver-toxic exposures including antiretroviral drugs or residual immune activation and chronic inflammation with treated HIV.
The effect of alcohol on liver fibrosis is particularly relevant for individuals with HIV and hepatitis C virus (HCV) co-infection, as both diseases have been independently associated with liver disease progression.10,11 A recent cross-sectional study among United States veterans demonstrated12 that alcohol use in the past year was associated with a significantly increased prevalence of advanced hepatic fibrosis (defined as a Fibrosis-4 score or FIB-4 > 3.25) among HIV infected, compared to HIV uninfected individuals, and HCV infected compared to HCV uninfected individuals. This same study found that the prevalence of fibrosis increased with increased alcohol consumption.
Although there is a clear association between alcohol use and advanced hepatic fibrosis among individuals with HIV, HCV, and HIV/HCV co-infection, it is unknown how alcohol use affects liver disease progression over time among individuals initiating ART. Furthermore, antiretroviral medications may have a direct toxic effect on the liver. Early studies examining both protease inhibitors (PI) and non-nucleoside reverse transcriptase inhibitors (NNRTI) demonstrated an increase in liver transaminases13–15 with these regimens, though newer PI and NNRTI appear to have less hepatotoxicity. We longitudinally examined the (1) effect of hazardous alcohol use on hepatic fibrosis, measured by FIB-4 Score, among HIV infected individuals initiating ART, and (2) whether alcohol's effect on fibrosis would vary by ART class and by HCV co-infection.
Methods
Study design
This is a prospective cohort study of individuals enrolled in the Johns Hopkins HIV Clinical Cohort (JHHCC), a longitudinal cohort of HIV-infected individuals receiving HIV primary care in the Johns Hopkins HIV Clinic. All patients are eligible to participate. Data collected on participants include demographic, clinical, laboratory, and pharmacy data. Information from clinical records is abstracted by trained staff. Laboratory data are obtained electronically. A more detailed description of the methods for the JHHCC is available elsewhere.16
Since July 2000, an Audio Computer-Assisted Self-Interview (ACASI) has been part of the data collection procedures of the JHHCC. The survey takes about 15 min to complete and collects patient-reported outcomes including alcohol use, illicit drug use, ART use, and ART adherence over the prior 6 months. A description of the ACASI methods has been published elsewhere.17 Written informed consent is obtained from the participants. This study has been approved by the Johns Hopkins University School of Medicine's Institutional Review Board.
Study inclusion
For this study, we included HIV-infected patients who completed an ACASI within 6 months of ART initiation between July 2000 and July 2012, had liver enzymes (AST and ALT) and platelets measured within 6 weeks of initiation of ART, and had at least one follow-up visit. ART was defined as any regimen that included a combination of: nucleoside reverse transcriptase inhibitors (NRTI), protease inhibitors (PI), non-nucleoside reverse transcriptase inhibitors (NNRTI), or integrase strand transfer inhibitors (INSTI).
Outcome
The outcome for this study was the level of liver fibrosis as measured using the Fibrosis-4 Score (FIB-4).18 This score was specifically developed to detect liver fibrosis in HIV or HCV infected patients and is calculated with the following formula:
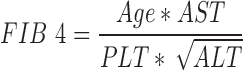 |
For descriptive purposes, FIB-4 was categorized as normal or mildly elevated (<1.45), moderately elevated (1.45–3.25), and elevated (≥3.25, indicating an 85% probability or more of advanced liver disease or cirrhosis).
Exposure
The primary exposure of interest was alcohol consumption, measured with a frequency/quantity and binge drinking questions in the ACASI. We classified individuals' alcohol consumption according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) categories: hazardous alcohol consumption as >7 standard drinks per week or more than 3 drinks on one occasion for women, and >14 standard drinks per week or more than 4 drinks on one occasion for men.19 Moderate alcohol use was defined as alcohol use below hazardous levels. No alcohol use was defined as none reported at the time of the ACASI. As examined before in this same cohort, changes in alcohol use over time were minimal and therefore we treated alcohol as a time-fixed exposure.20
Covariates
Covariates of interest included age at clinical visit, self-reported sex and self-reported race (categorized as African-American or other), type of ART, HIV-RNA level (time-varying), and HCV co-infection.
ART was categorized as PI-based (PI + NRTI with or without an integrase strand inhibitor (INSTI)) or NNRTI-based (NNRTI+ NRTI with or without an INSTI). Participants on both a PI and NNRTI (n = 36) were excluded from the analysis, as the number of people in this group was small. In addition, during the period of this study, few participants were on NRTI + INSTI alone (n = 16) and thus these individuals were excluded. The final sample size for our analysis was 533 patients.
