Abstract
The development of reliable tissue engineering methods using decellularized cadaveric or donor lungs could potentially provide a new source of lung tissue. The vast majority of current lung decellularization protocols are detergent based and incompletely removed residual detergents may have a deleterious impact on subsequent scaffold recellularization. Detergent removal and quality control measures that rigorously and reliably confirm removal, ideally utilizing nondestructive methods, are thus critical for generating optimal acellular scaffolds suitable for potential clinical translation. Using a modified and optimized version of a methylene blue-based detergent assay, we developed a straightforward, noninvasive method for easily and reliably detecting two of the most commonly utilized anionic detergents, sodium deoxycholate (SDC) and sodium dodecyl sulfate (SDS), in lung decellularization effluents. In parallel studies, we sought to determine the threshold of detergent concentration that was cytotoxic using four different representative human cell types utilized in the study of lung recellularization: human bronchial epithelial cells, human pulmonary vascular endothelial cells (CBF12), human lung fibroblasts, and human mesenchymal stem cells. Notably, different cells have varying thresholds for either SDC or SDS-based detergent-induced cytotoxicity. These studies demonstrate the importance of reliably removing residual detergents and argue that multiple cell lines should be tested in cytocompatibility-based assessments of acellular scaffolds. The detergent detection assay presented here is a useful nondestructive tool for assessing detergent removal in potential decellularization schemes or for use as a potential endpoint in future clinical schemes, generating acellular lungs using anionic detergent-based decellularization protocols.
Introduction
For many patients with end-stage lung diseases, lung transplantation remains the only available therapeutic option. Lung transplantation is complicated by acute and chronic rejection, side effects from immunosuppressive medications, and organ shortage.1 Ex vivo lung tissue engineering, using decellularized whole lungs or lobes as scaffolds for seeding with autologous cells derived from the transplant recipient, has been recently explored as a potential alternative.2 The majority of current lung decellularization approaches utilize detergent-based protocols to remove cells and cell debris while leaving the major airway and vascular architecture and extracellular matrix (ECM) composition of the native lung intact (reviewed in Wagner et al.2).
The most commonly utilized detergents include the nonionic detergent Triton X-100, the anionic detergents sodium deoxycholate (SDC) or sodium dodecyl sulfate (SDS), and the zwitterionic detergent 3-[(3-cholamideopropyl)dimethylammonio]-1-propanesulfonate (CHAPS).2,3 However, the success of subsequent recellularization of an acellular lung scaffold is, in part, based on the removal of the lysed cellular material and cytotoxic detergents after the decellularization process.4–10 This can be particularly challenging in large organs, such as porcine and human lungs, as large rinse volumes may be needed to wash out residual detergents. Removal of detergents, especially anionic detergents, from proteins and large acellular scaffolds is known to be difficult.3–8,11 At present, there are no widely used noninvasive assays to detect anionic detergent removal from decellularized lungs and thus investigators mainly use excision of small segments and culture of thin slices with one or two cell types to test cytocompatibility before recellularizing larger segments or whole lobes.2,3,5,6,10,12,13
In addition to cell-based cytocompatibility assays, others have previously assessed the presence of residual detergents in large lung scaffolds derived from porcine or human sources.5,7,8 Gilpin et al. used colorimetric analysis to detect residual SDS present in tissue sampled from decellularized human and porcine lungs using the Stains-All solution.5 The Stains-All solution was previously shown to be specific for SDS, among other potential contaminating ions and detergents, in a linear manner between 0% to 0.2% SDS,14 while the general concentration of SDS used throughout lung decellularization protocols is 0.1–1%.4,5,15 Increased detergent concentrations have been thought to be necessary for decellularizing lungs obtained from larger species and Triton X-100 has been used following SDS perfusion to remove residual SDS.4,5 However, this method of detergent analysis utilized excision of decellularized lung tissue, lyophilization, and enzymatic digestion of the tissue for detection of SDS. Whether Stains-All can be used for detection of other anionic detergents or in effluents is unknown.
Price et al. used Sudan III staining to detect residual detergents in situ in excised tissue slices from porcine lungs decellularized using a 0.1% Triton X-100 and 2% SDC-based protocol and found that porcine lungs decellularized using a manual-based perfusion technique retained more detergent than those decellularized using an automated bioreactor program.7 While this technique is useful for potentially visualizing detergent localization in the matrix, it requires destructive tissue excision.7 Sudan staining is also not specific for detergents and may stain other residual cellular materials, such as lipids, potentially complicating interpretation.16,17 Significantly, both of these techniques are destructive to the integrity of the complete scaffold and likely are not ideal to be used in a clinical translation scheme where detergent removal is important to confirm before recellularization.
Detection and removal of residual detergents has also been an important endpoint in generating acellular scaffolds other than lung.11,12,18 The SDC content in acellular human saphenous vein and in acellular porcine liver and kidney tissue has been assessed using an methylene blue (MB)-based assay, colorimetrically analyzing the presence of anionic detergents.12,18 Both methods, however, used excision and homogenization of acellular tissue for detergent detection and were not optimized for use with other decellularization agents. Agents such as chloride and sulfates have long been known from wastewater studies to potentially impact measurements in the MB-based detergent assays and contaminants have required extensive backwashes, multiple chloroform extraction steps, and use of large volumes to minimize these effects.19
In an effort to extend this method of anionic detergent detection to the lung decellularization field, we scaled down and optimized the MB detergent detection assay for use in whole lung perfusion decellularization effluents, taking into account other potential decellularization reagents that might interfere with detection. We were able to reliably and accurately detect at least two different anionic detergents, SDC and SDS, with high specificity both in the concentration range utilized in decellularization schemes and also in the presence of other commonly utilized decellularization agents and successfully applied this assay during detergent-based decellularization of both small and large lungs. We further determined detergent concentrations causing cytotoxic effects in four different relevant, representative human lung cell types that might be utilized in recellularization schemes and found that different cells have different detergent cytotoxicity thresholds. Therefore, the use of such an assay before recellularization studies could be critical in nondestructively determining the cytocompatibility of acellular scaffolds produced using either of these detergents.
Materials and Methods
Anionic detergent assay development and optimization
Concentrations of two anionic detergents widely utilized in whole lung and lobar decellularization schemes, SDC (Sigma-Aldrich, St. Lewis, MO) and SDS (Bio-Rad Laboratories, Berkeley, CA), were determined using an MB assay.14,18,20 To generate standard curves, 2% SDC and 1% SDS solutions were diluted in de-ionized (DI) water over a range consistent with use in decellularization schemes.4,5,7,8,10,13,20–29 The samples were then mixed with 0.0125% MB (Sigma-Aldrich) in DI water (w/v) at a ratio of either 1:1, 1:10, or 1:100 (sample:MB solution, v/v). After vortexing of the samples with MB, chloroform (Sigma-Aldrich) was added at a ratio of 1:2 (sample: chloroform, v/v). Samples were then vortexed for 1 min. Following a 5, 15, 30, or 60 min incubation period at room temperature, 150 μL of the bottom chloroform layer was extracted (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec) and the absorbance was measured in a Synergy HT Multi-Detection Microplate Reader (Biotek Instruments, Winooski, VT).
