Abstract
Background:
Bacterial infection by antibiotic-resistant Staphylococcus aureus strains is a worldwide concern and the development of novel antistaphylococcal agents is acutely needed. Lysostaphin, an example of such novel agents, is a bacteriocin secreted by S. simulans to kill S. aureus through proteolysis of the Staphylococcus cell wall.
Objectives:
The aim of this study was to evaluate the in vitro and in vivo antistaphylococcal activity of recombinant lysostaphin.
Materials and Methods:
The in vitro study of the recombinant lysostaphin activity against S. aureus was determined by turbidimetric assay. For in vivo investigation, two groups of rats were inoculated with 1.4 × 109 CFU S. aureus. Five days after the nasal instillation of S. aureus, treatment in one of the groups was performed with a single dose (200 μg/dose) of recombinant lysostaphin formulated in Eucerin-based cream.
Results:
Recombinant lysostaphin at 100 μg/mL concentration showed a significant decrease of the optical density compared to the control samples. The in vivo study demonstrated that a single dose (200 μg/dose) of recombinant lysostaphin cream significantly reduced nasal colonization in all the treated animals compared to the untreated ones.
Conclusions:
These results demonstrated that the recombinant lysostaphin produced in this study was able to kill nasal S. aureus in rats. It can be recommended for human clinical trial studies.
Keywords: Lysostaphin, Recombinant Protein, Staphylococcus aureus
1. Background
Lysostaphin is a glycine-glycine endopeptidase produced by Staphylococcus simulans which specifically cleaves the glycine-glycine bond unique to the inter-peptide cross-bridge of S. aureus cell wall. Due to its unique specificity, lysostaphin has a high potential for treating antibiotic-resistant staphylococcal infections. Staphylococcus aureus causes a wide range of infections from localized or systemic abscesses, septicemia, and endocarditis to septic emboli (1). Staphylococci have become the most common causes of nosocomial and community-acquired infections (2) and the emergence of multidrug-resistant variants has been a serious problem in the treatment of S. aureus infections (3).
The main niche for S. aureus in humans is the anterior nares (4) and nasal colonization of this pathogen promotes the risk of development of S. aureus infections. Almost 20% of people persistently carry S. aureus, 60% are intermittent carriers, and 20% are noncarriers in the anterior nares (5). Hospitalized patients (6), patients with diabetes mellitus, eczema, and those with diminished immunity (5) show high levels of S. aureus nasal colonization. This suggests that great populations are at risk for development of S. aureus infections and eradication of S. aureus from nose demonstrated to be effective in reducing this risk (7-9). Mupirocin ointment is the current standard care for the clearance of S. aureus nasal colonization, but resistance to this antibiotic has been shown (10). Therefore, this issue has promoted a search for new active therapeutic agents against this group of pathogens (11).
Unlike an antibiotic that interferes with bacterial growth, lysostaphin is highly effective in the lysis of S. aureus cells in all the metabolic stages. Lysostaphin is effective against both quiescent S. aureus cells including staphylococci in biofilms as well as actively dividing cells (12). Lysostaphin kills methicillin-resistant S. aureus (MRSA) (13), vancomycin-intermediate S. aureus (VISA) (14), and resistant strains of S. aureus to other antibiotics (15) through the digestion of the peptidoglycan pentaglycine interpeptide which causes disintegration of the cell wall and lysis of the bacteria. Previous studies have shown lysostaphin as an effective agent for the treatment of various staphylococcal infections, which has an in vitro inhibitory activity against many staphylococcal species (16). Previously, we produced a new recombinant lysostaphin (rLysostaphin) by Escherichia coli BL21 (DE3) pLysS expression system using pET32a vector (17).
2. Objectives
In this study, we evaluated the antistaphylococcal activity of rLysostaphin produced in our laboratory against S. aureus under in vitro and in vivo conditions.
3. Materials and Methods
3.1. Chemicals, Strain and Media
Commercial lysostaphin (sigma, USA) and S. aureus ATCC 25923 (health reference laboratory in Ministry of Health and Education) were used in this study. Tween 20 (sigma) and Mueller Hinton Broth (MHB) (Merck, Germany) were used for bacterial culture.