HCV status was obtained from laboratory records with individuals categorized as having HCV co-infection based upon having a positive antibody. The most valid definition of HCV status is a detectable HCV-RNA but not all individuals in our study had an HCV-RNA available at baseline. Moreover, for those that had HCV-RNA available at baseline (n = 159) the correlation between having a positive HCV antibody and detectable HCV-RNA was very strong (OR = 179.3, with a 14.6% misclassification rate). Previous research conducted in this same population found that the rates of sustained virologic response to HCV treatment were extremely low (0.7%).21
Statistical analysis
Descriptive statistics were calculated for outcome, exposure, and covariates for the entire cohort and stratified by alcohol consumption level. Differences between alcohol consumption groups at baseline were tested using ANOVA (or Kruskal-Wallis test for non-normally distributed covariates) or Fisher's exact tests as needed, according to variable type.
To study the association between alcohol consumption and liver fibrosis by type of ART, we used linear mixed effects models with the natural logarithm of FIB-4 (given the skewness of the FIB-4 distribution) in each visit as the outcome. First, we fitted a model with only time since ART initiation to see if FIB-4 changed over time. Second, we added alcohol consumption category (with moderate drinking as the reference category) and ART type (with non-PI based regimen as reference) as the main exposures to the model. Third, we tested if FIB-4 changes over time differed by alcohol consumption category. Fourth, we added the interaction of alcohol consumption category and ART type. Last, we tested if the interaction of alcohol consumption and ART was also associated with variations in FIB-4 change over time.
A random intercept and random time slope were included in all models. Adjusted models included age, race, sex, and time on ART as covariates, as well as the natural logarithm of FIB-4 at baseline. Given the log transformation of FIB-4, the interpretation of the exponentiated coefficient for alcohol consumption is the percent change in FIB-4 between levels of alcohol consumption at any time point during follow-up for individuals with the same baseline FIB-4 compared to the reference group (moderate alcohol consumption). We estimated model parameters using an unstructured covariance matrix and allowed the residuals to have an exponential correlation structure. We examined residuals (distribution, trends over time, and dispersion) to confirm the validity of the model assumptions.
Several additional analyses were conducted. First, given the potential for a confounding or mediational role of diabetes and body mass index (BMI), we performed a secondary analysis including them in our model. Second, we also examined the interaction between HCV, alcohol consumption, and ART type by including HCV as a covariate along with the interaction terms between HCV, alcohol consumption, and ART type. Third, given the potential role of HIV RNA as a mediator of this relationship, we performed mediation analyses by adding and removing HIV RNA as a predictor in our models and checking the change in the regression coefficients. Fourth, given the potential for changes in prescription patterns over time, we conducted a further analysis adding date of ART initiation into the model. Fifth, since ART prescription patterns may vary by CD4 nadir, we conducted a further analysis adding CD4 nadir into the model.
Data on key variables (alcohol, ART type, or FIB-4 at baseline) were missing in 52 participants (7.9%), but these participants did not differ from those with complete information so we chose to perform a complete-case analysis. All results presented below (including descriptive results in Table 1) include a sample size of 533 individuals with complete data on all variables.
Table 1.