All assays were conducted at the respective absorbance maximums of 630 nanometers (nm) for SDC:MB complexes or 650 nm for SDS:MB complexes in a polypropylene 96-well plate (Costar, Corning, New York City, NY). A maximum of eight samples were transferred and measured in the plate reader at one time to reduce potential error caused by evaporation of the chloroform. The protocol was performed in triplicate. Pure DI-water or PBS (Mediatech, Inc., Manassas, VA) containing no detergents served as the blank for respective experiments.
To analyze the effect of other ions and reagents commonly utilized during decellularization protocols,2,4,5,7,8,10,20,21,24–26 we determined the SDC concentration in the presence of a range of Triton X-100 (0–1%), 2 mM CaCl2 (Sigma-Aldrich), 1.3 mM MgSO4 (Sigma-Aldrich), a solution of both 2 mM CaCl2 (Sigma-Aldrich) and 1.3 mM MgSO4 (Sigma-Aldrich), or 1 M NaCl (Fisher Scientific, Hampton, NH). To assess the effect of PBS on assay performance, SDC and SDS standards were prepared in PBS in addition to DI water.
We further evaluated the influence of proteins on assay performance by adding bovine serum albumin (BSA) (Fisher Scientific) in three different concentrations, 5, 0.5, and 50 μg/mL to cover a broad range of protein concentrations that could be detected in decellularization effluents. We further analyzed the effect of different basic solutions on assay performance. Standard curves were prepared with pHs of 8, 10, and 12—a pH range that was recently used by Tsuchiya et al.30 to analyze the effect of pH during detergent-based decellularization.
Lung decellularization and effluent collection
Adult C57BL/6 mice (8–12 weeks, Charles River, Wilmington, MA) were maintained under standard laboratory conditions and euthanized by exposure to CO2 according to Institutional Animal Care and Use Committee (IACUC) and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards. Human lungs were obtained with permission from next of kin from autopsy services at the University of Vermont Medical Center, Burlington, VT, and only lungs or lung lobes from patients with no history of smoking or significant lung disease were used in this study. Porcine heart-lung blocs were obtained at the University of Vermont in Burlington, Vermont, from 12-week-old male Sus Scrofa pigs, courtesy of Helene Langevin, MD and Jeff Spees, PhD, under all appropriate IACUC and AAALAC standards.
Human lobes and mouse and pig lungs were decellularized under sterile conditions over 3 days by sequential instillation and rinsing through the trachea (main lobar bronchi for human lobes) and the right ventricle (main lobar artery and vein for human lobes) with DI water, 0.1% Triton X-100 (Sigma-Aldrich), 2% SDC, 1 M NaCl, and porcine pancreatic DNase (Sigma-Aldrich); human and porcine lungs were terminally sterilized in peracetic acid (PAA) (Sigma-Aldrich) followed by equilibration with a PBS storage solution (5X pen/strep, 50 mg/L gentamicin (Corning), 2.5 μg/mL amphotericin B (Corning) in 1X PBS (PBS-SS), as previously described.8,10,20,24
Histologic assessment of decellularization
Efficacy of decellularization in murine, porcine, and human lungs was assessed by fixing samples in 4% paraformaldehyde, embedding in paraffin, and mounting 5 μm sections before deparaffinization and hematoxylin and eosin (H&E) staining, as previously described.8,10,20,23,24 All images were acquired using an EVOS™ Digital Color Fluorescence Microscope (Advanced Microscope Group, Bothell, WA).
Assessment of protein concentrations in lung decellularization effluents
Protein concentration of effluent samples was measured using the Detergent Compatible Protein Detection Assay (BioRad, Hercules, CA) according to the manufacturer's instructions. Samples were measured in triplicates and compared to a BSA standard curve for quantification.
Assessment of residual anionic detergents in lung decellularization effluents
All effluent samples were initially measured in a 1:100 ratio with MB, as this assay protocol yielded linear standard curves for the entire potential range. If concentrations in the effluents were below 0.5%, a lower initial ratio of 1:10 (sample:MB) within its linear range was utilized to improve accuracy. The SDC concentration in the effluents was calculated using a linear regression of the respective standard curve.
Wash effluents were obtained at different stages of the decellularization process for mouse, pig, and human lungs and stored at 4°C until assessed for anionic detergent content. An overview of the sampling scheme is displayed in Supplementary Fig. S2. Mouse lungs were flushed with 3 mL DI water via the trachea and 3 mL DI water via the right ventricle. Those effluents were combined and stored at 4°C until analysis. Flushing was repeated five times (wash numbers 1–5) and each wash step was analyzed separately.
Human lung lobes and pig lungs were washed two to three times alternating via the airways and vasculature with DI water until fully inflated (approximately 1.5–2.5 L, depending on size) after each incubation step (wash numbers 1–6).8,10 Airway (wash number 1, 3, 5) and vascular washes (wash number 2, 4, 6) for human and porcine lungs were not combined and were analyzed individually. For calculation of detergent concentrations in effluents following PBS-SS washes and homogenates, PBS-SS was used as the blank.
Cell culture
Human bronchial epithelial cells (HBE) (courtesy of Albert van der Vliet, University of Vermont, originally from Drs. J. Yankaskas and R. Wu),31 human lung fibroblasts (HLF) (Lonza, Basel, Switzerland), human mesenchymal stem cells (hMSC) (courtesy of Dr. David McKenna, University of Minnesota), and a primary human pulmonary vascular endothelial cell line (CBF) (courtesy of Dr. Mervin Yoder, Indiana University) were expanded in standard 150 mm tissue culture plates (Corning) in their respectable media as previously described.8 All cells were cultured in a humidified incubator at 37°C, 5% CO2.
Cytotoxicity assessments
To determine cytotoxic thresholds from the different detergents and thus begin to establish minimum effluent detergent concentration criteria, we followed ISO 10993:5 (Standard, I. Biological Evaluation of Medical Devices: Tests for in vitro cytotoxicity) and used 70% viability compared to the cell cultivation medium (negative, nontoxic control) as cytotoxic threshold.32 hMSCs were seeded at 10,000 cells/well and CBFs, HBEs, and HLFs were seeded at 20,000 cells/well in 96-well plates and left to adhere overnight. Different cell numbers were used to account for the differences in cell size and to assure similar confluence after initial cell attachment. Media was then changed to media containing different amounts of detergents over a range of concentrations commonly used in decellularization protocols (SDC: 0.0–2.0%, SDS: 0.0–1.0%, Triton X-100: 0.0–0.1%; mixed at a ratio of 1:4 (sample:medium)) and incubated for 24 h.
