Abstract
Background:
Previous studies have reported endothelial dysfunction and inflammatory cytokine in heart failure patients (HF).
Objectives:
The purpose of this study was to determine the effects of creatine monohydrate and exercise on inflammatory and endothelial dysfunction markers among HF patients.
Patients and Methods:
One hundred patients were prospectively randomized into two groups: Intervention group which received 5 grams/day creatine monohydrate and exercised for 8 weeks; and control group which did not receive any interventions. Interleukine-6 (IL-6), high sensitivity C reactive protein (hs-CRP), P-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) were measured at the start and end of the study for both groups.
Results:
In total, 100 patients including 50 controls and 50 intervention group (54% male, mean EF of 34.2 ± 10.5% and 52% male, mean EF of 35.6 ± 12.7%, respectively) were analyzed. The serum levels of hs-CRP and IL-6 increased at the end of the study in the control group compared to the baseline, (7.5 ± 1.5 mg/L vs. 6.9 ± 1.3 mg/L, P < 0.05 and 3.0 ± 0.75 ng/L vs. 2.55 ± 0.9 ng/L, P < 0.05, respectively). However, compared to the baseline, the level of both markers decreased at the end of the study in the intervention group (6.3 ± 1.6 mg/L vs.7.5 ± 1.5 mg/L, P < 0.05 and 2.1 ± 0.8 ng/L vs.2.5 ± 0.5 ng/L, P < 0.05). Also, P-selectin and ICAM-1 levels increased at the end of study (56.9 ± 1.8 ng/L vs. 51.9 ± 1.5 ng/L, P < 0.05 and 368.1 ± 25.4 µg/L vs. 353.1 ± 10.4 µg/L, P < 0.05 respectively). Inversely, the levels of these markers decreased in the intervention group, at the end of study (49.7 ± 1.9 ng/l vs. 51.4 ± 2.1 ng/l, P < 0.05 and 342.7 ± 16.5 µg/l vs. 350.4 ± 14.7 µg/l, P < 0.05, respectively). VCAM-1 level was not decreased significantly at the end of the study in the intervention group (570.5 ± 78.4 µg/L vs. 575.3 ± 86.5 µg/L, P > 0.05).
Conclusions:
Combination of creatine monohydrate and exercise attenuated inflammation and endothelial dysfunction markers among heart failure patients.
Keywords: Heart Failure, Creatine Monohydrate, Exercise, Inflammatory Markers, Endothelial Dysfunction
1. Background
Heart failure (HF) is one of the main health problems among all societies; the prevalence of which increases along with age (1) and due to aging population (2, 3). In addition to higher mortality rate, higher costs of HF associated with recurrent hospitalization accompanied with disability (4), lower exercise tolerance (5) and lower health related quality of life (HRQL) are among CHF outcomes (6-8). Studies showed that endothelial dysfunction followed by considerable increase of endothelial-derived apoptotic microparticles is almost the most essential cause of clinical manifestations in HF patients (9). A study on rats reported that the endothelium dependent vasorelaxation to acetylcholine was reduced significantly in diabetic animals and exercise training or grape seed extract administration partially improves this response. However, exercise training in combination with grape seed extract restored the endothelial function completely (10).
Different studies have reported that systemic inflammatory markers are related to failure in the cardiac function and its worse prognosis (11, 12). Due to micronutrient losses associated with cachexia or chronic application of diuretics, CHF patients may suffer from micronutrients deficiency (8). Studies have reported that complementary micronutrients can prevent smooth muscle myopathy which has the main role in the physical dysfunction of heart among HF patients (13). Despite the importance of this subject, deficiency of micronutrients and energy carrier substances has not been well investigated among HF patients. Micronutrient deficiency can result in heart failure and disturbance of energy metabolism in cardiac myocytes (14). The serum and myocardial levels of micronutrients among HF patients compared to healthy individuals is significantly reduced (15). Creatine is a natural product in the human body that is either synthesized in kidneys, pancreas and liver or is absorbed daily via food regimens (16). Creatine and phosphocreatine (phosphorylated form of creatine), can prevent the depletion of adenosine three phosphate (ATP), provoke protein synthesis, prevent protein degradation and stabilize the biologic membrane (17). Intravenous injection of creatine improves the cardiac output among HF patients (18). It has been proved that HF patients have a reduced level of muscular creatine (19). It also was shown that application of creatine alone in HF patients increased the muscular content of creatine and phosphor-creatine considerably and therefore improved the traction and aerobic capability of muscles (20, 21). A study showed that creatine complement caused an increase in the levels of required energy for cardiac myocytes, increase cardiac contractibility and therefore improvement of cardiac function in HF patients (22). A review study on clinical trials showed the beneficial effects of creatine on the control of hypertension among relevant patients (23). The main objective of physical activities and cardiac rehabilitation is the improvement of practical abilities, remedy or reduction of symptoms associated with activity, reduction of disability, and reduction of mortality rate related to cardiovascular diseases (18, 24). Regular exercise increases the ability of skeletal muscles to extract oxygen and tissue metabolization, increases the coronary circulation and decreases the cardiac function. Epidemiologic studies have also reported that, regular exercise, due to its physiological effects, reduces the mortality rate associated with cardiovascular diseases (25, 26). Studies showed that physical activities in patients with severe heart failure caused an improvement of the peripheral vasomotor function, induction of androgenic regenerations via rising the surface of progenitor cells and therefore an improvement of the ejection fraction of left cardiac ventricle (27). The suitable effects of creatine monohydrate and physical training alone on improvement of cardiac function among HF patients have already been reported; however, there is no report on the combination effects of these interventions on cardiac output of HF patients.