3.2. Recombinant Lysostaphin Production
The rLysostaphin was produced and purified according to a previous study (17) and was dialyzed in pH 7.2 phosphate buffer for storage at -20°C. Briefly, E. coli BL21 (DE3) pLysS was transformed with pET32a-lys and grown in LB broth (Merck, Germany) supplemented with Ampicillin (100 μg/mL) and chloramphenicol (35 μg/mL) at 37°C with agitation. For the expression of the recombinant protein, 500 μL of the culture was added to 50 mL LB broth (per liter containing: 10 g yeast extract (Difco, USA), 20 g Bacto tryptone broth (Difco, USA), 0.2% (mass/vol) glucose, 10 g NaCl, 1 g KCl, 0.5 g MgCl2, 0.5 g CaCl2, 100 μg/ mL ampicillin and 35 μg/mL chloramphenicol) and the mixture was incubated at 37°C and 200 rpm shaking with vigorous agitation until the optical density reached 0.6 at 600 nm. The expression of the rLysostaphin protein was then induced by the addition of isopropyl thio-β-D-galactosidase (IPTG) to a final concentration of 1 mM and the incubation was continued for four hours. The expressed protein was purified by Ni-NTA agarose resin affinity chromatography according to the manufacture’s instruction (Qiagen, USA). The quality and quantity of the purified rLysostaphin was analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE 12%) and Bradford methods, respectively.
3.3. In vitro Study
The in vitro antimicrobial activity assay of rLysostaphin was conducted according to Satishkumar et al. (18). Briefly, the pellet of S. aureus culture was resuspended in sterile PBS and washed twice with 10 mM PBS; then, a bacterial suspension with an optical density (OD600) of 0.55 was prepared. One milliliter of the suspended bacteria equivalent to 107 CFU (confirmed by pour plate method) was added to each tube containing PBS and MHB, separately. Six tubes including bacterial suspension without enzyme (negative control), bacteria with commercial lysostaphin (positive control), and bacteria with purified rLysostaphin (case) in PBS and MBH, separately, were subjected for the in vitro study. The turbidity assay was performed with 100 μg/mL of lysostaphin (Sigma) and purified rLysostaphin, respectively. Then, the samples were incubated under shaking at 37°C for five hours and the rate of cell lysis was monitored by measuring the OD600 at different intervals and compared with the control samples.
3.4. In vivo Study
Ethical approval was obtained from Arak University of Medical Sciences Ethics Committee (91-128-6).
3.4.1. Nasal Colonization Model
Cotton rat nasal colonization model is an adaption of the mouse nasal colonization model described by Kiser et al. (19). In this study, nasal colonization was performed by S. aureus (ATCC 25923) in Wistar male rats. For the in vivo study, 6 - 7-week rats were inoculated intranasally with 1.4 × 109 CFU in 10 μL of S. aureus suspension, as previously reported by Schaffer et al. (20). The procedure was similar to the method employed by Kokai-Kun et al. (21). Briefly, S. aureus was grown overnight in MHB at 37°C to a mid-log phase. The bacteria were pelleted and suspended in PBS at a concentration of 1.4 × 109 CFU/mL. One milliliter of the bacterial suspension was resuspended in 10 μL of PBS for each rat. The rats were anesthetized with a combination of ketamine and xylazine hydrochloride (25 and 2.5 mg/kg) (21). Each animal received 10 μL of cell suspension (~ 1.4 × 109 CFU S. aureus) intranasally which was distributed equally in each nostril of rat. Nasal colonization was evaluated by excision of the nose tissue and quantitative cultures from a separate group of rats which were sacrificed five days after the nasal colonization of S. aureus.
3.5. Lysostaphin Treatment
Intranasal treatment was performed five days after the nasal instillation of S. aureus. The treatment was performed with a single dose of purified rLysostaphin (200 μg/animal of lysostaphin), formulated in Eucerin-based cream. rLysostaphin cream was slowly injected into the nostrils of the anaesthetized rats with a 2-mL syringe. Twenty four hours after the nasal treatment, animals were sacrificed and their noses were washed with 500 μL of PBS containing 0.5% Tween 20 and were vortexed severely for 30 seconds to release bacterial colonization. Then, 50 μL of the supernatant was plated on blood agar supplemented with 7.5% NaCl to inhibit the growth of nonstaphylococcal bacteria. The plates were incubated for 48 hours at 37°C and then the S. aureus colonies were counted.