Cytotoxicity was subsequently assessed using a WST-1 assay (G-Biosciences, CytoScan, St. Louis, MO) according to the manufacturer's instructions. Cells were washed with PBS before incubation with WST-1 at a concentration of 15 μL/mL cultivation medium for 2 h. About 2.5% Triton X-100 was used as a cytotoxic (positive) control and media alone was used as a negative control. Values were normalized to the medium (negative) control and displayed in percentage (%) of control values.
To assess effects on cell viability of effluents and to assess removal of the cytotoxic detergent from the extracellular environment of the acellular scaffolds, cells were incubated for 24 h with either the first or final DI water effluents following SDC incubation of human lung decellularization (SDC1 and SDC6, respectively), the first or final PBS-SS wash (PBS-SS1 and PBS-SS6, respectively), or homogenates as described below (sample:medium ratio 1:4). Cell viability was assessed as described above.
Preparation of homogenates from decellularized lungs for cytotoxicity assessment
Decellularized human and pig lung segments of about 2 cm3 were homogenized in PBS-SS using a Polytron (Kinematica, Lucerne, Switzerland). To remove large pieces of homogenized tissue, solutions were centrifuged followed by filtration (MILLEX GP, pore size 0.22 μm, PES membrane, Merck Millipore Ltd., Tullagreen, Carrigtwohill, Co Cork, Ireland) before cytotoxicity assessment. SDC concentration was determined in these samples using the optimized protocol with PBS-SS as background. The potential effect of filtration on detergent concentration was assessed before and after filtering standard curves.
Statistical analyses
All values are shown as mean with standard deviation and calculated with Graph Pad Prism 6 (GraphPad Software; La Jolla, CA). The nonlinear standard curves were fit with a one-site total binding curve. For comparison of two groups, an unpaired t-test, with Welch's correction was used. More than two groups were compared with ANOVA and Bonferroni post-test. The WST data were analyzed with a one-sample t-test to the hypothetical value of 70%. A p-value lower then 0.05 was considered statistically significant.
Results
Optimization of anionic detergent assay
We first attempted to use a scaled down version of an MB detergent assay originally developed to detect anionic detergent in wastewater.19,33 We determined the absorption maximum from the absorption spectra for each MB-detergent complex (SDC and SDS) in chloroform and found that SDC's absorption peak occurred at about 635 nm, while SDS's occurred at 650 nm (Supplementary Fig. S3). In all future measurements, we used 630 nm for SDC and 650 nm for SDS detection for compatibility with commonly utilized spectrophotometers.
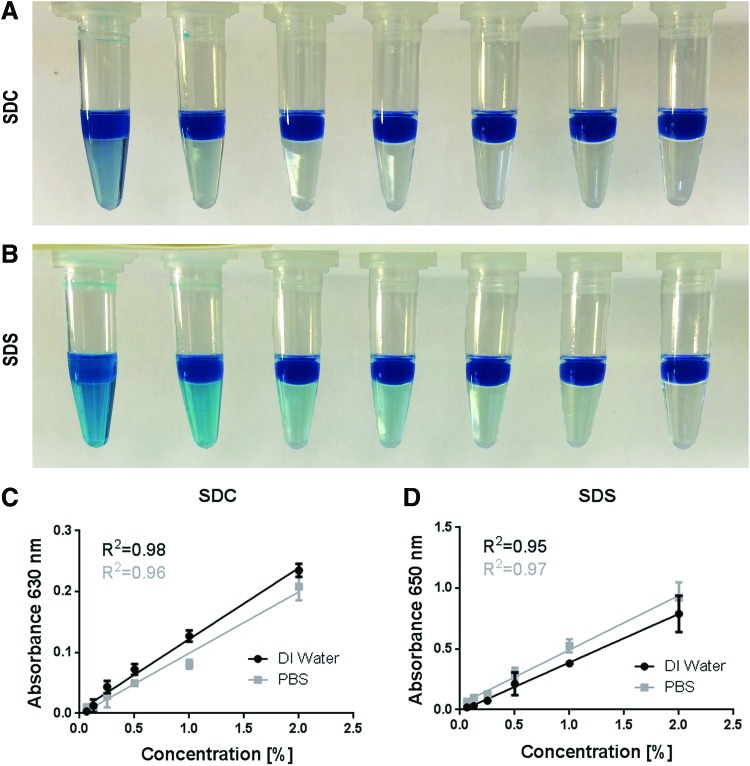
Owing to a previous report, which measured SDC in decellularized human saphenous veins, we began with a 1:1 ratio of MB to anionic detergent solution and found that standard curves from 0% to 2% SDC displayed nonlinear behavior (data not shown).18 We then began varying the initial dilution and increased the ratio of aqueous fraction to chloroform to 1:2 to see whether linear curves could be generated. We found that at a dilution of 1:10, the SDC standard curve was linear up to 0.5% and that a dilution of 1:100 achieved linearity for SDC over the complete range of SDC commonly used in lung decellularization protocols (Fig. 1 and Supplementary Fig. S4). We also could observe qualitative differences in the amount of blue color extracted into the chloroform layer over the same range (Fig. 1A).
FIG. 1.
Chloroform extraction and quantification of MB-SDC or MB-SDS complexes. (A) From left to right 2%, 1%, 0.5%, 0.25%, 0.125%, 0.0625%, and 0% SDC using the optimized detergent detection assay with a 1:100 dilution (sample:MB) is displayed following mixing with chloroform. (B) From left to right 1%, 0.5%, 0.25%, 0.125%, 0.0625%, 0.03125%, and 0% SDS using the optimized detergent detection assay with a 1:1000 dilution (sample:MB) is displayed following mixing with chloroform. (C, D) Standard curve of SDC or SDS concentration and optical density in both deionized (DI) water and PBS. SDC samples were measured at 630 nm and SDS samples at 650 nm according to their respective maximum absorptions. Mean ± SD, samples measured in triplicates. Linear curve fits shown for each DI water or PBS. MB, methylene blue; SDC, sodium deoxycholate; SDS, sodium dodecyl sulfate. Color images available online at www.liebertpub.com/tec
We next similarly assessed SDS and found that a 1:1000 dilution was needed for linear SDS detection (Fig. 1D and Supplementary Fig. S5). As anticipated from the different maximum absorbance peaks between SDC and SDS, the shade of blue extracted into the chloroform qualitatively differed between SDC and SDS (Fig. 1A, B). Standard curves for SDC (1:100) and SDS (1:1000) generated at higher concentrations were nonlinear, exhibiting saturation curve binding behavior at higher detergent concentrations (Supplementary Figs. S4 and S5). Higher concentrations of MB could not be assessed owing to increased viscosity and subsequent loss of precision in assay performance (data not shown).
Despite these low dilutions and volumes, we were able to precisely and reliably generate linear standard curves of both SDC and SDS across a wide range of concentrations commonly utilized in whole lung decellularization protocols.