Baseline Characteristics of HIV-Infected Individuals Initiating ART
| All N = 533 | Not drinker N = 328 (61.5%) | Moderate N = 154 (28.9%) | Hazardous N = 51 (9.6%) | p Value* | |
|---|---|---|---|---|---|
| Median follow-up—years [IQR] | 1.65 [1.30–1.80] | 1.65 [1.26–1.80] | 1.65 [1.31–1.80] | 1.72 [1.43–1.85] | 0.405 |
| Median visits [IQR] | 10 [7–18] | 10 [7–18] | 9 [6–13] | 12 [8–30] | 0.049 |
| Mean age at HAART initiation (SD) | 42.80(10.21) | 44.11(9.86) | 40.37(10.93) | 41.68(8.84) | <0.001 |
| Men, % | 58.9 | 54.6 | 70.8 | 51 | 0.001 |
| Race, % | |||||
| African American | 84.2 | 85.1 | 82.5 | 84.3 | 0.487 |
| Other | 15.8 | 14.9 | 17.5 | 15.7 | |
| BMI, mean (SD) | 25.80(7.48) | 25.81(7.23) | 26.15(8.46) | 24.75(6.38) | 0.616 |
| BMI WHO categories, % | |||||
| <18.5 | 10.4 | 9.8 | 9.8 | 15.8 | 0.879 |
| 18.5–24.9 | 42.4 | 42.5 | 45.1 | 34.2 | |
| 25–29.9 | 26.6 | 27.2 | 25.5 | 26.3 | |
| >30 | 20.6 | 20.5 | 19.6 | 23.7 | |
| CD4 nadir cells/mm3 Median [IQR] | 181 [57–303] | 196 [56–302] | 163 [48–310] | 173 [70–290] | 0.594 |
| CD4 nadir pre-HAART categories, % | |||||
| >500 | 5.1 | 4.9 | 5.2 | 5.9 | 0.959 |
| 351 ≤ 500 | 12.4 | 12.8 | 12.4 | 9.8 | |
| 201 ≤ 350 | 29.6 | 30.9 | 28.1 | 25.5 | |
| ≤200 | 52.9 | 51.4 | 54.2 | 58.8 | |
| Baseline HIV-RNA copies/mL Median [IQR] | 61187 [13727–168932] | 60162 [12122–185684] | 62472 [16357–151974] | 63997 [14251–124307] | 0.974 |
| Baseline HIV-RNA categories, % | |||||
| <10,000 | 22 | 22.3 | 21.6 | 21.6 | 0.858 |
| 10,000–99,999 | 42.2 | 41 | 42.5 | 49 | |
| >99,999 | 35.8 | 36.7 | 35.9 | 29.4 | |
| Baseline AST median [IQR] | 33.0 [24.0–54.0] | 33.0 [23.0–55.0] | 33.0 [23.0–48.0] | 36.0 [27.0–70.0] | 0.373 |
| Baseline ALT median [IQR] | 24.0 [17.0–41.0] | 25.0 [17.0–42.2] | 24.0 [16.0–40.0] | 25.0 [17.3–43.0] | 0.791 |
| Baseline PLT median [IQR] | 203.0 [164.0–256.0] | 197.0 [158.0–248.5] | 213.0 [175.0–267.0] | 207.0 [167.0–266.0] | 0.308 |
| Median FIB 4 at baseline [IQR] | 1.4 [0.9–2.1] | 1.5 [1.0–2.2] | 1.2 [0.8–1.8] | 1.5 [0.9–2.3] | <0.001 |
| FIB-4 category at baseline | |||||
| <1.45 | 52.9 | 49.4 | 63 | 45.1 | 0.044 |
| 1.45–3.25 | 34.7 | 36.9 | 28.6 | 39.2 | |
| ≥ 3.25 | 12.4 | 13.7 | 8.4 | 15.7 | |
| Number of drinking days/week, % | |||||
| <1 | 80.9 | 100 | 62.3 | 24.5 | <0.001 |
| 1–3 | 13.4 | 0 | 33.1 | 28.6 | |
| >3 | 5.7 | 0 | 4.6 | 46.9 | |
| HAART regimen,% | |||||
| PI+NRTI | 48.2 | 47.1 | 51 | 47.1 | 0.818 |
| NNRTI+NRTI | 50.9 | 51.7 | 49 | 51 | |
| Any INSTIa, % | 1.7 | 2.4 | 0.6 | 0 | 0.369 |
| Diabetes,% | 8.8 | 11 | 3.9 | 9.8 | 0.025 |
| Hepatitis C,% | 40.9 | 44.5 | 27.9 | 56.9 | <0.001 |
INSTI, Integrase Inhibitor.
p Values are for ANOVAs (for comparison of means), Kruskar Wallis tests (for comparison of medians) and χ2 tests (for comparison of proportions).
Analyses were performed using STATA IC 13.1 (StataCorp, College Station, TX).
Results
Study population
Baseline characteristics of participants by level of alcohol consumption are shown in Table 1. Sixty-one percent of the population reported no current alcohol use, 29% reported moderate use, and 10% reported hazardous alcohol use. Overall, 12% of the study sample had elevated FIB-4 levels (FIB-4 > 3.25) at baseline (ART initiation). Participants were followed for a median 1.7 years, similarly across the three categories of alcohol consumption. During the follow-up, patients had on average 10 measurements of FIB-4, with hazardous drinkers having slightly higher number of measurements (p = 0.049). Moderate drinkers were significantly younger (mean age of 40.2, compared to 44.3 in both not drinkers and hazardous drinkers, p < 0.001), significantly more likely to be men (74% were men, compared to 55% and 48% in nondrinkers and hazardous drinkers, p = 0.001).