2. Objectives
The aim of the current study was to assess the effects of complementary creatine monohydrate and regular training (in combination) on inflammatory and endothelial dysfunction markers among patients with HF.
3. Patients and Methods
3.1. Trial Design
This study was a double blind clinical trial among HF patients who attended the physiotherapy and sport medicine clinics in Ilam city, during 2013 - 2014. The protocol of this study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of Ilam university of medical sciences and written informed consent was obtained from all patients at the beginning of the study. Also this study was registered in Iranian Registry of Clinical Trials (clinical trial Registration code IRCT IRCT2014061718128N1). The blinding was for participants and those who measured the demographic, anthropometric and laboratory results.
3.2. Patients’ selection
Among the patients who attended the physiotherapy and sports medicine clinics in Ilam city, 100 HF patients were selected during 2013 - 2014. HF patients were selected according to the criteria of New York cardiovascular associates (USA). Inclusion criteria were: being outpatient, age > 18 years, LVEF ≤ 45%, end diastolic residual > 96 mL/m2, cardiac functional class ≥ II according to NYHA classification and exclusion criteria were: patients with uncontrolled arrhythmia, persistent and severe chest pain, glucosamide or mineral or vitamin consumption, suffering from known renal failure, arterial abnormalities, sensitivity to sulfur components and patients who did not regularly participate in the study activities, inability to stand on treadmill, history of hospitalization in the recent 12 months, uncontrolled hypertension, changing the drug regimen during the past 3 months, valve disorders, existence of pulmonary diseases, anaemia, immunosuppressive therapy, atrial fibrillation, pregnancy and suffering from infectious diseases. After completion of a consent form, patients were divided into intervention and control groups randomly. Demographic and anthropometric data of each participant was taken via a validated questionnaire. The questionnaire included some queries about patients’ demographical data, history of cardiovascular and other related diseases, history of past medical or surgical interventions, anthropometric measurements and some laboratory measurements such as lipid profile.
At the start of the study all steps of the project were explained for the participants and each patient completed an informed consent form. In the next step all demographic data and basal as well as laboratory measurements of participants were collected and recorded in their individual files. During the study all routine drugs of patients were continued. Weight and height sizes of patients were measured using a digital scale (Omron, model HN-283, Japan) and a tapeline respectively by the same expert individual. BMI was calculated as weight (kg) divided by height (m2). The blood pressure was measured for all participants at the sitting and standing positions by the same person and the mean pressure obtained for these positions was considered as final blood pressure for the patient. The ejection fraction was measured for each participant using echocardiography, Philips I E33 (Philips medical systems, Andover, MA, USA) by the same cardiologist.
3.3. Exercise Protocol
All the patients participated in a program of 8 weeks exercise as well as a prepared diet regimen advised by a food specialist. For determination of functional capacity, using Naughton method (28), an exercise test was applied for each patient via a treadmill (Track Master-US) by a cardiologist. According to the Naughton protocol, patients were encouraged to walk on the treadmill and their heart electrical activities were recorded by an attached ECG at the same time. The Naughton protocol is a sub-maximal exercise test designed to keep patients in a heart rate zone that is lower than the maximum heart rate. There are several stages, each one slightly more intense than the last. The heart rate gradually increases throughout the test with an endpoint target zone that is 80 to 90 percent of the maximum heart rate.