Effect of other decellularization components on assay performance
It has previously been reported that detection of detergents in water samples by the MB assay can be significantly impacted by contaminating ions.33,34 We first tested the linearity of standard curves of both anionic detergents in the presence of PBS, as many decellularization protocols utilize PBS as a final wash rinse.2,3 While we observed a trend of decreased absorbance with SDC standard curves in PBS versus SDC in DI water, these differences were not statistically different (Fig. 1C). In contrast, we observed a slight, but nonsignificant, increase in absorbance measurements with SDS standard curves made in PBS versus DI water (Fig. 1D). Importantly, linearity was maintained in PBS for both anionic detergents.
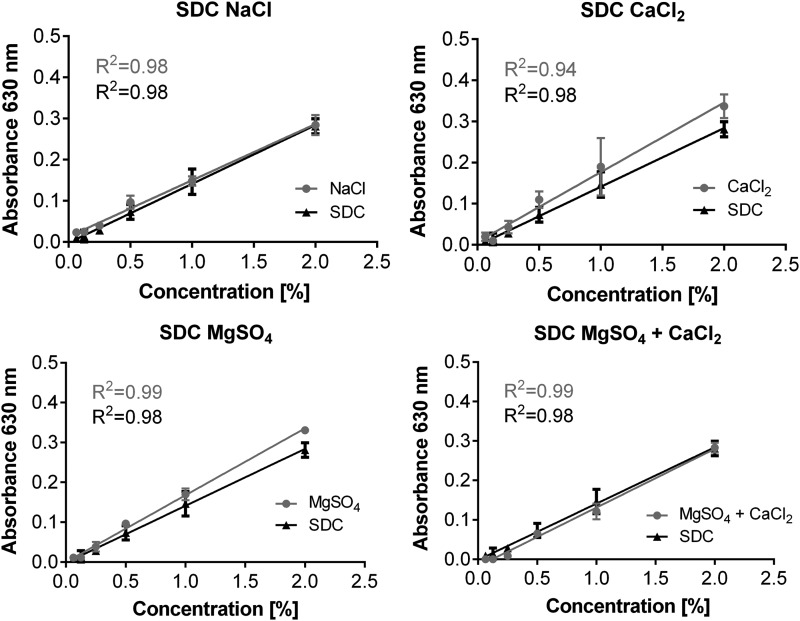
We next tested each of the potential decellularization components that might affect assay performance in SDC-based protocols (1 M NaCl, 2 mM CaCl2, 1.3 mM MgSO4, 2 mM CaCl2 + 1.3 mM MgSO4, and PBS).7,8,10,20–22,24–27 Similar to our findings in PBS, we found that none of these components, at these concentrations, affected the linearity of the assay nor resulted in statistically significant differences between the DI water and decellularization solution at any concentrations (Fig. 2). The impact of the various decellularization solutions at 0.5%, a more realistic upper concentration limit that might be encountered in actual usage of the assay is summarized in Table 1.
FIG. 2.
Effect of commonly utilized decellularization agents on standard curves. Representative standard curves for determination of the SDC concentration in the presence of different decellularization agents (2 mM CaCl2, 1.3 mM MgSO4, 2 mM CaCl2 + 1.3 mM MgSO4, and 1 M NaCl). Mean ± SD, samples measured in triplicates.
Table 1.
Summary of Common Additives in Decellularization Protocols and Their Effect on Assay Performance
| Additive | Concentration | R2 | Δ at 0.5% SDC |
|---|---|---|---|
| NaCl | 1 M | 0.98 | +0.023 |
| CaCl2 | 2 mM | 0.94 | +0.037 |
| MgSO4 | 1.3 mM | 0.98 | +0.023 |
| CaCl2 + MgSO4 | 2 mM +1.3 mM | 0.98 | −0.008 |
| BSA | 5 mg/mL | 0.95 | −0.016 |
| 0.5 mg/mL | 0.93 | −0.002 | |
| 50 μg/mL | 0.96 | −0.006 |
R2 is the value of the linear regression for the SDC standard curve with the given additive.
Δ at 0.5% SDC is the difference between the mean value of 0.5% SDC in deionized water and the 0.5% SDC with the additive.
BSA, bovine serum albumin; SDC, sodium deoxycholate.
We further analyzed the specificity of the assay for anionic detergents by using a nonionic detergent (Triton X-100) commonly used in SDC and SDS decellularization protocols.4,5,7,20,21,22,24,26 Use of Triton X-100 (concentration range 0.0015625–1%) did not result in any absorbance detection up to 1% Triton X-100 (Supplementary Fig. S6). Further, there was no significant difference between the SDC standard prepared in DI water versus in a 0.1% Triton X-100 solution (Supplementary Fig. S6B). Interestingly, the addition of 0.1% Triton X-100 to the SDS standard led to a significant decrease in absorbance at concentrations of 0.5 and 1% SDS (Supplementary Fig. S6C).
We next analyzed the influence of different basic pH solutions on assay performance. Neither pH 10 nor 12 led to a significant difference in absorbance measurement compared to the SDC standard prepared in DI water (final solution pH 8) (Supplementary Fig. S7).
To determine general influences of proteins in decellularization wash effluents on assay performance, we evaluated the SDC concentration with the addition of BSA. We first determined the amount of protein detected in wash effluents during human lung decellularization with a commercially available detergent compatible protein detection assay. We found the highest protein concentration to be about 0.5 mg/mL in the wash steps after incubation with 2% SDC (Supplementary Fig. S8A). None of the different concentrations of BSA added to the SDC solutions, covering a broad range potentially detected during lung decellularization, including 10x higher than values that we measured in our protocol (i.e., 5 mg/mL) led to significant differences in absorbance measurements in the MB detergent assay (Supplementary Fig. S8B and Table 1).
Optimization of assay performance
It has been previously reported that the incubation time of MB with anionic detergents may impact the reliability of the assay performance.34 Therefore, we further evaluated whether the incubation time would influence our anionic detergent detection ability. Following vortexing, we incubated the samples with chloroform for 5, 15, 30, and 60 min. Longer incubation times were found to correspond to higher absorbance values (Supplementary Fig. S9). However, we only observed significant differences between incubation times at an SDC concentration of 2% and linearity remained unaffected during all incubation times. While we saw significant differences between the shorter incubation times (comparing 5 to 15, 5 to 60, and 15 to 60 min), there was no significant difference between 30 and 60 min, indicating that most of the MB:detergent complexes had entered the chloroform phase after 30 min.
These results show that the assay is suitable to specifically and reliably detect anionic detergents, like SDC, in effluents during decellularization without interference of other additives like NaCl, CaCl2, MgSO4, and Triton X-100. A stepwise protocol of the optimized assay is depicted in Table 2.
Table 2.
