Abstract
Objective
The aim of this study was to compare pregnancy outcomes in euthyroid women who were anti-TPO Ab+ with those who were anti-TPO Ab−.
Design
This observational study comprised 1,000 women in the age group of 25–35 years, having normal thyroid function tests, normotensive, non-diabetic, singleton pregnancy and attending Gyn. OPD/ANC up to 20 weeks’ gestation and those who were already in the process of abortion. anti-TPO Ab levels of >50 IU/ml were considered as anti-TPO Ab+.
Setting
This study was conducted in the SMS Medical College’s attached hospital, Jaipur from April 2012 to September 2013.
Main Outcome
The estimation of the proportion of anti-TPO Abs in the hospital-based population and the comparison of pregnancy and neonatal outcomes in anti-TPO Ab+ and Ab− euthyroid women were done.
Results
The main result showed increased rates in miscarriages (13.33 vs. 2.34 %, P < 0.001), LBWs (25 vs. 5.12 %, P < 0.001), preterm deliveries [<34 weeks] (5 vs. 1.80 %, P > 0.05) in anti-TPO Ab+ women.
Conclusions
The current study revealed that anti-TPO Abs are strongly associated with miscarriage and LBW irrespective of their gestational age. However, we did not find any correlation with the other complication as found in the studies by Abbassi-Ghanavati, Negro et al.
Keywords: Thyroid peroxidase, Antibodies, Euthyroid, Pregnancy
Introduction
Pregnancies in women with thyroid gland disorders may have increased rates of pregnancy complications including abortion, preterm delivery, placental abruption, and abnormal neuropsychological development in offspring [1–4]. Subclinical and overt hypothyroidisms are most commonly associated with adverse outcomes, and together, they are thought to represent a continuum of autoimmune thyroiditis [5– 7]. In these autoimmune diseases, structural or functional damage is produced by humoral and cell-mediated immune reaction with normal components of the body. Production of specific antibodies, i.e., auto antibodies, is an integral part of response. Autoimmune thyroid disease is common, affecting approximately 1 % of the population, while subclinical, focal thyroiditis, and circulating thyroid antibodies can be found in about 15 % of otherwise healthy subjects who are euthyroid [8–10].
Detection of specific antibodies is also playing an increasingly significant role in diagnosis and treatment. Tissue damage may be confined to certain organ, in which case auto antibodies are referred to as organ specific; an example would be thyroid [11, 12]. Although many types of antithyroid antibodies have been described, the most common is the group of antibodies directed against various parts of the thyroid peroxidase molecule.
Materials and Methods
This hospital-based observational study was conducted in the Department of Obstetrics & Gynecology, Gangori hospital attached to SMS Medical College, Jaipur from April 2012 to September 2013.
Selection of Cases
This study comprised 1,000 women in the age group of 25–35 years, having normal thyroid function tests, normotensive, non-diabetic, and singleton pregnancy. Women having heart disease, liver disease, renal disease, chronic hypertension, epilepsy, severe anemia, antiphospholipid antibody syndrome, anatomical abnormalities in uterus, cervix, and congenitally anomalous of fetus, and those who smoke and chew tobacco, were excluded from this study.
Study Participants
Women registered in antenatal clinic before 20 weeks of gestation and women who were already in the process of abortion were also included in the study and followed-up till delivery.
Sample Size
Since the incidence of anti-thyroid peroxidase antibody is low, 1,000 cases attending Gynae OPD/antenatal clinic were included in this study, and pregnancy outcomes were noted as abortion/miscarriage, preterm delivery, term delivery (>37 weeks) PIH, preeclampsia, placental abruption, and mode of delivery (Vaginal, LSCS). Neonatal outcomes were noted as assessment of Apgar score at 1 and 5 min, birth weight, neonatal death, IUD, meconium, need of admission of neonate to NICU, and stay period in NICU [13–15].