Levels of liver enzymes and platelets were similar for nondrinkers and moderate drinkers and higher (lower in the case of platelets) for hazardous drinkers (p = 0.066, p = 0.721, and p = 0.081 for AST, ALT, and platelets, respectively). FIB-4 was higher in hazardous drinkers (40% and 16.8% had a minimally elevated and elevated FIB-4 at baseline, respectively), compared to 37.2% and 12.8% among nondrinkers and 27% and 8.8% among moderate drinkers (p = 0.023).
The proportion of individuals with HCV co-infection was highest in hazardous drinkers (60%, compared to 45% and 28% in nondrinkers and moderate drinkers, respectively, p < 0.001). Diabetes rates were also higher in nondrinkers and hazardous drinkers (p = 0.032).
Table 2 shows the most common antiretroviral medications by date of ART initiation and regimen. For PI-based regimens, the most common medication before 2001 was Nelfinavir (in 63% of regimens), whereas from 2002 the most common medications were ritonavir-boosted PI regimens of Lopinavir or Atazanavir (73% and 20% in 2002–2004, 25% and 52% in 2005–2007, respectively), and later Atazanavir or Darunavir (48% and 40% in 2008–2010, 37% and 57% in 2010–2012, respectively). For NNRTI based regimens, Efavirenz was the most commonly used medication, in more than 90% of all patients in NNRTI-based regimens. The most common NRTI or combination thereof in all regimens was Zidovudine/Lamivudine from 1999 to 2001 (45% of regimens), Lamivudine in 2002 to 2004 (25%), and Tenofovir/Emtricitabine from then on (54% of regimens from 2005 onwards).
Table 2.
Most Commonly Prescribed Medication by ART Regime and Date of ART Initiation
| PIs in PI based regimens | NNRTIs in NNRTI based regimens | NRTIs in PI or NNRTI based regimens | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| 1999–2001 | Nelfinavir (63%) | Indinavir/RTV (27%) | Efavirenz (95%) | Nevirapine (4%) | 3TC/ZDV (45%) | Stavudine (18%) |
| 2002–2004 | Lopinavir/RTV (73%) | Atazanavir/RTV (20%) | Efavirenz (90%) | Nevirapine (10%) | Lamivudine (25%) | Tenofovir (24%) |
| 2005–2007 | Atazanavir/RTV (52%) | Lopinavir/RTV (25%) | Efavirenz (100%) | FTC/TDF (48%) | 3TC/ABC (19%) | |
| 2008–2010 | Atazanavir/RTV (48%) | Darunavir/RTV (40%) | Efavirenz (98%)a | FTC/TDF (53%) | 3TC/ABC (22%) | |
| 2010–2012 | Darunavir/RTV (57%) | Atazanavir/RTV (37%) | Efavirenz (100%) | FTC/TDF (76%) | 3TC/ABC (12%) | |
The other 2% NNRTIs in the 2008-2010 period correspond to Etravirine.
FTC/TDF, fixed dose combination of Emtricitabine and Tenofovir; RTV, Ritonavir; 3TC/ABC, fixed dose combination of Lamivudine and Abacavir; 3TC/ZDV, fixed dose combination of Lamivudine and Zidovudine.
ART, alcohol consumption, and FIB-4
Controlling for age, sex, and race, hazardous drinkers had FIB-4 levels 15.6% higher than moderate drinkers (95% CI 1.8–31.2%, p = 0.025), while nondrinkers did not have a significant elevation in FIB-4 compared to moderate drinkers (95% CI −2.6–14.4%, p = 0.189). There was a significant difference in FIB-4 levels according to alcohol level and ART class (Table 3). Among patients taking PI-based regimens, hazardous drinking was associated with FIB-4 levels 26.90% higher (95% CI 4.83–53.63%, p = 0.015) compared to moderate drinkers on NNRTI regimens (reference group). Nondrinkers on PI-based regimens had no significant elevation (9.62%, 95% CI −2.69–23.48%, p = 0.131) of FIB-4 compared to the reference group (NNRTI moderate drinkers). Among patients taking NNRTI-based regimens, neither hazardous nor nondrinkers had significantly different FIB-4 levels compared to moderate drinkers (hazardous drinkers: 1.83%, 95% CI −15.31–22.42%, p = 0.848; nondrinkers: 7.09%, 95% CI −4.62–20.27%, p = 0.248). The interaction coefficient of hazardous drinking and ART regimen was statistically significant (p = 0.037), while the interaction between nondrinking and ART regimen was not (p = 0.854).
Table 3.