Also, each patient was evaluated by echocardiography for determination of left ventricular ejection fraction. The intensity of the exercise program for patients was determined upon the 60% - 80% of their maximum heart rates, recorded during the exercise test and in accordance with the New York cardiovascular associates criteria (29). Exercise program included 24 exercise sessions (60 - 90 minutes/ 3 times weekly). Each exercise session was started with 0 - 20 minutes warming, 20 - 40 minutes aerobic exercise with the severity of 60% - 80% of the maximum pulse rate (under Electocardiography monitoring if needed), 5 - 10 minutes cooling and finally 20 minutes relaxation. All the exercise programs were under monitoring of a sport medicine specialist and an expert nurse. Intervention group received creatine monohydrate supplement daily as much as 5 grams/day dissolved in water for 8 weeks and control group did not receive this supplement. Patients continued their routine nutritional regimen during the study.
3.4. Laboratory Measurements
In order to measure different markers including IL-6, hs-CRP, P-selectine, ICAM-1, and VCAM-1 and compare the results, 2 blood samples were taken from each patient before and after the intervention. All the participants were fasted for 12 - 14 hours, one day before and at the last day of the intervention and were referred to the laboratory in this condition. 10 mL venous blood was taken from each patient at a sitting position and two times during the study, one before and another after the intervention. After centrifugation of blood samples at 3,000 rpm for 10 minutes, their separate serum sections were kept at -80° until analysis. The serum levels of total cholesterol, TG, LDL-C and HDL-C were measured using Selecta 2-autoanalyser (Pars Azmon Co., Iran). The serum level of hs-CRP was measured by nephelometric method expressed as mg/dL. The serum level of IL-6 was measured via ELISA method and Bender Med system, Germany with a sensitivity of 92% pg/mL. The serum markers related to the endothelial function, including P-selectine, ICAM-1 and VCAM-1 were measured by ELISA method (BioSource co., San Diego, USA).
3.5. Statistical Analysis
Measurement tools for this study included a questionnaire, laboratory reports and standard kits. Data were analyzed using SPSS 16. To compare the mean results of different variables, before and after intervention, student t-test was applied and between intervention and control groups, independent t-test and Mann Whitney test were applied accordingly. Mann Whitney was applied to compare the data with non-normal distributions. A P value of < 0.05 was considered as significant.
4. Results
4.1. Patients’ Demographic Data
All characteristic data of participants are shown in Table 1. Totally, 100 participants including 50 cases and 50 controls were analyzed. There were no significant differences between the age, gender and BMI of the cases and controls (P = 0.06, P = 0.2, P = 0.2, respectively). Regarding the cardiovascular risk factors, diabetes showed the highest prevalence (50%) among control group followed by positive history of IHD, dyslipidemia and smoking (44%, 38% and 30%, respectively). However, the most prevalent risk factor among intervention group was dyslipidemia (44%) followed by positive history of IHD, smoking and diabetes (36%, 32% and 26%, respectively). There was no significant difference between the cardiovascular risk factors among the two groups.
Table 1. Characteristics of Participants According to Intervention and Control Groupsa,b,c.
| Variable | Control | Case | P Value |
|---|---|---|---|
| Gender, male/female, % | 54/46 | 52/48 | 0.056 |
| Age, y | 60.95 ± 12.01 | 57.82 ± 10.87 | 0.179 |
| BMI, kg/m 2 | 29.93 ± 3.58 | 28.91 ± 4.74 | 0.209 |
| DM | 25 (50) | 13 (26) | 0.311 |
| IHD | 22 (44) | 18 (36) | 0.270 |
| Smoking | 15 (30) | 16 (32) | 0.500 |
| Dyslepidmia | 19 (38) | 22 (44) | 0.342 |
| Triglyceride, mg/dL | 123.73 ± 44.53 | 140.72 ± 37.54 | 0.276 |
| Cholesterol, mg/dL | 123.57 ± 47.54 | 131.90 ± 48.43 | 0.509 |
| HDL, mg/dL | 36.55 ± 12.55 | 34.56 ± 18.65 | 0.267 |
| LDL, mg/dL | 97.45 ± 24.73 | 98.42 ± 26.54 | 0.851 |
| SBP, mmhg/min | 101.43 ± 32.44 | 102.33 ± 45 | 0.113 |
| DBP, mmhg/min | 76.54 ± 34.54 | 72.55 ± 25.65 | 0.065 |
| Ejection fraction | 34.22 ± 10.5 | 35.55 ± 12.65 | 0.354 |
| NYHA class | |||
| II | 35 (70) | 34 (68) | 0.453 |
| III | 13 (26) | 16 (32) | 0.342 |
| Medication therapy | |||
| Clopidgrol | 46 (92) | 45 (90) | 0.500 |
| Beta blocker | 43 (86) | 39 (78) | 0.218 |
| ACE inhibitor | 47 (94) | 47 (94) | 0.661 |
| Statin | 37 (74) | 18 (36) | 0.000 |
| Digital | 27 (54) | 20 (40) | 0.115 |
aAbbreviations: ACE, Angiotensin converting enzyme; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL, high density lipoprotein; IHD, ischemic heart diseases; LDL, low density lipoprotein; SBP, systolic blood pressure.
bData are presented as mean ± SD or No. (%).
cN = 50.