3.6. Statistical Analysis
The statistical analysis of data was performed using Mann-Whitney test by SPSS software (version 18, SPSS Inc. Chicago, IL).
4. Results
4.1. Turbidimetric Assay and Antistaphylococcal Activity
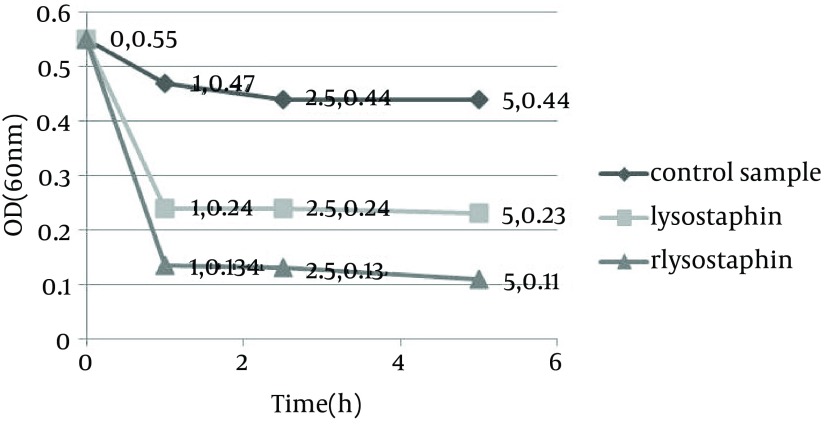
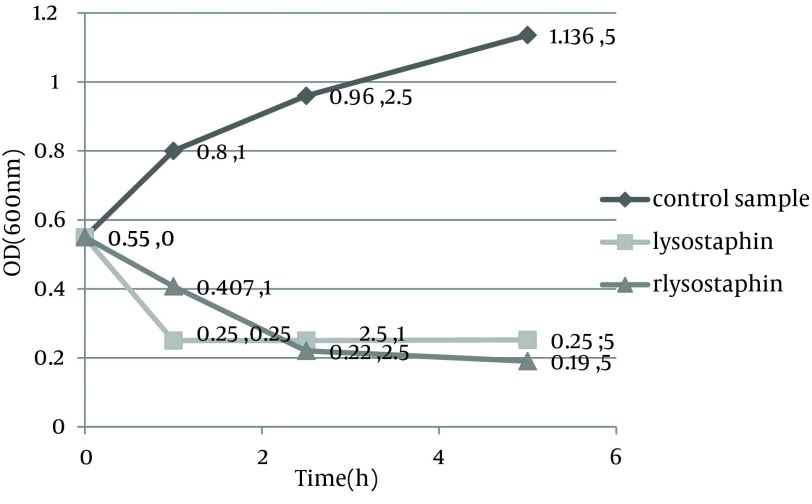
Monitoring of the optical density showed a significant decrease in the optical density from 0.55 to 0.11 in PBS and from 0.55 to 0.19 in MHB medium by purified rLysostaphin compared to negative control samples with change in optical density from 0.55 to 0.44 in PBS and from 0.55 to 1.136 in MHB medium. In addition, the results of optical density assay indicated that the decrease in turbidity with rLysostaphin was more than the commercial lysostaphin in both PBS and MHB after five hours. Figures 1 and 2 illustrate the results of cell lysis in three samples at different time courses in both PBS and MHB.
Figure 1. turbidity assay results for three samples at different time courses in phosphate buffered saline.
Figure 2. turbidity assay results for three samples at different time course in Muller-Hilton broth.
4.2. Nasal Colonization in Wistar Rats by Staphylococcus aureus
To determine the nasal colonization, we inoculated the Wistar rats with 1.4 × 109 CFU/animal S. aureus (ATCC25923). Five days after the instillation of S. aureus, the animals were sacrificed and nasal colonization was determined on blood agar plates supplemented with 7.5% NaCl. All the rats became intranasally colonized by S. aureus, with an average of 2417 CFU/nose. These results indicated a high level of nasal colonization in Wistar rats.