Percent FIB-4 Difference by Alcohol and ART Regimen Class Adjusted for Age, Sex, Race, and Time on ART
| ΔFIB-4 | (95% CI) | p Value* | |
|---|---|---|---|
| Drinking category* | |||
| NNRTI nondrinker | 7.09% | (−4.65;20.27%) | 0.248 |
| NNRTI moderate (ref.) | 0 (ref.) | ||
| NNRTI hazardous | 1.82% | (−15.31;22.42%) | 0.848 |
| PI nondrinker | 9.62% | (−2.69;23.48%) | 0.131 |
| PI moderate | 3.94% | (−9.14;18.90%) | 0.573 |
| PI hazardous | 26.90% | (4.83;53.63%) | 0.015 |
p Value represents comparison between each group and the reference (moderate drinkers on NNRTI based regimens).
Regarding changes over time, on average, study participants had an unadjusted relative increase in FIB-4 of 2.6% per year (95% CI −0.4–5.6%). Neither hazardous nor nondrinkers had different rates of increase in FIB-4 per year when compared to moderate drinkers (hazardous: 2.4% per year, p = 0.627; nondrinkers: 4.2% per year, p = 0.06). We also found that the rate of change of FIB-4 was similar across alcohol consumption and ART categories (p-value for the three-way interaction = 0.586 and 0.369 for nondrinkers and hazardous drinkers, respectively).
Interaction between ART type, HCV status and alcohol consumption
HCV co-infected individuals had, on average, FIB-4 levels 29.2% higher compared to individuals without hepatitis C (95% CI 19.8–39.2%, p < 0.001). Allowing for the interaction (Table 4) between HCV status, alcohol consumption, and ART regimen, non-HCV co-infected hazardous drinkers on PI-based regimens had a large and significant elevation in FIB-4 of 44.95% (95% CI 4.74–100.60%, p = 0.025) compared to the reference group (no hepatitis C co-infection, moderate drinkers on NNRTI regimens). However, nondrinkers did not have a significant elevation (95% CI −5.63–32.80%, p = 0.195) compared to the reference. Both hazardous and nondrinkers on NNRTI regimens had no significant change in FIB-4 levels (p = 0.897 and p = 0.780, respectively) compared to moderate drinkers. HCV co-infected individuals showed significant and large elevations of FIB-4 compared to the reference.
Table 4.
Percent FIB-4 Difference by Hepatitis C Infection, Alcohol, and ART Class Adjusted for Age, Sex, and Race, Compared to the Reference Group (Hepatitis C Negative, Moderate Drinkers, NNRTI ART Regimen)
| Hepatitis C negative | Hepatitis C positive | |||||
|---|---|---|---|---|---|---|
| ΔFIB-4 | (95% CI) | p Value | ΔFIB-4 | (95% CI) | p Value | |
| Drinking category* | ||||||
| NNRTI nondrinker | 1.08% | (−14.05;18.88%) | 0.897 | 66.99% | (40.15;98.98%) | <0.001 |
| NNRTI moderate (ref.) | 0 (ref.) | 35.81% | (6.25;73.59%) | 0.004 | ||
| NNRTI hazardous | 4.06% | (−21.27;37.56%) | 0.780 | 70.32% | (26.96;128.48%) | 0.001 |
| PI nondrinker | 11.95% | (−5.63;32.80%) | 0.195 | 68.90% | (41.84;101.12%) | <0.001 |
| PI moderate | 3.24% | (−13.72;23.54%) | 0.728 | 75.09% | (37.46;123.02%) | <0.001 |
| PI hazardous | 44.95% | (4.74;100.60%) | 0.025 | 68.73% | (26.98;124.21%) | <0.001 |
p Value represents comparison between each group and the reference (hepatitis C negative, moderate drinkers on NNRTI based regimens).
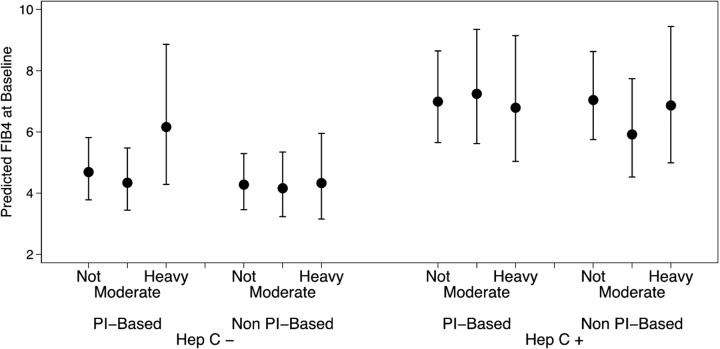
Figure 1 shows the predicted FIB-4 by alcohol consumption, ART type, and HCV status. HCV-infected individuals had higher elevations of FIB-4 compared to non-infected individuals. Moreover, hazardous drinkers had significantly higher FIB-4 levels compared to moderate drinkers only among individuals on PI-based regimens. Nonetheless, the alcohol and ART interaction was statistically similar in both HCV coinfection categories (p value for the three-way interaction = 0.283 and 0.200 for nondrinkers and hazardous drinkers, respectively), meaning that the association between alcohol consumption and ART type was similar in HCV-infected individuals and non HCV-infected individuals.