Sampling Procedure
All the patients attending Gynae OPD/antenatal clinic were subjected to serum T3, T4, and TSH levels. Out of these, euthyroid patients were selected on the basis of normal serum TSH and T4 levels. anti-TPO Ab levels were measured in them, and the patients were divided into anti-TPO Ab+ and anti-TPO Ab− groups who were compared for any adverse maternal and neonatal outcome.
Statistical Analysis
The qualitative data were analyzed using chi-square test, and quantitative data (mean and standard deviation) were compared using T test and Z test.
Method
All pregnant women <20 weeks and women who had been aborted in our hospital have undergone the measurement of serum TSH level and were reflexly assayed for free T4 concentration. Serum aliquots were analyzed for thyroid peroxidase antibody concentration using a chemiluminescent immunoassay Immulite 2000—for quantitative measurements of T4, TSH, and anti-TPO Ab in our central lab.
Women with both abnormal TSH and free T4 levels were excluded [16–18]. Those who were normal for TSH and T4 were assayed for anti-TPO Ab concentration using chemiluminescent immunoassay. The analytical sensitivity of thyroid peroxidase assay is 5 IU/ml, and its coefficient of variation is 9.8 % within run and 11.3 % between run. Anti-TPO Ab level more than 50 IU/ml is abnormal, and those women were considered as antithyroid peroxidase antibody positive [19–21].
Serum TSH level >3 mu/l and normal range free T4 were considered as subclinical hypothyroidism [11]. Normal serum TSH level and free T4 <0.86 ng/dl were considered as maternal hypothyroxinemia [22, 23]. Samples were retrieved from women.
Normal-range Serum TSH: 0.08 mIU/l.
Free T4: 0.86–1.9 ng/dl [11].
Observation
Pregnancies in women with thyroid gland disorders may have increased rates of pregnancy complications, including abortion, preterm delivery, placental abruption, and abnormal neuropsychological development in offspring [1–4]. Although many types of antithyroid antibodies have been described, the most common is the group of antibodies directed against various parts of the thyroid peroxidase molecule [24–26].
Most pregnant women with abnormally elevated serum levels of thyroid peroxidase antibodies are clinically and biochemically euthyroid, but they may be at increased risk of spontaneous abortion, and preterm delivery and other pregnancy complications [11, 27].
The current study was conducted to estimate the proportion of antithyroid peroxidase antibodies in the hospital-based population and to compare pregnancy outcome in anti-TPO Ab+ and anti-TPO Ab−euthyroid women.
Because high serum TSH levels have been associated with adverse maternal and fetal outcomes, a particular strength of our study was the ability to remove the confounding effect of TSH level on adverse outcomes. Thus, a clear association between thyroid antibody positivity and adverse maternal and neonatal outcomes could be demonstrated, even among euthyroid women.
The following are the observations in our study, evaluating a link between anti-thyroid peroxidase antibodies and adverse pregnancy outcomes.
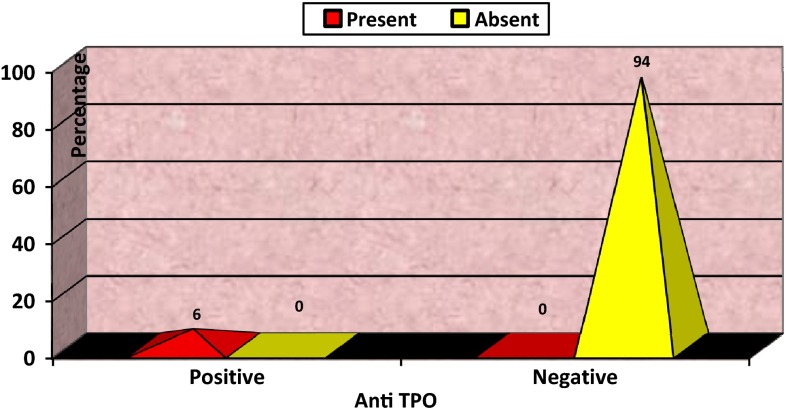
In our study, women positive for anti-TPO Ab were 6 %, and the rest 94 % women were found to be Ab− (Fig. 1).