The mean serum level of TG was higher among cases than the controls (140.7 mg/dL vs. 123.7 mg/dL) with no significant difference (P = 0.3). The serum levels of cholesterol were also higher among control group compared to the intervention group; however, thee serum level of HDL was higher among intervention group compared to control group but both non-significant.
The mean ± SD serum level of inflammatory markers as well as endothelial function results and other variables in both the interventional and control groups are shown in Table 1.
4.2. Inflammatory Markers
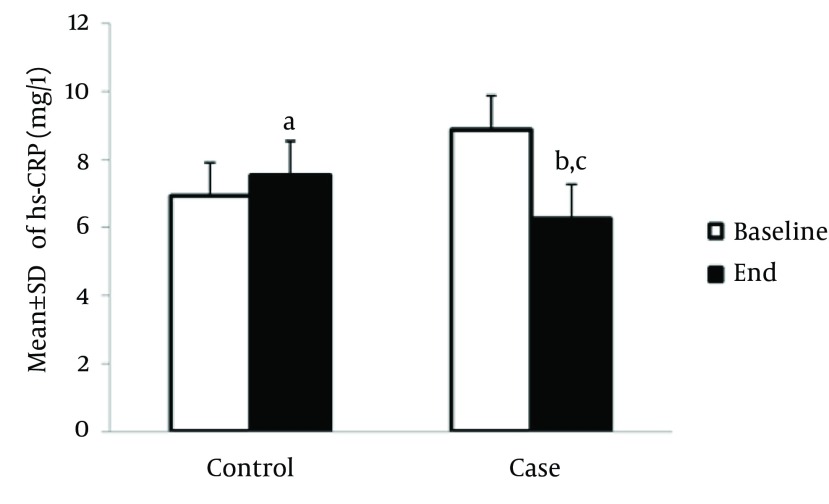
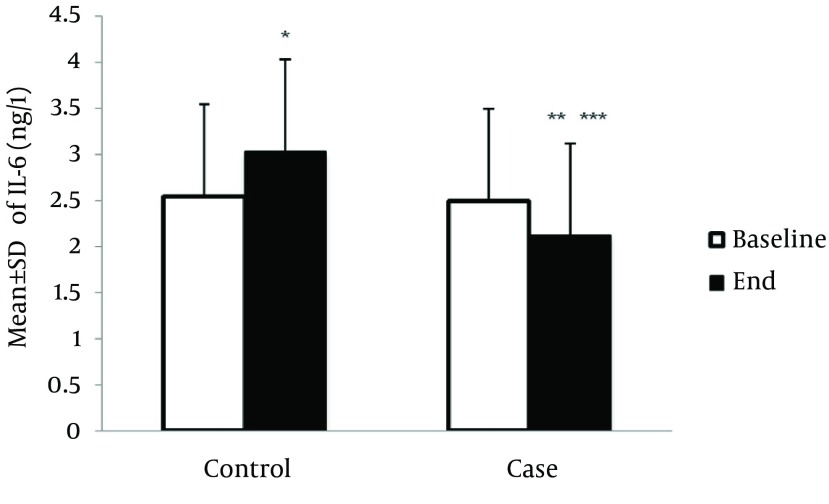
Serum level of hs-CRP at the end of study was significantly elevated among HF patients compared to the baseline (7.5 ± 1.5 mg/L vs. 6.9 ± 1.3 mg/L, P < 0.05). However, this result was not observed among cases and hs-CRP level was significantly decreased compared to the hs-CRP level at the end of study (6.3 ± 1.6 mg/L vs.8.86 ± 2.3 mg/L, P < 0.05). After 8 weeks, hs-CRP level in the case group significantly decreased compared to the control group (6.26 ± 1.6 mg/L vs.7.52 ± 1.54 mg/L, P < 0.05) (Figure 1). Also, at the end of study, the serum level of IL-6 (ng/L) among the controls significantly increased compared to the baseline level (3. ± 0.75 ng/L vs. 2.55 ± 0.9 mg/L, P < 0.05). Among the intervention group, the serum level of IL-6 significantly decreased, compared to the baseline level (2.1 ± 0.8 ng/L vs. 2.5 ± 0.5 ng/L, P < 0.000, respectively) (Figure 2).