FIG. 1.
Predicted FIB-4 at baseline by HCV infection status, type of ART, and alcohol consumption. Solid circle markers represent the point estimate for change, and error bars indicate 95% confidence intervals.
Secondary analyses
Adding diabetes and body mass index to the models above did not change these associations (data not shown). Mediation analyses with HIV RNA showed only a slight attenuation of the associations described above (data not shown). Adding date of ART initiation or CD4 nadir to the model did not change the results (data not shown).
Discussion
In this large clinic based study of PLWH initiating ART, we found that hazardous drinking was associated with progression of liver fibrosis as determined using FIB-4. In addition, the effect of hazardous drinking was only significant among those initiating PI-based ART. Those individuals co-infected with HCV had a higher overall risk of progression of liver fibrosis but this increased risk did not seem to interact with the alcohol-ART association.
Our finding that hazardous alcohol consumption is associated with increased FIB-4 is consistent with previous studies. A recent study in the Veterans Aging Cohort Study (VACS) found that among HIV-positive people, those with hazardous or binge alcohol use (both included in the hazardous drinking category in our analysis) had a significant increase in markers of liver fibrosis, including FIB-4 compared to individuals with non-hazardous alcohol consumption.12 Among the mechanisms they proposed for this association is the potential for direct hepatotoxicity of HIV,22 which may be accentuated by the diminished effect of ART therapy with hazardous alcohol consumption.20 However, our mediation analysis results do not support this hypothesis, as changes in viral load with alcohol consumption were not mediating this association.
Prior research has demonstrated increased liver toxicity with the use of PI-based therapies,15,23 and Lim et al.12 has suggested that there is an interaction between alcohol consumption and antiretroviral therapy that results in increased liver toxicity.24 Our results support this hypothesis, as we found that hazardous alcohol drinkers on PI-based regimens were more likely to have elevated FIB-4 levels compared to moderate drinkers. This association was not present in individuals who were on NNRTI-based regimens. A potential mechanism for this interaction is oxidative stress on the liver, or PI-induced endoplasmic reticulum stress,25 believed to be potentiated by alcohol consumption through the modulation of calcium homeostasis26 and proapoptotic factors.27 Another potential mechanism may be increased blood alcohol concentration caused by alterations in ethanol metabolism by ARTs, but previous research has shown that commonly used ARTs (Ritonavir and Efavirenz) are not associated with increased blood alcohol concentration.28 Future research targeting the underlying mechanism of this association between alcohol use, PI and increased fibrosis will be important.
In our study, we found higher levels of FIB-4 in individuals with hazardous alcohol consumption compared to those with moderate alcohol consumption only if they were using a PI-based regimen. Our analysis did not support an acceleration of the increase in FIB-4 over time in hazardous drinkers, that is, the rate of increase in FIB-4 over time was similar across alcohol/ART categories. It should be noted that due to the nature of our models (where the outcome is the natural logarithm of FIB-4), a lack of an effect in the log scale means that hazardous drinkers actually have an acceleration in their increase in FIB-4 compared to moderate and nondrinkers because hazardous drinkers actually begin with higher levels of FIB-4.
Our finding of increased fibrosis even among current nondrinkers may be secondary to the inclusion of sick abstainers in this category, prior drinkers who were no longer drinking for health reasons.29 As we did not have a measure of past alcohol use for the nondrinkers, we were not able to distinguish between true abstainers and sick quitters.29 In addition, among HIV/HCV-infected individuals, there may be under-reporting of alcohol use,30 given that they are frequently counseled on alcohol reduction. Future studies that examine past alcohol use and/or include an alcohol biomarker such as phosphatidylethanol to verify self-reported current alcohol use, may further clarify the underlying mechanisms of this finding.31
Prior studies have also indicated that there is a significant interaction between HCV co-infection and PI use on liver toxicity.15,23 However, these studies used high-dose ritonavir, while the majority of individuals in our study were on 100 mg of ritonavir used to boost protease inhibitors, and we did not find this interaction with newer protease inhibitors. Subsequent studies have shown no liver toxicity with ritonavir-boosted regimens.14 We did find, however, that the FIB-4 increased irrespective of regimen type among those with HCV co-infection, likely due to the direct effect of HCV viremia on liver fibrosis, which may be more potent than regimen type or level of alcohol use and therefore hinder our ability to find this interaction.