Fig. 1.
Distribution of women according to anti-TPO antibody
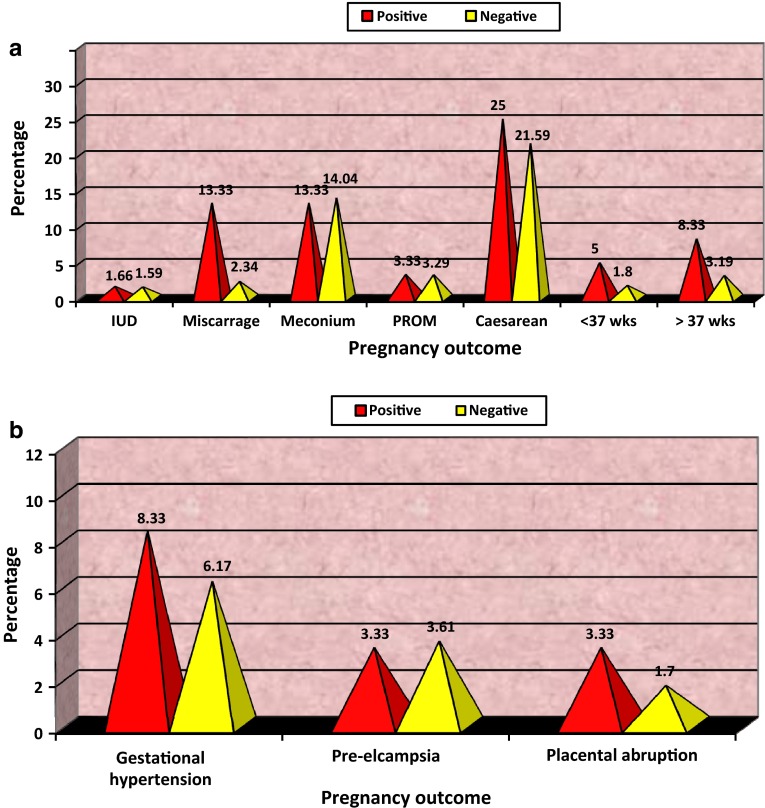
Pregnant women with TPO Ab levels of more than 50 IU/mL, whom we termed anti-TPO Ab+, have a more than fivefold increased risk of miscarriage compared with women identified to be anti-TPO Ab− (Fig. 2a).
Fig. 2.
Distribution of women according to pregnancy outcome
We found significant association between TPO Ab status and birth weight. The percentages of babies born with low birth weight were 25 % and 5.12 % in anti-TPO positive women and in anti-TPO negative women, respectively (Table 1).
Table 2.
Pregnancy complication and outcome
| Pregnancy complication | Anti-TPO antibody | X 2 | D.f | P value | Significance | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Gestational hypertension | 5 (8.33) | 58 (6.17) | 0.395 | 1 | >.05 | NS | |
| Pre eclampsia | 2 (3.33) | 34 (3.61) | 0.059 | 1 | >.05 | NS | |
| Placental abruption | 2 (3.33) | 16 (1.70) | 0.172 | 1 | >.05 | NS | |
| IUD | 1 (1.66) | 15 (1.59) | 0.223 | 1 | >.05 | NS | |
| Miscarriage | 8 (13.33) | 22 (2.34) | 23.421 | 1 | <.001 | HS | |
| Meconium | 8 (13.33) | 132 (14.04) | 0.023 | 1 | >.05 | NS | |
| PROM | 2 (3.33) | 22 (3.29) | 0.015 | 1 | >.05 | NS | |
| Caesarean | 15 (25.00) | 202 (21.59) | 0.391 | 1 | >.05 | NS | |
| Preterm delivery | <34 weeks | 3 (5.00) | 17 (1.80) | 1.528 | 1 | >.05 | NS |
| 34–37 weeks | 5 (8.33) | 30 (3.19) | 3.024 | 1 | >.05 | NS | |
We found no significant association between anti-TPO Ab status and other adverse pregnancy outcomes including hypertensive disorders, premature rupture of membranes, placental abruption, meconium, caesareans section, intra uterine death, neonatal death, NICU admission, and preterm and very preterm deliveries (Table 2).