4.3. Treatment of Nasal Colonization With Staphylococcus aureus by Purified Lysostaphin Cream
Five days after the rats were colonized with S. aureus, a group of animals were treated with a single dose (200 μg/animal) of lysostaphin cream. Twenty four hours after the instillation of cream, nasal colonization was determined, as described in materials and methods. A single treatment with lysostaphin cream significantly (P = 0.004) reduced S. aureus nasal colonization in all the animals, but not eradicated. The treated animals remained colonized (Mean: 452/6 ± 312/4 CFU/nose) whereas the mean S. aureus colony count in untreated animals was 2385 ± 677 CFU/nose (Table 1).
Table 1. Staphylococcus aureus Nasal Colonization of Rats Following rLysostaphin Intranasal Treatment With a Single Dose (200 μg/animal) of Lysostaphin Cream a.
| rLysostaphin-Treated Animals | Untreated Animals |
|---|---|
| 760 | 2300 |
| 880 | 1620 |
| 460 | 1840 |
| 330 | 2150 |
| 98 | 3300 |
| 188 | 3100 |
a Data are presented as No. of colonization, CFU/nose.
4.4. Statistical Analysis
Significant (P < 0.05) difference between the obtained results for two Wistar rat groups was determined by the Mann-Whitney test.
5. Discussion
Lysostaphin was first identified in S. simulans (Schindler and Schuhardt, 1964). Unlike an antibiotic that interferes with bacterial growth, lysostaphin is highly effective in lysing S. aureus cells throughout the metabolic stage. Earlier methods for the production of lysostaphin endopeptidase aimed to purify it from crude extract of S. simulans (11). Nowadays, lysostaphin expressed in E. coli is commercially available from different companies. It is essential for genetic studies on Staphylococcus, for DNA isolation (22), formation of protoplasts, and segregation of Staphylococcus strains (23). In our study, the in vitro and in vivo antibacterial activity of expressed rLysostaphin in E. coli and purified in laboratory against S. aureus was evaluated. The results indicated that purified rLysostaphin was highly effective on S. aureus cell lysis; thus, has a potential for employment in research applications. Studies on lysostaphin as an antistaphylococcal agent are abundant. However, the availability of new rLysostaphin produced in our laboratory provided an opportunity to investigate the enzymatic activity of lysostaphin.
In this study, turbidimetric assay was performed in PBS and MHB. The results indicated that purified rLysostaphin and commercial lysostaphin had enzymatic activity in both media. Commercial lysostaphin activity was almost the same in PBS and MHB, but the turbidity of the cell suspension decreased faster in PBS using rLysostaphin. Comparing the turbidimetric assay results between rLysostaphin and commercial lysostaphin showed that rLysostaphin decreased the turbidity more than commercial lysostaphin because commercial lysostaphin had its maximum activity only within the first hour of the reaction and almost got inactive after one hour at 37°C, whereas rLysostaphin had activity within the five hours under in vitro conditions.
In this study, the ability of rLysostaphin to clear S. aureus nasal colonization was also evaluated. Nasal carriage is a known risk factor for staphylococcal infections (24). Topical lysostaphin treatment has been applied safely and effectively to eliminate the nasal carriage of S. aureus in human (25). A previous study reported the efficacy of lysostaphin cream for the clearance of nasal colonization of S. aureus in cotton rat model (21). Cotton rat (Sigmodon hispidus) is a well model for consistent and high levels of nasal colonization (26). In this study, the efficacy of purified rLysostaphin cream in nasal decolonization of S. aureus in Wistar rats was determined through rat colonization model. According to the results, a high level of S. aureus colonization was observed in this group of animals. Therefore, they have the potency to be used in other nasal colonization experiments.
A single application (dose: 200 μg/animal) of rLysostaphin cream significantly decreased S. aureus nasal colonization in all the animals compared to the control group. Therefore, rLysostaphin cream could be instilled in nose and the in vivo susceptibility of S. aureus to the rLysostaphin cream in nose illustrated the ability of the enzyme to retain the cell lysis activity in cream for at least 24 hours. These findings suggest that purified rLysostaphin could be efficient in the prevention of nasal colonization by S. aureus in carrier individuals and hospitalized patients.
The application of a single dose of recombinant lysostaphin produced in this study significantly reduced the number of S. aureus in the nose of Wistar rats. Therefore, higher doses or application of several doses during several days may be effective for its eradication.
Acknowledgments
This study was conducted with financial assistance from Arak University of Medical Sciences, Iran, and we are grateful for their invaluable contribution to this study. This paper is extracted from the thesis (No. 772) by Mrs. Leila Farhang Nia, a master student of Biotechnology at Arak University of Medical Sciences, Iran.