A second reason for the absence of this interaction may be a lack of statistical power to detect three-way interactions with a sample of 533 patients (as three-way interaction requires a fourfold increase in sample size compared to two-way interactions to achieve the same power).32 Studies with a larger sample size may be needed to detect these three-way interactions between alcohol consumption, ART regimen, HCV co-infection, and liver fibrosis. Newer treatments for HCV and higher rates of sustained virological response and cure provide an important opportunity to decrease liver disease progression. Recent research has found that newer treatments for HCV may interact directly with boosted-PI regimens, making the choice of ART in HCV infected individuals a non-trivial one.33 It is worth noting that data analyzed for this study ranged from the years 2000 to 2012, when only less effective treatments for HCV were available and therefore these results may shift when these newer treatments become more widely available. Effective treatment of both HIV and HCV is needed since these two infections may have synergistic effects on mortality.34
Limitations
As examined previously,20 most people in our cohort have stable alcohol consumption and therefore we decided to use alcohol consumption as a time-fixed variable measured at baseline. Therefore, we cannot exclude the possibility that our results may be driven by people with changes in alcohol consumption not captured by our time-fixed operationalization of this exposure. Similarly, we treated ART as a time-fixed variable that was set at initiation. Therefore, we cannot exclude the possibility that our results may be driven by people with changes in alcohol consumption or ART regimen not captured by our time-fixed operationalization of these exposures. Our cohort is largely urban and African American so generalization to other populations may be limited.
Regarding our outcome, FIB-4 is an indirect composite measure of liver fibrosis and not a gold standard, but it has previously been shown to be a good marker of liver fibrosis in HIV-infected populations.18 It is also important to note that the FIB-4 Score includes platelets, which can be affected directly by alcohol use independent of liver toxicity, resulting in an elevation in FIB-4 not entirely related to liver fibrosis. Nonetheless, we have no reason to believe this effect would be different by type of ART, our main finding in this study. In addition, our nondrinker category likely included individuals with past alcohol use.
In addition, though we added the date of ART initiation to our models to adjust for baseline differences in hepatotoxicity between older PIs and newer PIs (atazanavir and darunavir), questions remain as to whether these two medications have the same associations. Nonetheless, from 2008, more than 90% of PI prescriptions were either atazanavir or darunavir, meaning that an adjustment for date of ART initiation should control for these potential differences in the associations. Lastly, we cannot exclude the possibility of unmeasured confounding in our study, including confounding on the associations between alcohol or type of ART therapy and our outcome, including differential prescription behaviors (increased prescription of PI-based regimens) towards individuals with hazardous alcohol consumption. Nonetheless, previous literature has shown results consistent with ours.
In conclusion, in this sample of HIV-infected individuals who have recently initiated ART, PI-based regimens were associated with an increase in liver fibrosis among those with hazardous alcohol consumption. This association was not observed in HIV-infected individuals on non-PI based regimens. We believe that our study has clinical implications for the treatment of HIV among hazardous drinking individuals. Given the potential increase in hepatic fibrosis among hazardous drinkers using protease inhibitors, screening for and counseling on alcohol reduction, and consideration of alcohol pharmacotherapy is an important component of comprehensive HIV care. In addition, for those with persistent hazardous alcohol use, alternate agents including NNRTIs and INSTIs, with less potential hepatotoxicity could be considered.
Acknowledgments
Dr. Usama Bilal was supported by a La Caixa-Postgraduate Fellowship and a Johns Hopkins Center for a Livable Future-Lerner Fellowship during the completion of this research.
This study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (Grant Nr: U24AA020801 [McCaul, Chander, Hutton, Bilal] and R01AA016893 [Chander, Moore, Lau]), the National Institute on Drug Abuse (Grant Nr: U01DA036935 [Moore, Chander, Lau] and K24DA034621 [Sulkowski]), the National Institute of Allergy and Infectious Diseases (Grant Nr: P30AI094189 [Moore, Lau]).