Table 1.
Neonatal outcome
| Neonatal outcome | Anti-TPO Ab+ | Anti-TPO Ab− | ||
|---|---|---|---|---|
| Birth weight | LBW (SGA + Preterm) | 13 (25 %) | 47 (5.12) | |
| NBW | 39 (75 %) | 871 (94.88) | ||
| Neonate | Expired | 1 (1.67 %) | 7 (0.74) | |
| Survived | 59 (98.33 %) | 933 (99.26) | ||
| ICU | Required | 9 (15 %) | 147 (15.63) | |
| Apgar Score at 1 min and 5 min | ≥ 7 | 43 (82.69) | 696 (75.82) | |
| < 7 | 9 (17.31) | 222 (24.18) | ||
Most of the women in our study were in the age group of 25–27 years. Mean maternal ages in our study were 27.37 + 2.42 years in anti-TPO antibodies positive group and 28.00 + 2.63 in anti-TPO negative group. In our study, nearly two-thirds of women were urban. Both the groups had higher frequencies of urban population (66.67 vs. 33.33 %) and higher literacy rate, and most of the women in both groups belonged to middle socioeconomic status.
Religionwise, most of the women in our study were Hindu. In antibody positive group, Hindu and Muslim women were 65 and 35 %, respectively. In antibody negative group, 72.03 % were Hindu, and 27.97 % were Muslim women.
In the study of Mini Abbassi-Ghanavati in 2011, women who were anti-TPO Ab+were older, heavier, and more often parous than women with anti-TPO Ab−.
In our study also, incidence of anti-TPO Ab+ women was higher in multiparous women compared with primigravida. Glinoer et al. [3] found a doubling in the preterm delivery rate in thyroid antibody-positive Belgian women (16 vs. 8 %, P 0.005). Ghafoor et al. [4] reported that TPO Ab+ women had a significantly higher rate of preterm delivery compared with women who were TPO Ab+ (26.8 vs. 8.0 %, P < 0.01). Similarly, a prospective trial by Negro et al. [28] reported a significant increase in preterm delivery in southern Italy in TPO Ab+ women compared with women who were TPO Ab− (22.4 vs. 8.2 %).
On the other hand, a retrospective study by Tierney et al. [24] performed in Australia found no difference in preterm deliveries between women who were thyroid antibody positive and women who were thyroid antibody negative.
Likewise, in a nested case–control study performed in New Jersey by Stagnaro-Green et al. [11] reported a similar rate of thyroid antibody positivity in women who delivered at term compared with women with preterm delivery.
In our study, rates of preterm delivery at <34 weeks were 5 vs. 1.80 %, respectively, in thyroid antibody positive and antibody negative women, while preterm delivery rates between 34 and 37 weeks in both groups were 8.33 vs. 3.19 %. So, we can see that although preterm delivery rate was more in antibody positive group, it was not significant (P > 0.05), but there was significant difference among birth weights of newborns in both groups (P < 0.001).
Roberto Negro, et al. [29] assessed 245 women who were euthyroid (TSH_2.5 mIU/l) and thyroid peroxidase positive in the first trimester and 3,348 women who were euthyroid and thyroid peroxidase negative in the first trimester and reported no significant difference in low birth weight babies between the two groups (6.5 vs. 4.8 %, P −0.229).
Abbassi-Ghanavati et al. [30] found no difference in low birth weight babies among anti-TPO antibodies positive and negative women.
In our study, we observed that the presence of thyroid peroxidase antibodies significantly affected birth weights of newborns irrespective of gestational age (P < 0.001). Incidence of low birth weight babies was 25 % in thyroid peroxidase positive women while, it was only 5.12 % in thyroid peroxidase negative women as shown in Table 1.