Footnotes
Funding/Support:Funding for this work was provided by Arak University of Medical Sciences.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Control CfD. National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24(5):380–8. [PubMed] [Google Scholar]
- 3.Kumar JK. Lysostaphin: an antistaphylococcal agent. Appl Microbiol Biotechnol. 2008;80(4):555–61. doi: 10.1007/s00253-008-1579-y. [DOI] [PubMed] [Google Scholar]
- 4.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goslings WR, Buchli K. Nasal carrier rate of antibiotic-resistant staphylococci; influence of hospitalization on carrier rate in patients, and their household contacts. AMA Arch Intern Med. 1958;102(5):691–715. doi: 10.1001/archinte.1958.00260220007002. [DOI] [PubMed] [Google Scholar]
- 7.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346(24):1871–7. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 8.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344(1):11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 9.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, et al. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35(4):353–8. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 10.Bannerman TL, Peacock SJ, Murray PR, Baron EJ, Jorgensen JH, Landry ML, et al. Staphylococcus, Micrococcus, and other catalase-positive cocci. Man Clin Microbiol. 2006;1(Ed. 9):390–411. [Google Scholar]
- 11.Schindler CA, Schuhardt VT. Lysostaphin: A New Bacteriolytic Agent for the Staphylococcus. Proc Natl Acad Sci. 1964;51(3):414–21. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JA, Kusuma C, Mond JJ, Kokai-Kun JF. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob Agents Chemother. 2003;47(11):3407–14. doi: 10.1128/AAC.47.11.3407-3414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dajcs JJ, Hume EB, Moreau JM, Caballero AR, Cannon BM, O'Callaghan RJ. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Invest Ophthalmol Vis Sci. 2000;41(6):1432–7. [PubMed] [Google Scholar]
- 14.Patron RL, Climo MW, Goldstein BP, Archer GL. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 1999;43(7):1754–5. doi: 10.1128/aac.43.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhakta M, Arora S, Bal M. Intraspecies transfer of a chloramphenicol-resistance plasmid of staphylococcal origin. Indian J Med Res. 2003;117:146–51. [PubMed] [Google Scholar]
- 16.Bastos M, Coutinho BG, Coelho MLV. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals. 2010;3(4):1139–61. doi: 10.3390/ph3041139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhangnia L, Ghaznavi-Rad E, Mollaee N, Abtahi H. Cloning, Expression, and Purification of Recombinant Lysostaphin From Staphylococcus simulans. Jundishapur J Microbiol. 2014;7(5):e28489. doi: 10.5812/jjm.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satishkumar R, Sankar S, Yurko Y, Lincourt A, Shipp J, Heniford BT, et al. Evaluation of the antimicrobial activity of lysostaphin-coated hernia repair meshes. Antimicrob Agents Chemother. 2011;55(9):4379–85. doi: 10.1128/AAC.01056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiser KB, Cantey-Kiser JM, Lee JC. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun. 1999;67(10):5001–6. doi: 10.1128/iai.67.10.5001-5006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, et al. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun. 2006;74(4):2145–53. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokai-Kun JF, Walsh SM, Chanturiya T, Mond JJ. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob Agents Chemother. 2003;47(5):1589–97. doi: 10.1128/AAC.47.5.1589-1597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klesius PH, Schuhardt VT. Use of lysostaphin in the isolation of highly polymerized deoxyribonucleic acid and in the taxonomy of aerobic Micrococcaceae. J Bacteriol. 1968;95(3):739–43. doi: 10.1128/jb.95.3.739-743.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geary C, Stevens M. Rapid lysostaphin test to differentiate Staphylococcus and Micrococcus species. J Clin Microbiol. 1986;23(6):1044–5. doi: 10.1128/jcm.23.6.1044-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31(1):13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 25.Quickel KJ, Selden R, Caldwell JR, Nora NF, Schaffner W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl Microbiol. 1971;22(3):446–50. doi: 10.1128/am.22.3.446-450.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faith RE, Montgomery CA, Durfee WJ, Aguilar-Cordova E, Wyde PR. The cotton rat in biomedical research. Lab Anim Sci. 1997;47(4):337–45. [PubMed] [Google Scholar]