Figure 1. Effect of Creatine + Exercise on the Serum Level of hs-CRP (mg/L) in Heart Failure Patients.
A: P < 0.05 at the end of the study compared to the study baseline in controls. B: P < 0.05 at the end of the study compared to the study baseline in intervention group. C: P < 0.05 at the end of the study in the intervention group compared to the control group.
Figure 2. Effect of Creatine + Exercise on the Serum Level of IL-6 (ng/L) in Heart Failure Patients.
* P < 0.05 vs. baseline of study in controls group; ** P < 0.05 vs. baseline of study in cases group; *** P < 0.05 vs. the end of study in control group.
4.3. Markers of Endothelial Dysfunction
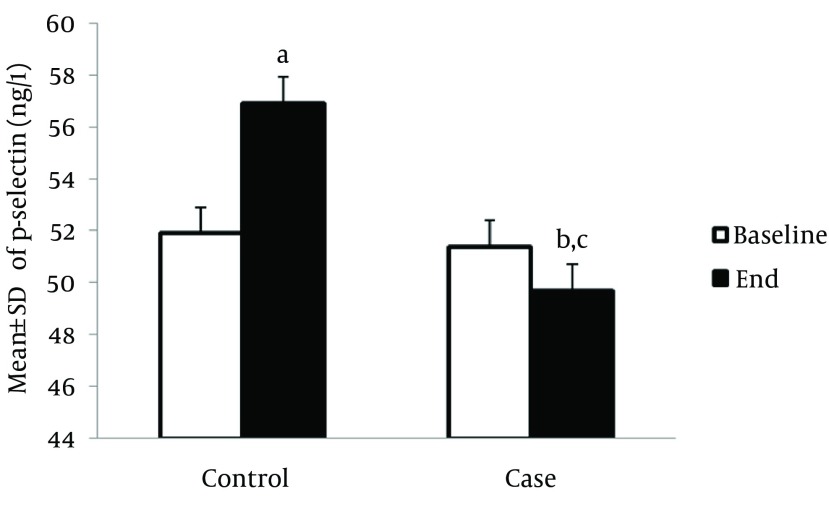
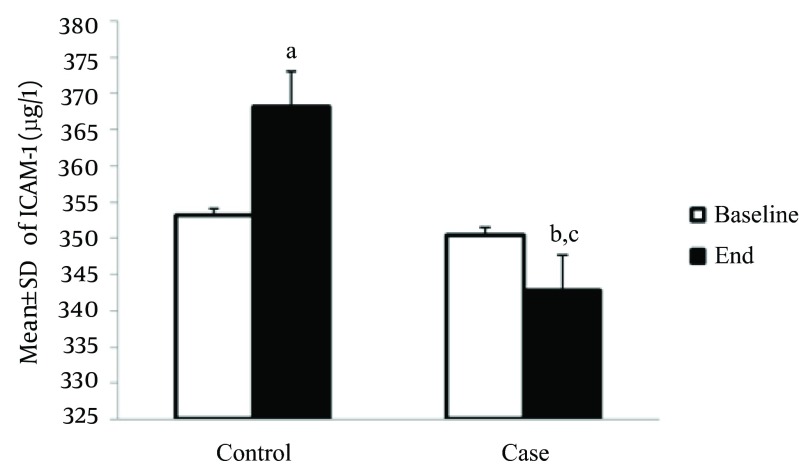
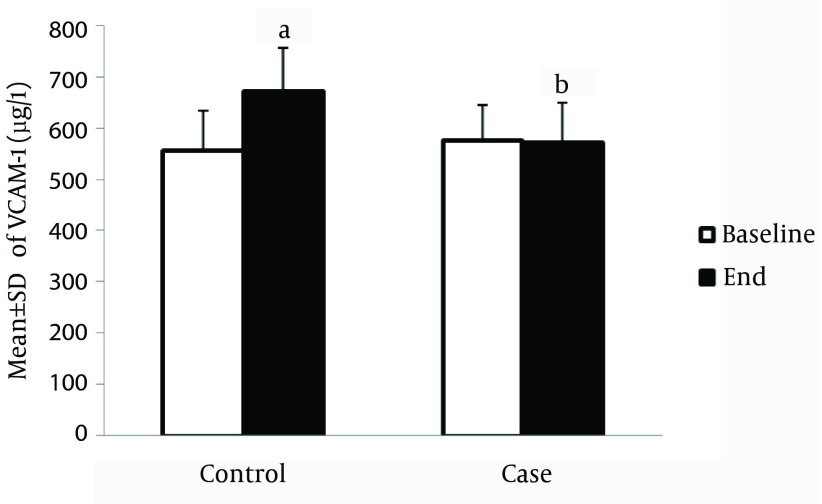
The serum level of P-selectin in the control group was significantly higher compared to the baseline, at the end of study (56.9 ± 1.8 ng/L vs. 51.9 ± 1.5 ng/L, P < 0.05); however, this marker was significantly reduced in the treatment group after the interventions (49.7 ± 1.9 ng/L vs. 51.4 ± 2.1 ng/L, P < 0.05). In addition, the serum level of the P-selectin marker was significantly lower among the intervention group compared to the control group, at the end of the study (49.7 ± 1.9 ng/L vs. 56.9 ± 1.8 ng/L, P < 0.05) (Figure 3). Also, the serum level of ICAM-1 was increased in the control group compared to the baseline level of this marker, at the end of the study (368.1 ± 25.4 µg/L vs. 353.1 ± 10.44 µg/L, P < 0.05); however, this marker was significantly reduced in the treatment group after the interventions (350.5 ± 14.7 µg /L vs. 342.7 ± 16.5 µg/L, P < 0.05). In the intervention group, the serum level of ICAM-1 was significantly lower compared to control group, at the end of study (342.7 ± 16.5 µg/L vs. 350.4 ± 14.7 µg/L and 368.1 ± 25.4 µg/L, P < 0.05, respectively) (Figure 4). The serum level of VCAM-1 marker in control patients was significantly increased compared to the baseline level, at the end of study (670.4 ± 68.6 µg/L vs. 555.9 ± 77.5 µg/L, P < 0.05) (Figure 5).
Figure 3. Effect of Creatine + Exercise on the Serum Level of P-Selectin (ng/L) in Heart Failure Patients.
Figure 4. Effect of Creatine + Exercise on the Serum Level of ICAM-1 (µg/L) in Heart Failure Patients.
Figure 5. Effect of Creatine + Exercise on the Serum Level of VCAM-1 (µg/L) in Heart Failure Patients.
5. Discussion
The suitable effects of creatine monohydrate and physical training alone on improvement of cardiac function among HF patients have already been reported by various studies (15-17); however, there is no report on the combination effects of these interventions on cardiac function of HF patients. This study was launched to assess the combination effects of creatine monohydrate and regular exercise on the inflammatory markers and endothelial function among patients with HF. According to the results of this study, patients with chronic cardiac failure had a progressive inflammatory trend and functional disorder of endothelial cells. Our study showed that combination of complementary creatine monohydrate and exercise in HF patients could reduce the serum level of inflammatory markers such as hs-CRP or IL-6. The mean serum levels of inflammatory markers and those related to endothelial dysfunction were considerably increased among control group at the end of the study compared to their status at the start of the study. Conversely, study results showed the