Optimized Methylene Blue Detergent Assay for Detection of Anionic Detergents in Whole Organ Decellularization
| 1 | Transfer 1 μL sample (1:10 prediluted for SDS) to tube |
| 2 | Add 99 μL MB 0.0125% |
| 3 | Mix |
| 4 | Add 200 μL chloroform |
| 5 | Vortex 1 min |
| 6 | Incubate 30 min, room temperature |
| 7 | Transfer 150 μL of bottom layer to 96-well-plate (PP) |
| 8 | Read absorbance at 630 nm (650 nm for SDS) |
MB, methylene blue; SDS, sodium dodecyl sulfate.
Detection of SDC during lung decellularization and remaining in acellular lung scaffolds
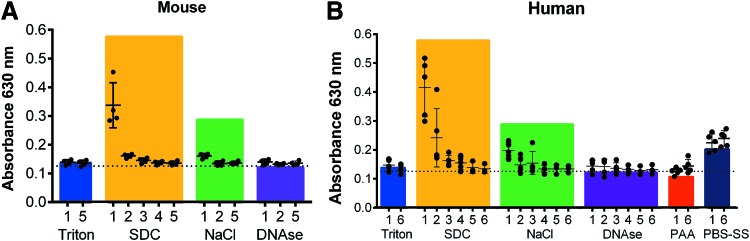
We next sought to determine whether this assay could detect changes in the effluent of decellularized lungs in a Triton X-100 and SDC detergent-based protocol, which we and others have used in rodent, primate, porcine, and human lungs.7,8,10,20–22,24–26 As previously described, decellularized scaffolds generated from mouse, porcine, and human lungs were evaluated by H&E staining and were found to have maintained gross lung architecture and removal of both cellular bodies and no intact nuclear staining (Supplementary Fig. S10). We first used the optimized assay to measure the absorbance values of each decellularization solution at baseline to determine whether any of the solutions might produce false positives (i.e., significant absorbance in the absence of SDC). We found that both the 1 M NaCl solution and the PBS-SS used during decellularization lead to slight, but significant, increases in absorbance measurements when compared to pure DI water (Supplementary Fig. S11A). Interestingly, in addition to increased absorbance over DI water controls, we also observed a slight shift in the absorbance maximum for non-SDC containing solutions compared with the maximum peak for SDC-containing solutions (Supplementary Fig. S11B). This likely indicates formation of a different MB complex. Taken together, this strongly suggests that using this optimized assay, the MB:SDC complex is preferentially formed in the presence of these potential contaminating ions at these concentrations and is the complex detected in the chloroform layer.
We next collected effluents at each DI water wash step of the decellularization protocol and determined the anionic detergent concentration at these different steps for mouse, human, and pig lungs (Fig. 3 and Supplementary Fig. S12). In the DI water washes immediately following Triton X-100 incubations, no absorbance was detected at 630 nm, matching our finding that Triton X-100 does not interfere with the assay (Supplementary Fig. S6). We were, however, able to detect SDC in the early DI water wash steps immediately following the SDC incubation step, which then decreased with subsequent DI water washes. We found that murine lungs were almost free of SDC following the first wash step, but that the SDC remained longer and was more difficult to wash out in the human and pig lungs (Fig. 3 and Supplementary Fig. S12). No detectable SDC was found in the final DI water wash effluents following SDC incubation.
FIG. 3.
Detection of detergent in various effluents of mouse lung and human lung lobes during perfusion decellularization. (A) Absorbance at 630 nm (SDC) using the methylene blue detergent assay was measured in effluents from mouse lung decellularization (n = 4). (B) Absorbance at 630 nm (SDC) was measured in effluents from human lung lobe decellularization (n = 5). The colored bars show the mean absorbance values of the pure solutions used during decellularization (n = 3–5). T, Triton; S, SDC; N, NaCl; D, DNase; PAA, peracetic acid; PBS-SS, PBS storage solution. Mean ± SD, samples measured in triplicates. Color images available online at www.liebertpub.com/tec
The first wash step after NaCl incubation showed a slight increase in absorbance for all species, but values remained lower than the absorbance of the pure 1 M NaCl solution, likely due to dilution with DI water. We did not detect any absorbance above background in DI water wash effluents following DNase incubation or PAA. As expected from absorbance measurements of pure solutions (Supplementary Fig. S11), a similar effect of increased absorbance was detected in the PBS-SS solution washes (PBS-SS1-6) for human and porcine lungs. The absorbance measurements of the PBS-SS washes was higher than in the washes following DNase treatment and tended to increase with each consecutive wash step, normalizing around the absorbance value of the pure PBS-SS (Fig. 3 and Supplementary Fig. S11).
To confirm that the lack of SDC detected in final effluent measurements was not due to SDC retention in the tissue, we also performed this same assay on excised, homogenized segments of acellular lungs from humans. We found that SDC concentrations detected in the homogenates from decellularized human lung lobes generally correlated with those found in the final effluents of the corresponding human lobe (i.e., little or no detergent detected in either the final effluents or homogenates). Further, we confirmed that the use of filtration does not alter detergent concentrations (Supplementary Table S1 and Supplementary Fig. S13).
Determination of cytotoxic detergent thresholds
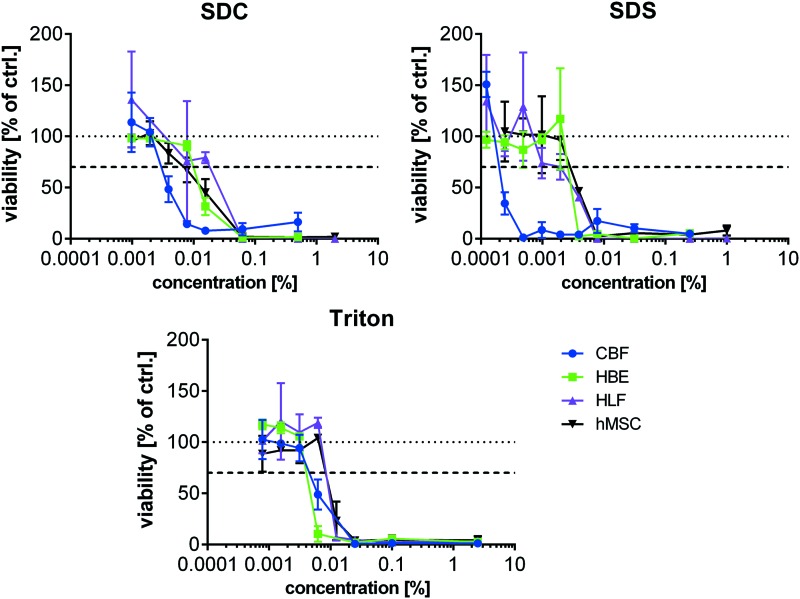
As a number of different cell types will be investigated for use in recellularization of decellularized lung scaffolds, we assessed the in vitro cytotoxicity of three commonly utilized detergents across a broad range of potential detergent concentrations in four representative human lung cells: epithelial (HBE), vascular endothelial (CBF), and fibroblasts (HLF), and a bone marrow-derived stromal progenitor cell (hMSC). Interestingly we found that cells displayed differential responses to the different detergents with CBFs displaying higher sensitivity than the other cells to cytotoxic effects in all detergents (Fig. 4 and Table 3). Similar to the CBF cells, HBEs were more sensitive to Triton X-100 while hMSCs and HLFs were slightly more resistant. SDS was similarly toxic to HBE, hMSC, and HLFs. HLFs showed the highest resistance against SDC while HBEs and hMSCs were similarly sensitive. Overall, SDS and Triton X-100 induced cytotoxicity at lower concentrations than SDC.