Mannisto et al. [18] found an increase in perinatal death in anti-TPO Ab positive women compared with anti-TPO Ab negative women. Haddow et al. [31] reported an increase in preterm premature rupture of membranes. Abbassi-Ghanavati et al. [30] reported a threefold increase in placental abruption. Neonatal death was 2 % in anti-TPO Ab positive women compared with anti-TPO Ab negative women.
In our study, the incidence of preterm rupture of membranes was comparable in both Ab positive women and in Ab negative women (3.33 vs. 3.29 %, P > 0.05), and it was found as statistically not significant. We also noted twofold increase in placental abruption in anti-TPO Ab positive women compared with anti-TPO Ab negative women (3.33 vs. 1.70 %), but it was statistically not significant (P > 0.05).
No significant difference was noted in our study regarding neonatal death among anti-TPO Ab positive and anti-TPO Ab negative women (1.67 vs. 0.74 %, P > 0.05).
Abbassi-Ghanavati et al. [30] reported no difference in Apgar score of newborns in thyroid peroxidase Ab positive and negative women (1 vs. 1 %). Roberto Negro et al. noted low Apgar score among thyroid peroxidase antibodies positive and negative women (0.5 vs. 06 %, P 0.571).
In our study, no significant difference was noted in Apgar score among anti-thyroid peroxidase antibodies positive and negative women (P > 0.05).A meta-analysis by Prummel et al. showed that anti-TPO antibody positive women were associated with a twofold increased risk of miscarriage as shown in Table 1.
In our study, pregnant women positive for anti-thyroid peroxidase antibody had a more than fivefold increased risk of miscarriage compared with women identified to be anti-thyroid peroxidase antibody-negative (13.33 vs. 2.34 %), and this difference was highly significant (P < 0.001) as shown in Fig. 2a.
Roberto Negro et al. noted no significant difference in incidences of gestational hypertension, preeclampsia, NICU admission, and caesarean, among antibody positive and negative women. In our study, the prevalence of gestational hypertension was 8.33 % in anti-thyroid peroxidase antibody positive and 6.17 % in antibody negative women (P > 0.05 not significant). No significant differences were noted in incidences of caesarean rate and preeclampsia among anti-thyroid peroxidase antibodies positive and negative women (caesarean 25 vs. 21.59 %, P > 0.05 and preeclampsia 3.33 vs. 3.61 %, P > 0.05).
The need for neonatal ICU admission was nearly same in both groups, 15 % in antibody positive group and 15.63 % in antibody negative (P > 0.05). In this study, we noticed that the presence of anti-thyroid peroxidase antibodies had no significant adverse effect on intrauterine death (1.66 vs. 1.59 %, P > 0.005).
No significant difference was noted in Apgar score among anti-thyroid peroxidase antibodies positive and negative women (P > 0.05). There were 82.69 % babies of antibody positive women and 75.82 % babies of antibody negative women having Apgar score of >7. Low Apgar i.e., <7 score was seen in 17.31 and 24.18 % babies of antibody positive and negative women, respectively, as shown in Table 1.
Conclusion
With this observational study, we concluded that anti-thyroid peroxidase antibodies are associated with some adverse pregnancy outcomes.
Anti-thyroid peroxidase antibodies are present in 6 % of the hospital-based obstetric population.
Anti-thyroid peroxidase antibody increases the rates of miscarriage and low birth weight irrespective of their gestational age.
Anti-thyroid peroxidase antibody is associated with very preterm delivery.
No significant association was noted among anti-thyroid peroxidase antibody and pregnancy induced hypertension, placental abruption, IUD, meconium, premature rupture of membrane, caesarean section, Apgar score, neonatal death, NICU admission, and preterm delivery.
The current study revealed the fact that anti-TPO antibodies are strongly associated with miscarriage and low birth weight of babies irrespective of their gestational age. However, we did not find any correlation with other complication as studies by other authors like Abbassi-Ghanavati et al. and Negro et al. have found.
Compliance with ethical requirements and conflict of interest
We ensure that accepted principles of ethical and professional conduct have been followed in this study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. The authors declare that they have no conflict of interest.