effective role of exercise and creatine monohydrate in decreasing the mean serum levels of inflammatory markers, particularly IL-6, among the intervention group. Also, comparing the mean serum levels of inflammatory markers between control and intervention groups showed a significant decreasing effect of regular exercise in combination with complementary creatine monohydrate on these markers among HF patients. The serum levels of markers associated with endothelial dysfunction were also affected by regular exercise and creatine monohydrate. Similar to serum inflammatory markers, markers of endothelial dysfunction were also increased at the end of the study compared to the start of the study among the control group; however, among the intervention group, the serum levels of p-selectin and VCAM-1 were decreased, though not significantly, and the serum level of ICAM-1 was significantly decreased at the end of the study. The difference between the intervention and control groups for all the markers of endothelial dysfunction was significant. Mitochondrial dysfunction reduces the energy production and increases the oxidative stress and, via this phenomenon, causes a change in the DNA structure, lipids and proteins. Creatine monohydrate can improve the mitochondrial function and, therefore, amends the aforesaid conditions (30, 31). Creatine is a phosphate donor, existence of which is essential for an adequate level of intracellular adenosine triphosphate (ATP) (32). Different studies reported that serum levels of creatine phosphate are reduced during cardiac failure and the proportion of creatine to ATP is a better predictive factor for left ventricular ejection fraction (32). Although, several review studies have reported the beneficial effects of creatine monohydrate in CHF patients; however, the associated mechanisms are still unknown (33). For example, Witte and Clark investigated the effects of q10 coenzyme and creatine monohydrate on respiratory criteria and physical functions of respiratory system among CHF patients and reported that the intervention group had a better oxygen saturation rate than the control group and also patients receiving these complements had significantly better physical activities compared to the control group (31). These results were in accordance with the findings of the current study. The same results were also reported by Allard and others who also reported an increase in the peak VO2, anaerobic threshold and O2 pulse in addition to aforesaid conditions among HF patients (32). Most studies have reported the beneficial effects of exercise on inflammatory process and endothelial dysfunction among patients with cardiovascular diseases. For example an in vitro study on rats reported that the endothelium dependent vasorelaxation to acetylcholine was reduced significantly in diabetic animals and exercise training or grape seed extract administration partially improves this response. Also, the combination of exercise training with grape seed extract restored the endothelial function completely (10). The results of this study could indicate the beneficial effects of exercise and anti-oxidant compounds on the chronic diseases related to endothelial dysfunction such as cardio vascular diseases. Some other studies have also reported the anti-oxidant properties (34), improvement of endothelial dysfunction (35) and anti-inflammatory effects (36) of exercise, alone, among patients with heart failure.
