Figure 2.
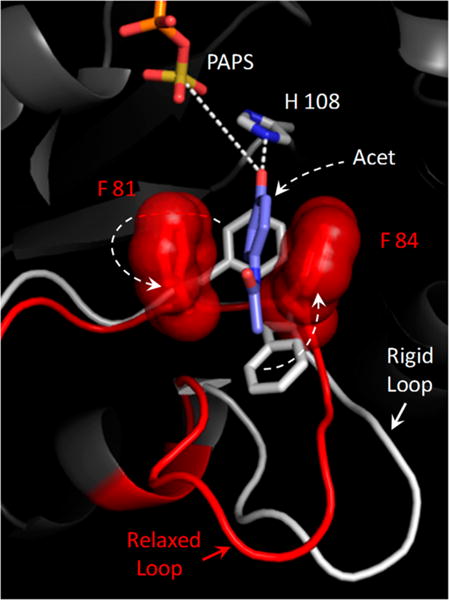
Structural basis of positive synergy. The active-site structural changes predicted to occur when a positive-synergy substrate (acetaminophen, Acet) binds to SULT1A1 are shown. The white segment indicates the disposition of the active-site “lower lip” when Acet is docked into a neutral-synergy conformation (see the text) in which the backbone is held fixed. The red segment indicates how the lower lip switches into what appears to be a positive-synergy conformation when the backbone becomes mobile and the system can “relax” around Acet. This conformational change shifts F81 and F84 into positions that sandwich the benzyl ring of the substrate. Consistent with experimental findings, the molecular “clamp” that forms seems likely to both enhance the affinity of the positive-synergy substrates and to increase the rate of turnover by positioning the nucleophilic hydroxyl for both activation (by hydrogen bonding to H108) and attack at the sulfuryl group. Simulations performed with additional positive- and neutral-synergy substrates suggest that only positive-synergy substrates induce the rearrangement of F81 and F84.