FIG. 4.
Detergent-induced toxicity in different human cell lines. Viability of HBE, CBF, HLF, and hMSC in response to various amounts of different detergents (SDC, SDS, and Triton X-100) was determined by WST-1 conversion after cultivation for 24 h. 2.5% Triton X-100 served as positive (cytotoxic) control while cultivation medium served as negative control (set to 100%). 70% viability and thereby critical, cytotoxic concentrations are displayed in Table 3. Mean ± SD, samples measured in triplicates. HBE, Human bronchial epithelial; HLF, human lung fibroblasts; hMSC, human mesenchymal stem cell. Color images available online at www.liebertpub.com/tec
Table 3.
Estimated Cytotoxic Thresholds for Each Cell Type for Detergents Commonly Used in Whole Lung Decellularization
| Cell type | % SDC | % SDS | % Triton |
|---|---|---|---|
| CBF | 0.002 | 0.00012 | 0.0031 |
| HBE | 0.0078 | 0.002 | 0.0031 |
| HLF | 0.016 | 0.002 | 0.0063 |
| hMSC | 0.0078 | 0.002 | 0.0063 |
Evaluation of effluent cytotoxicity
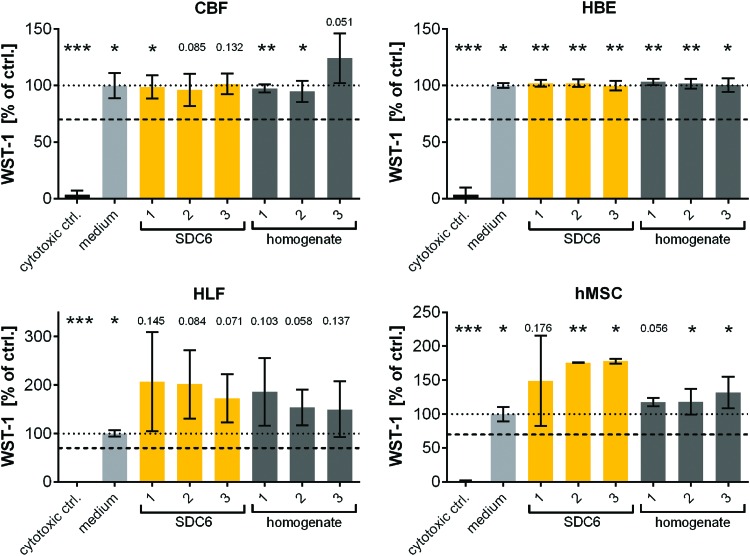
To test the potential for establishing a nondestructive means of evaluating scaffold cytotoxicity for the decellularization protocol, effluents from the first and last DI water wash step after incubation with SDC (SDC1 and SDC6), the first and last PBS-SS wash step of pig lung lobe decellularizations (PBS-SS1 and PBS-SS6) and supernatants from homogenized acellular human lung tissue from different samples were tested with the four different cell lines. We found that while SDC1 induced significant toxicity in all cell lines, neither SDC6, any PBS-SS wash step or supernatants from homogenized acellular lung tissue induced significant cell toxicity (Fig. 5 and Supplementary Fig. S14). This matches our previous finding that we were unable to detect any SDC content in the final PBS-SS wash effluents using our optimized anionic detergent detection protocol or cytotoxic levels in homogenates (Fig. 3 and Supplementary Table S1). This further validates our previous collective results in successfully recellularizing intact small segments of acellular porcine and human lungs generated using this decellularization protocol with all four cell types.8,10,23 This confirms that there are no residual toxins, including anionic detergents, which are present at a level in final PBS-SS effluents or in the decellularized scaffolds that significantly compromises viability of these cell types, and that effluent samples can be used to test cytocompatibility.
FIG. 5.
Cytotoxicity effects in different cell lines (HBE, CBF, HLF, and hMSC) of homogenized decellularized lungs and effluents collected after the last DI water wash step following incubation with SDC (SDC6). Viability of different cell lines (HBE, CBF, HLF, and hMSC) was determined by WST-1 after 24 h incubation with either SDC6 effluents or homogenized decellularized lung lobes. Both effluents and homogenized decellularized lung lobes were mixed 1:4 with medium. 2.5% Triton X-100 served as a cytotoxic (positive) control in both cases while cultivation medium served as the negative control (set to 100%). The threshold for cytotoxicity was set at 70%. Mean ± SD, n = 2–6. One sample t-test *: sign. to 70% viability, *p < 0.05, **p < 0.01, ***p < 0.001, other significance values are indicated by discrete p-values. Color images available online at www.liebertpub.com/tec
Discussion
Ex vivo bioengineering of functional lung tissue is a growing area of research that offers a potential solution to the high demand for lung tissue for transplantation. Traditionally, engineered tissue requires a scaffold, or three-dimensional (3D) matrix, which could be biologically derived or synthetically fabricated. Scaffolds can be subsequently seeded with autologous stem, progenitor, or other cells obtained from the ultimate transplant recipient to avoid immune complications and the usage of immunosuppressive medication.2,35 This method has been successfully used clinically in regeneration of the trachea and in preclinical animal models of tissue regeneration such as skin, vasculature, cartilage, bone, heart, and liver.28,29,35–37 To date, even extremely advanced manufacturing technologies, such as 3D printing, have not been successful in recreating the complex 3D architecture of the lung and acellular lung scaffolds remain the most promising current approach.
Commonly utilized approaches for whole lung decellularization include the perfusion of the native lung vasculature and in some protocols, also the airways, with solutions, including hypo- and hypertonic salt solutions, detergents, and DNase to lyse and remove cells and cellular debris.2 However, detergent removal can be challenging, particularly in lungs and other highly vascularized organs, such as kidney and liver, derived from large species such as porcine and human sources.4–8,12 While others have successfully used techniques to identify residual detergents in homogenates generated from these acellular organs, the specificity of some of these techniques in detecting specific anionic detergents remains unclear.7,12 Further, all of these techniques utilized destructive methods and thus are not optimal for clinical translation schemes or in nondestructively assessing cytocompatibility before costly and complex recellularization experiments. Further, the applicability of these protocols to be used in different laboratories using different anionic detergent schemes remains unknown and they have not all been optimized for use with other decellularization agents. Other noninvasive-based techniques, such as high performance liquid chromatography, have also been used to detect detergents in simple decellularized scaffolds,11 but may not be readily available in all laboratories or conducive for cost-effective routine testing.