Dr. Aruna Meena
is working as a Senior Resident in the Department of Obstetrics and Gynaecology in SMS Medical College, Jaipur since July 2014. She had started her research work since 2012. She had passed her MBBS from the Govt Medical College, Kota, Rajasthan in 2009. She had passed M.S in June 2014 from the SMS Medical College, Jaipur. She has participated in clinical photography held in 2013 GYNECON, RSCOG at Udaipur. Recently, she has reported a case of congenial mullerian duct anomaly, and will submit the same for publication as soon as possible. She is also doing research work on the use of uterine ballon therapy in menorrhagia. 
References
- 1.Potlukova E, Potluka O, Jiskra J, et al. Age as a risk factor for AITD in pregnancy. J Clin Endocrinol Metab. 2012;97(6):1945–1952. doi: 10.1210/jc.2011-3275. [DOI] [PubMed] [Google Scholar]
- 2.Rushworth FH, Backos M, Rai R, et al. Prospective pregnancy outcome in untreated recurrent miscarries with thyroid autoantibodies. Hum Reprod. 2011;15:1637–1639. doi: 10.1093/humrep/15.7.1637. [DOI] [PubMed] [Google Scholar]
- 3.Thangaratinam S, Tan A, Knox E, et al. Thyroid auto-antibodies are strongly associated with miscarriage and preterm birth: a meta-analysis of evidence. BMJ. 2011;342:d2616. doi: 10.1136/bmj.d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagis T, Gokcel A, Saygili ES. Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid. 2011;11:1049–1053. doi: 10.1089/105072501753271743. [DOI] [PubMed] [Google Scholar]
- 5.Gyamfi C, Wapner RJ, D’Alton ME. Thyroid dysfunction in pregnancy: the basic science and clinical evidence surrounding the controversy in management. Obstet Gynecol. 2009;113(3):702–707. doi: 10.1097/AOG.0b013e3181996fe5. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura H, Usa T, Motomura M, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008;31(10):861–865. doi: 10.1007/BF03346432. [DOI] [PubMed] [Google Scholar]
- 7.Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab. 2008;4:394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Yp, Ying Y, Wu HT, et al. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9(2):121–5. doi:10.7150/ijms.3467. [DOI] [PMC free article] [PubMed]
- 9.Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun. 2012;38:J275–J281. doi: 10.1016/j.jaut.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Voell M, Rahlff I, et al. Longterm follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18:755–760. doi: 10.1089/thy.2008.0008. [DOI] [PubMed] [Google Scholar]
- 11.Abbassi-Ghanavati M, Casey BM, Spong CY, et al. Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstet Gynecol. 2010;116:381–386. doi: 10.1097/AOG.0b013e3181e904e5. [DOI] [PubMed] [Google Scholar]
- 12.Cebecauer L, Radikova Z, Rovensky J, et al. Increased prevalence and coincidence of antinuclear and antithyroid antibodies in the population exposed to high levels of polychlorinated pollutants cocktail. Endocr Regul. 2009;43:75–81. [PubMed] [Google Scholar]
- 13.McElduff A, Morris J. Thyroid function tests and thyroid autoantibodies in an unselected population of women. Int J Med Sci. 2012;9:121–5. http://www.medsci.org. [DOI] [PubMed]
- 14.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. 2011;96:E920–E924. doi: 10.1210/jc.2011-0026. [DOI] [PubMed] [Google Scholar]