A review study by Babista and co-workers reported that exercise and physical activities could improve chronic inflammation, inhibit the pre-inflammatory cytokine generation and reduce the oxidative stress among HF patients (36). Linke and others showed that exercise could reduce the oxidative stress in skeletal muscles of patients with HF, via increasing the activities of scavenger enzymes (34). Also, Fu and colleagues reported that regular walking improves the pulse rates of CHF patients considerably in comparison with control patients. They also reported that walking could decrease homocystein, improve the lifestyle and endothelial function among intervention group patients of HF compared to control group (37). These results were in accordance with the findings of our study. A study performed by Erbs and others on CHF patients reported that 12 weeks exercise resulted in improvement of the vasomotor function of peripheral vessels, improvement of left ventricular function and ejection fraction, revival of neovascularisation for skeletal muscles and endothelial function (27). There were no studies in the literature to show the effects of creatine monohydrate on the inflammatory characteristics or endothelial dysfunction in HF patients and most studies have shown the effects of this complement on the contraction of cardiac muscles or on the ergogenic properties of creatine (18, 38, 39). All these beneficial effects have been associated with only regular exercise among HF patients and mostly were in accordance with our findings. In addition to regular exercise we investigated a combination of this physical activity and application of an essential micronutrient among HF patients. This is the first study to investigate the effects of this combination among HF patients and this pilot study revealed an effective role of this combination for HF patients among the interventional group compared to control group. One of the limitations of the current study was the lack of evaluation of participants for left ventricular function and ejection fraction via echocardiography.
According to the findings of this study, combination of creatine monohydrate and regular exercise can improve the inflammatory process and endothelial function of patients with chronic heart failure. Therefore, this method can be suggested as a nondrug treatment for control of destructive effects, created by inflammatory process and endothelial dysfunction, among CHF patients.
Acknowledgments
We gratefully thank the School of Medicine, Ilam University of medical sciences and vic chancellor of researches and technology for their valuable helps on this study.
Footnotes
Authors’ Contribution:Farajollah Hemati and Asghar Rahmani designed and monitored the study, contributed in conducting the study, data acquisition, laboratory analyses, and data interpretation. Koroush Soleimannejad and Zahra Khalighi were involved in data analysis and revision of the manuscript. Khairollah Asadollahi was involved in conducting the study, data acquisition, writing up of the article and data interpretation. All authors contributed to revisions of the manuscript and reviewed the final version.
Funding/Support:This work was financially supported by Ilam University of medical sciences.