The MB detergent detection assay has been primarily used in detecting anionic detergent concentrations in different applications such as wastewater, environmental water, and in drug testing of urine samples.19,33,38 In the presence of anions, such as the anionic detergents SDC and SDS, electrostatic forces between the positively charged MB and anionic detergents generate a chloroform extractable complex, which correlates with the amount of anionic detergent in the sample. Without formation of this complex, the water soluble MB remains immiscible in chloroform. However, there are many variables that can affect assay performance and previous MB assays used in wastewater studies required large volumes (in the mL range) and extensive backwashing with chloroform to minimize these effects.18,33,38 By doubling the initial ratio of the amount of chloroform to the total aqueous fraction typically used in the MB detergent assay, we did not require extensive backwashing to eliminate interference from potential contaminating ions or compounds.19,39 We also found that we were able to scale down the entire assay to μL amounts by using a detergent:MB solution ratio of 1:100 for SDC and 1:1000 for SDS, respectively, and that we could generate linear curves for the entire potential anionic detergent concentration range used in most lung decellularization schemes.
We were successfully able to use this assay to assess anionic detergent removal using a Triton X-100, SDC perfusion based lung decellularization protocol. The MB detergent assay has previously been reported to have interference from anions such as chloride or sulfates, however, we did not observe significant changes in standard curves prepared using solutions containing chloride or sulfates using our optimized assay at the concentrations used in decellularization solutions. We did, however, observe slight batch to batch variations in the background absorbance measurements of our PBS-SS, presumably due to differences in the antibiotics and antimycotic lots. Sulfates may also be present in proteins, such as proteoglycans (e.g., heparan sulfates). In a previous report, mass spectrometry proteomic analysis was used to detect proteins in effluents using a similar Triton X-100, SDC detergent-based protocol as we used here.34 Sulfated proteoglycans were detected at the highest amounts following the 1 M NaCl wash. However, we did not see a large increase in absorbance in these DI water wash effluents above the background of the 1 M NaCl solution, indicating that our optimized assay is specific for anionic detergent detection. The use of this assay to assess anionic detergent amounts throughout the decellularization protocol, confirms the removal of anionic detergent using the volumes and flow rates we previously reported for generating cytocompatible porcine and human acellular lungs.8,10
Another important endpoint in evaluating decellularized scaffolds is the determination of its capability to support survival and cellular growth during recellularization. An unexpected finding of this study was that the four different representative cell lines/types assessed had different cytotoxic thresholds to the various detergents. Recellularization schemes will likely require multiple cell types and therefore it is important to test multiple cell types when assessing cytocompatibility of decellularized scaffolds. HMSCs and HLFs were more resistant to all three detergents, while HBEs and CBFs were unable to tolerate higher concentrations of detergents. Homogenized scaffolds, the last DI water wash following SDC incubation (SDC6), and the PBS-SS washes did not induce significant cytotoxicity in the four different cell types, suggesting that there is no significant detergent left in the scaffold generated using our protocol that would negatively impact recellularization. This is in line with our previous findings that recellularized segments support growth and proliferation in healthy murine, macaque, porcine, and human scaffolds up to 28 days.2,10,22,25,26
In addition to the potential for residual detergents to induce cytotoxicity in recellularization strategies, the detergents used and the manner in which they are utilized can dramatically impact the final matrix composition and immune response upon implantation. Detergents utilized have been shown to variably induce matrix metalloproteinase activity, resulting in potential degradation of remaining ECM proteins in the scaffold.3,15,20,21 The pH at which detergents are used has also recently been shown to have adverse effects in in vivo implantation studies.30 We found no influence of basic pH changes on the detection of SDC with our MB assay, thereby proving its potential application in decellularization protocols using basic conditions. Use of acidic pH was not feasible because of precipitation of SDC at acidic pHs. Decellularization using CHAPS at a more physiologic pH was shown to minimize the host inflammation response when acellular rodent lung scaffolds were implanted into immunocompetent rodents, despite the presence of increased DNA content in these scaffolds, which is thought to be immunogenic.3 It remains unknown whether a recellularized construct would mask this inflammatory response by covering immunogenic epitopes or whether recellularization could remodel a scaffold to minimize or eliminate immunogenicity.
At present, there is no consensus for an optimal decellularization protocol. Reduction of large volume DI water rinses may be beneficial for preserving matrix components, including matrix-bound growth factors, which have been shown to be necessary for inducing differentiation of embryonic stem cell-derived definitive endodermal cells seeded onto acellular lung.40 As each large organ may be different in size, especially for human-derived lungs, this assay may be one easy way to determine the minimal DI water washes with respect to anionic detergent removal. Thus, at present, careful monitoring of the removal of detergents is a critical endpoint in detergent-based protocols and should be assessed in addition to other proposed endpoints such as retention of major ECM components and removal of visible cell nuclei.3 The assay we developed here can be readily used in all laboratories using common reagents and equipment as a cost-effective means to detect removal of anionic detergents in effluents during decellularization protocols, such as SDC and SDS.
Supplementary Material
Acknowledgments
The authors are grateful to Helene Langevin, MD and Jeff Spees, PhD, University of Vermont, the Langevin lab for their assistance in obtaining porcine lungs, and the autopsy staff at The University of Vermont Medical Center; Mervin Yoder, MD, Indiana University for the CBF cells; Albert van der Vliet, PhD for the HBE cells; Ethan Griswold, Richard Hommel, Wyatt Chia, Sean Wrenn, Jacob Dearborn, Amelia Payne, and Jordan Appenzeller for help with the measurements of the detergent effluents, lung tissue homogenizations, and lung decellularizations.
Support
Studies were supported by NIH-NHLBI ARRA RC4HL106625 (DJW), NIH-NHLBI R21HL108689 (DJW), and the UVM Lung Biology Training grant T32 HL076122 from the NHLBI. D.E. Wagner is currently supported by the Whitaker International Fellows and Scholars Program and the Helmholtz Munich Postdoctoral Fellowship Programme.
Disclosure Statement
D.J.W. receives research funding from Athersys, Inc. and United Therapeutics Corp. to conduct research studies. The other authors have no competing financial interests.
Portions of this were presented in poster form at the American Thoracic Society 2015 Conference.