- 15.Lee RH, Spencer CA, Mestman JH, et al. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200(3):260-e1–260-e6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Sripada S, Saravelos SH, et al. Thyroid peroxidase antibody in women with unexplained recurrent miscarriage: prevalence, prognostic value, and response to empirical thyroxine therapy. Fertil Steril. 2012;98:378–382. doi: 10.1016/j.fertnstert.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Fister P, Gaberscek S, Zaletel K, et al. Thyroid function in the third trimester of pregnancy and after delivery in an area of adequate iodine intake. Int J Gynaecol Obstet. 2011;112(1):52–55. doi: 10.1016/j.ijgo.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy RL, Malabu UH, Jarrod G, et al. Thyroid function and pregnancy: before during and beyond. J Obstet Gynecol. 2010;30(8):774–783. doi: 10.3109/01443615.2010.517331. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann M, Hoermann R, Francis C, et al. Measuring thyroid peroxidase antibodies on the day nulliparous women present for management of miscarriage: a descriptive cohort study. Reprod Biol Endocrinol. 2013;11:40. doi: 10.1186/1477-7827-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestgaard M, Nielsen VR, Rasmussen AK, et al. Thyroid peroxidase antibodies in pregnant women with type 1 diabetes :impact on thyroid function, metabolic control and pregnancy outcome. Acta Obstet Gynecol Scand. 2008;87(12):1336–1342. doi: 10.1080/00016340802499261. [DOI] [PubMed] [Google Scholar]
- 21.Mascanfroni I, Montesinos MM, Susperreguy S, et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22(4):1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- 22.Mehran L, Tohidi M, Sarvghadi F, et al. Management of thyroid peroxidase antibody euthyroid women in pregnancy: comparison of the american thyroid association and the endocrine society guidelines. J Thyroid Res. 2013;2013:6. Article ID 542692. doi:10.1155/2013/542692. [DOI] [PMC free article] [PubMed]
- 23.Friedrich N, Schwarz S, Thonack J, et al. Association between parity and autoimmune thyroiditis in a general female population. Autoimmunity. 2008;41:174–180. doi: 10.1080/08916930701777629. [DOI] [PubMed] [Google Scholar]
- 24.Toulis KA, Goulis DG, Venetis CA, et al. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol. 2010;162(4):643–652. doi: 10.1530/EJE-09-0850. [DOI] [PubMed] [Google Scholar]
- 25.Brix TH, Hansen PS, Kyvik KO, et al. Aggregation of thyroid autoantibodies in twins from opposite-sex pairs suggests that microchimerism may play a role in the early stages of thyroid autoimmunity. J Clin Endocrinol Metab. 2009;94:4439–4443. doi: 10.1210/jc.2009-0813. [DOI] [PubMed] [Google Scholar]
- 26.Hodkinson CF, Simpson EE, Beattie JH, et al. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55–70 years. J Endocrinol. 2009;202(1):55–63. doi: 10.1677/JOE-08-0488. [DOI] [PubMed] [Google Scholar]
- 27.Camargo RY, Tomimori EK, Neves SC, et al. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo. Braz Eur J Endocrinol. 2008;159:293–299. doi: 10.1530/EJE-08-0192. [DOI] [PubMed] [Google Scholar]
- 28.Haddow JE, Cleary-Goldman J, McClain MR, et al. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and preterm delivery. Obstet Gynecol. 2010;116(1):58–62. doi: 10.1097/AOG.0b013e3181e10b30. [DOI] [PubMed] [Google Scholar]
- 29.Koleva H, Stuart S, O’Hara MW, et al. Risk factors for depressive symptoms during pregnancy. Arch Womens Ment Health. 2011;14(2):99–105. doi: 10.1007/s00737-010-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debieve F, Duliere S, Bernard P, et al. To treat or not to treat euthyroid autoimmune disorder during pregnancy? Gynecol Obstet Invest. 2009;67:178–182. doi: 10.1159/000185689. [DOI] [PubMed] [Google Scholar]
- 31.Anna L, Teresa JM, Ana C. Undiagnosed thyroid dysfunction, & thyroid antibodies in Mediterranean population. Endocr J. 2010;38(3):391–396. doi: 10.1007/s12020-010-9397-2. [DOI] [PubMed] [Google Scholar]
- 32.Shields B, Hill A, Bilous M, et al. Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid function. J Clin Endocrinal Metab. 2009;94(2):570–574. doi: 10.1210/jc.2008-0380. [DOI] [PubMed] [Google Scholar]