References
- 1.Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC, et al. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54(18):1660–73. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1(2):125–33. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchionni N, Di Bari M, Fumagalli S, Ferrucci L, Baldereschi G, Timpanelli M, et al. Variable effect of comorbidity on the association of chronic cardiac failure with disability in community-dwelling older persons. Arch Gerontol Geriatr. 1996;23(3):283–92. doi: 10.1016/s0167-4943(96)00737-6. [DOI] [PubMed] [Google Scholar]
- 5.Masoudi FA, Rumsfeld JS, Havranek EP, House JA, Peterson ED, Krumholz HM, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10(5):368–73. doi: 10.1016/j.cardfail.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274–9. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 7.Heo S, Moser DK, Lennie TA, Zambroski CH, Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart Lung. 2007;36(1):16–24. doi: 10.1016/j.hrtlng.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Witte KKA, Clark AL, Cleland JGF. Chronic heart failure and micronutrients. J Am College Cardiol. 2001;37(7):1765–74. doi: 10.1016/s0735-1097(01)01227-x. [DOI] [PubMed] [Google Scholar]
- 9.Berezin AE, Kremzer AA, Samura TA, Martovitskaya YV. Circulating endothelial-derived apoptotic microparticles in the patients with ischemic symptomatic chronic heart failure: relevance of pro-inflammatory activation and outcomes. Int Cardiovasc Res J. 2014;8(3):116–23. [PMC free article] [PubMed] [Google Scholar]
- 10.Badavi M, Abedi HA, Sarkaki AR, Dianat M. Co-administration of Grape Seed Extract and Exercise Training Improves Endothelial Dysfunction of Coronary Vascular Bed of STZ-Induced Diabetic Rats. Iran Red Crescent Med J. 2013;15(10):e28578. doi: 10.5812/ircmj.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michowitz Y, Arbel Y, Wexler D, Sheps D, Rogowski O, Shapira I, et al. Predictive value of high sensitivity CRP in patients with diastolic heart failure. Int J Cardiol. 2008;125(3):347–51. doi: 10.1016/j.ijcard.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ, Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, et al. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12(1):61–5. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, et al. L-Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283(3):C802–10. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- 14.Tulchinsky TH. Micronutrient deficiency conditions: global health issues. Public Health Rev. 2010;32(1):243–55. doi: 10.1186/s40985-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felker GM. Coenzyme Q10 and statins in heart failure: the dog that didn't bark. J Am Coll Cardiol. 2010;56(15):1205–6. doi: 10.1016/j.jacc.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 16.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 17.Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53(2):161–76. [PubMed] [Google Scholar]
- 18.Ferraro S, Maddalena G, Fazio S, Santomauro M, Lo Storto M, Codella C, et al. Acute and short-term efficacy of high doses of creatine phosphate in the treatment of cardiac failure. Current Ther Res. 1990;47(6):917–23. [Google Scholar]
- 19.Mancini DM, Ferraro N, Tuchler M, Chance B, Wilson JR. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol. 1988;62(17):1234–40. doi: 10.1016/0002-9149(88)90266-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuethe F, Krack A, Richartz BM, Figulla HR. Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie. 2006;61(3):218–22. [PubMed] [Google Scholar]
- 21.Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf CJ, Nyquist O, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30(3):413–8. doi: 10.1016/s0008-6363(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 22.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102(3):808–13. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horjus DL, Oudman I, van Montfrans GA, Brewster LM, Horjus DL. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst Rev. 2011;9(11):CD005184. doi: 10.1002/14651858.CD005184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51(17):1619–31. doi: 10.1016/j.jacc.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini MA, Mandegar H, Zand BM. Assessment the effects of cardiac rehabilitation program on occurance of clinical manifestation and rehospitalization of patients after coronary bypass grafting surgery [Persian]. J Ahwaz Uni Medi Sci. 2004;39(4):7–12. [Google Scholar]
- 26.O'Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80(2):234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 27.Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3(4):486–94. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 28.Pashkow FJ. Clinical cardiac rehabilitation: a cardiologist's guide. Philadelphia: Lippincott Williams & Wilkins; 1993. [Google Scholar]
- 29.American Association of Cardiovascular and Pulmonary Rehabiltation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. Champaign: Human Kinetics; 2003. [Google Scholar]
- 30.Tarnopolsky MA. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv Drug Deliv Rev. 2008;60(13-14):1561–7. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Witte KK, Clark AL. Micronutrients and their supplementation in chronic cardiac failure. An update beyond theoretical perspectives. Heart Fail Rev. 2006;11(1):65–74. doi: 10.1007/s10741-006-9194-4. [DOI] [PubMed] [Google Scholar]
- 32.Allard ML, Jeejeebhoy KN, Sole MJ. The management of conditioned nutritional requirements in heart failure. Heart Fail Rev. 2006;11(1):75–82. doi: 10.1007/s10741-006-9195-3. [DOI] [PubMed] [Google Scholar]
- 33.Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26(21):2238–44. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 34.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111(14):1763–70. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 35.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular Physical Exercise Corrects Endothelial Dysfunction and Improves Exercise Capacity in Patients With Chronic Heart Failure. Circulation. 1998;98(24):2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 36.Batista Junior ML, Lopes RD, Seelaender MC, Lopes AC. Anti-inflammatory effect of physical training in heart failure: role of TNF-alpha and IL-10. Arq Bras Cardiol. 2009;93(6):643–51. [PubMed] [Google Scholar]
- 37.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167(1):41–50. doi: 10.1016/j.ijcard.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 38.Schaufelberger M, Eriksson BO, Held P, Swedberg K. Skeletal muscle metabolism during exercise in patients with chronic heart failure. Heart. 1996;76(1):29–34. doi: 10.1136/hrt.76.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews R. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19(4):617–22. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]