References
- 1.Kotloff R.M., and Thabut G. Lung Transplantation. Am J Respir Crit Care Med 184, 159, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Wagner D.E., Bonvillain R.W., Jensen T., Girard E.D., Bunnell B.A., Finck C.M., Hoffman A.M., and Weiss D.J. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18, 895, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crapo P.M., Gilbert T.W., and Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyette J.P., Gilpin S.E., Charest J.M., Tapias L.F., Ren X., and Ott H.C. Perfusion decellularization of whole organs. Nat Protocols 9, 1451, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Gilpin S.E., Guyette J.P., Gonzalez G., Ren X., Asara J.M., Mathisen D.J., Vacanti J.P., and Ott H.C. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant 33, 298, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Nichols J.E., Niles J., Riddle M., Vargas G., Schilagard T., Ma L., Edward K., La Francesca S., Sakamoto J., Vega S., Ogadegbe M., Mlcak R., Deyo D., Woodson L., McQuitty C., Lick S., Beckles D., Melo E., and Cortiella J. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A 19, 2045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price A.P., Godin L.M., Domek A., Cotter T., D'Cunha J., Taylor D.A., and Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng Part C Methods 21, 94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D.E., Bonenfant N.R., Sokocevic D., DeSarno M.J., Borg Z.D., Parsons C.S., Brooks E.M., Platz J.J., Khalpey Z.I., Hoganson D.M., Deng B., Lam Y.W., Oldinski R.A., Ashikaga T., and Weiss D.J. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 35, 2664, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., and Niklason L.E. Tissue-engineered lungs for in vivo implantation. Science 329, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner D.E., Bonenfant N.R., Parsons C.S., Sokocevic D., Brooks E.M., Borg Z.D., Lathrop M.J., Wallis J.D., Daly A.B., Lam Y.W., Deng B., DeSarno M.J., Ashikaga T., Loi R., and Weiss D.J. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35, 3281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cebotari S., Tudorache I., Jaekel T., Hilfiker A., Dorfman S., Ternes W., Haverich A., and Lichtenberg A. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs 34, 206, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Bao J., Wu Q., Zhou Y., Li Y., Wu X., Shi Y., Li L., and Bu H. Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical-scale bioengineering engrafts. Xenotransplantation 22, 48, 2015 [DOI] [PubMed] [Google Scholar]
- 13.O'Neill J.D., Anfang R., Anandappa A., Costa J., Javidfar J., Wobma H.M., Singh G., Freytes D.O., Bacchetta M.D., Sonett J.R., and Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 96, 1046, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusconi F., Valton E., Nguyen R., and Dufourc E. Quantification of sodium dodecyl sulfate in microliter-volume biochemical samples by visible light spectroscopy. Anal Biochem 295, 31, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., and Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16, 927, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Fischer B. Über die Fettfärbung mit Sudan III und Scharlach-R. Centrantralbl f Pathol 13, 943, 1902 [Google Scholar]
- 17.Sabnis R.W.S. Handbook of Biological Dyes and Stains. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2010, p. 425 [Google Scholar]
- 18.Mathapati S., Galla S., Sankaranarayanan K., Shanker Verma R., Mammencherian K., and Guhathakurta S. Qualitative and quantitative detection of sodium deoxycholic acid in decellularized tissue. Indian J Thorac Cardiovasc Surg 26, 129, 2010 [Google Scholar]
- 19.Koga M., Yamamichi Y., Nomoto Y., Irie M., Tanimura T., and Yoshinaga T. Rapid determination of anionic surfactants by improved spectrophotometric method using methylene blue. Anal Sci 15, 563, 1999 [Google Scholar]
- 20.Wallis J.M., Borg Z.D., Daly A.B., Deng B., Ballif B.A., Allen G.B., Jaworski D.M., and Weiss D.J. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 18, 420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price A.P., England K.A., Matson A.M., Blazar B.R., and Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 16, 2581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonvillain R.W., Danchuk S., Sullivan D.E., Betancourt A.M., Semon J.A., Eagle M.E., Mayeux J.P., Gregory A.N., Wang G., Townley I.K., Borg Z.D., Weiss D.J., and Bunnell B.A. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A 18, 2437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner D.E., Fenn S.L., Bonenfant N.R., Marks E.R., Borg Z., Saunders P., Oldinski R.A., and Weiss D.J. Design and synthesis of an artificial pulmonary pleura for high throughput studies in acellular human lungs. Cell Mol Bioeng 7, 184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly A.B., Wallis J.M., Borg Z.D., Bonvillain R.W., Deng B., Ballif B.A., Jaworski D.M., Allen G.B., and Weiss D.J. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A 18, 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokocevic D., Bonenfant N.R., Wagner D.E., Borg Z.D., Lathrop M.J., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., Hoffman A.M., and Weiss D.J. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 34, 3256, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonenfant N.R., Sokocevic D., Wagner D.E., Borg Z.D., Lathrop M.J., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., and Weiss D.J. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 34, 3231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth A.J., Hadley R., Cornett A.M., Dreffs A.A., Matthes S.A., Tsui J.L., Weiss K., Horowitz J.C., Fiore V.F., Barker T.H., Moore B.B., Martinez F.J., Niklason L.E., and White E.S. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186, 866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.-L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M.L., and Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16, 814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott H.C., Matthiesen T.S., Goh S.-K., Black L.D., Kren S.M., Netoff T.I., and Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya T., Balestrini J.L., Mendez J., Calle E.A., Zhao L., and Niklason L.E. Influence of pH on Extracellular Matrix Preservation During Lung Decellularization. Tissue Eng Part C Methods 20, 1028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yankaskas J.R., Haizlip J.E., Conrad M., Koval D., Lazarowski E., Paradiso A.M., Rinehart C.A., Jr., Sarkadi B., Schlegel R., and Boucher R.C. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol 264, C1219, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Standard I. Biological Evaluation of Medical Devices. Tests for in vitro Cytotoxicity. Switzerland, Geneva: ISO/TC 194, 2009 [Google Scholar]
- 33.Abbott D.C. The colorimetric determination of anionic surface-active materials in water. Analyst 87, 286, 1962 [Google Scholar]
- 34.George A.L., and White G.F. Optimization of the methylene blue assay for anionic surfactants added to estuarine and marine water. Environ Toxicol Chem 18, 2232, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Badylak S.F., Weiss D.J., Caplan A., and Macchiarini P. Engineered whole organs and complex tissues. Lancet (London, England) 379, 943, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Totonelli G., Maghsoudlou P., Garriboli M., Riegler J., Orlando G., Burns A.J., Sebire N.J., Smith V.V., Fishman J.M., Ghionzoli M., Turmaine M., Birchall M.A., Atala A., Soker S., Lythgoe M.F., Seifalian A., Pierro A., Eaton S., and De Coppi P. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 33, 3401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macchiarini P., Jungebluth P., Go T., Asnaghi M.A., Rees L.E., Cogan T.A., Dodson A., Martorell J., Bellini S., Parnigotto P.P., Dickinson S.C., Hollander A.P., Mantero S., Conconi M.T., and Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Jones J.T., and Esposito F.M. An assay evaluation of the methylene blue method for the detection of anionic surfactants in urine. J Anal Toxicol 24, 323, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Lukanov B., and Firoozabadi A. Specific ion effects on the self-assembly of ionic surfactants: a molecular thermodynamic theory of micellization with dispersion forces. Langmuir 30, 6373, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Shojaie S., Ermini L., Ackerley C., Wang J., Chin S., Yeganeh B., Bilodeau M., Sambi M., Rogers I., Rossant J., Bear , Christine E., and Post M. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep 4, 419, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.