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62:S38–S46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: Review and meta-analysis. J Acquir Immune Defic Syndr 2009;52:180–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conen A, Wang Q, Glass TR, et al. . Association of alcohol consumption and HIV surrogate markers in participants of the Swiss HIV cohort study. J Acquir Immune Defic Syndr 2013;64:472–478 [DOI] [PubMed] [Google Scholar]
- 4.Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr 2006;43:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chander G, Josephs J, Fleishman JA, et al. . Alcohol use among HIV-infected persons in care: Results of a multi-site survey. HIV Med 2008;9:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvan FH, Bing EG, Fleishman JA, et al. . The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. J Stud Alcohol 2002;63:179–186 [DOI] [PubMed] [Google Scholar]
- 7.Matthews GV, Neuhaus J, Bhagani S, et al. . Baseline prevalence and predictors of liver fibrosis among HIV-positive individuals: A substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16:129–136 [DOI] [PubMed] [Google Scholar]
- 8.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 2010;26:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braithwaite RS, McGinnis KA, Conigliaro J, et al. . A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res 2005;29:1190–1197 [DOI] [PubMed] [Google Scholar]
- 10.Di Martino V, Rufat P, Boyer N, et al. . The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: A long-term retrospective cohort study. Hepatology 2001;34:1193–1199 [DOI] [PubMed] [Google Scholar]
- 11.Kirk GD, Mehta SH, Astemborski J, et al. . HIV, age, and the severity of hepatitis C virus–related liver disease: A cohort study. Ann Inter Med 2013;158:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JK, Tate JP, Fultz SL, et al. . Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis 2014;58:1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macias J, Neukam K, Mallolas J, et al. . Liver toxicity of initial antiretroviral drug regimens including two nucleoside analogs plus one non-nucleoside analog or one ritonavir-boosted protease inhibitor in HIV/HCV-coinfected patients. HIV Clin Trials 2012;13:61–69 [DOI] [PubMed] [Google Scholar]
- 14.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS 2004;18:2277–2284 [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: Role of hepatitis C and B infections. Hepatology 2002;35:182–189 [DOI] [PubMed] [Google Scholar]
- 16.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: The Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol 1998;17:S38–S41 [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS 2002;16:767–774 [DOI] [PubMed] [Google Scholar]
- 18.Sterling RK, Lissen E, Clumeck N, et al. . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325 [DOI] [PubMed] [Google Scholar]
- 19.NIAAA. Helping Patients Who Drink Too Much: A Clinician's Guide. 2005
- 20.Kowalski S, Colantuoni E, Lau B, et al. . Alcohol consumption and CD4 T-cell count response among persons initiating antiretroviral therapy. JAIDS 2012;61:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta SH, Lucas GM, Mirel LB, et al. . Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 2006;20:2361–2369 [DOI] [PubMed] [Google Scholar]
- 22.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis 2003;188:1192–1204 [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000;283:74–80 [DOI] [PubMed] [Google Scholar]
- 24.Soriano V, Puoti M, Garcia-Gascó P, et al. . Antiretroviral drugs and liver injury. AIDS 2008;22:1–13 [DOI] [PubMed] [Google Scholar]
- 25.Zha BS, Wan X, Zhang X, et al. . HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS One 2013;8:e59514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology 2012;56:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinton M, Pandak W, Hylemon P, Zhou H. Cellular mechanisms by which alcohol promotes HIV protease inhibitor-induced liver lipotoxicity. FASEB J April 2015 2015;29 (Supplement):715.22. [Google Scholar]
- 28.McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med 2013;7:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet 1988;2:1267–1273 [DOI] [PubMed] [Google Scholar]
- 30.Roux P, Cohen J, Lascoux-Combe C, et al. . Determinants of the underreporting of alcohol consumption by HIV/HCV co-infected patients during face-to-face medical interviews: The role of the physician. Drug Alcohol Depend 2011;116:228–232 [DOI] [PubMed] [Google Scholar]
- 31.Walther L, de Bejczy A, Löf E, et al. . Phosphatidylethanol is superior to carbohydrate-eeficient transferrin and γ-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcoholism Clin Expl Res 2015;39:2200–2208 [DOI] [PubMed] [Google Scholar]
- 32.Heo M, Leon AC. Sample sizes required to detect two-way and three-way interactions involving slope differences in mixed-effects linear models. J Biopharm Stat 2010;20:787–802 [DOI] [PubMed] [Google Scholar]
- 33.Cope R, Pickering A, Glowa T, Faulds S, Veldkamp P, Prasad R. Majority of HIV/HCV patients need to switch antiretroviral therapy to accommodate direct acting antivirals. AIDS Patient Care STDs 2015;29:379–383 [DOI] [PubMed] [Google Scholar]
- 34.Marcus JL, Leyden WA, Chao CR, et al. . Differences in response to antiretroviral therapy by sex and hepatitis C infection status. AIDS Patient Care STDs 2015;29:370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]