Abstract
Context:
Early postprandial hyperglycemia and delayed hypoglycemia remain major problems in current management of type 1 diabetes (T1D).
Objective:
Our objective was to investigate the effects of pramlintide, known to suppress glucagon and delay gastric emptying, on postprandial glucose fluxes in T1D.
Design:
This was a single-center, inpatient, randomized, crossover study.
Patients:
Twelve patients with T1D who completed the study were analyzed.
Interventions:
Subjects were studied on two occasions with or without pramlintide. Triple tracer mixed-meal method and oral minimal model were used to estimate postprandial glucose turnover and insulin sensitivity (SI). Integrated liver insulin sensitivity was calculated based on glucose turnover. Plasma glucagon and insulin were measured.
Main Outcome Measure:
Glucose turnover and SI were the main outcome measures.
Results:
With pramlintide, 2-hour postprandial glucose, insulin, glucagon, glucose turnover, and SI indices showed: plasma glucose excursions were reduced (difference in incremental area under the curve [iAUC], 444.0 mMmin, P = .0003); plasma insulin concentrations were lower (difference in iAUC, 7642.0 pMmin; P = .0099); plasma glucagon excursions were lower (difference in iAUC, 1730.6 pg/mlmin; P = .0147); meal rate of glucose appearance was lower (difference in iAUC: 1196.2 μM/kg fat free mass [FFM]; P = .0316), endogenous glucose production was not different (difference in iAUC: −105.5 μM/kg FFM; P = .5842), rate of glucose disappearance was lower (difference in iAUC: 1494.2 μM/kg FFM; P = .0083). SI and liver insulin sensitivity were not different between study visits (P > .05).
Conclusions:
Inhibition of glucagon and gastric emptying delaying reduced 2-hour prandial glucose excursions in T1D by delaying meal rate of glucose appearance.
We investigated the effects of pramlintide on postprandial glucose fluxes in T1D. Pramlintide reduced 2-hour prandial glucose excursions in T1D by delaying meal rate of glucose appearance.
Type 1diabetes (T1D) is characterized by immune-mediated complete or near-complete destruction of β cells. Current insulin-based therapies for the treatment of the disorder are associated with significant glucose variability (1), with early postprandial hyperglycemia and late postprandial hypoglycemia continuing to pose major challenges to optimal glucose control in T1D. Postprandial hyperglycemia in T1D could be due to a combination of lack of postprandial glucagon suppression and a mismatch between onset and offset of insulin action and meal glucose appearance (2). Hyperglucagonemia is present in the postprandial state in T1D with absent endogenous insulin secretion (3). Early postprandial hyperglycemia is also likely because of a mismatch between insulin effect and rapidity of meal glucose appearance; that is, the delay in the onset of insulin action after sc administration delays insulin effects on stimulating glucose disappearance (Rd) and inhibiting endogenous glucose production. On the other hand, late postprandial hypoglycemia is likely due to the delayed offset of insulin action after sc administration on Rd when most of the systemic appearance of meal glucose is already completed.
We have published extensively on the use of the triple tracer meal method to measure meal glucose kinetics (3–5). Applying established scintigraphic techniques, we have also measured gastric emptying (GE) in healthy controls and T1D patients (5, 6). Manipulation of plasma glucagon concentrations and GE using a probe such as pramlintide would permit the assessment of the relative importance of contributing factors to postprandial hyperglycemia in T1D.
Pramlintide has been approved for therapy of T1D for several years. Pramlintide is an analog of islet amyloid polypeptide (IAPP), a hormone cosecreted with insulin by β cells that sends satiety signals to the brain to reduce food intake (7), delays gastric emptying (8), and lowers postprandial plasma glucagon concentrations (9). Though plasma IAPP may be detectable in some patients with T1D, plasma IAPP does not increase after stimulatory testing with either meal ingestion or glucagon (10, 11). The peptide sequence of pramlintide has been modified from that of IAPP resulting in the absence of amyloidogenic potential (12–14). Prandial carbohydrate physiology in the context of meal time pramlintide administration has been previously studied with the dual tracer method (15, 16). Applying triple tracer approach, we have recently reported that in healthy subjects, a single dose of 30 μg pramlintide delayed GE and peak meal rate of glucose appearance (MRa), lowered the rate of endogenous glucose production (EGP), and improved whole body insulin sensitivity (SI) (5).
Furthermore, even though it is well known that appropriate neuroendocrine communications between the gut, pancreas and brain are necessary to regulate blood glucose (17, 18), no study has yet explored the impact of delayed GE on other islet hormones such as pancreatic polypeptide (PP) and somatostatin, gastrointestinal (GI) and islet peptide such as ghrelin and GI peptides such as glucagon like peptide–1(GLP-1), especially in T1D.
Thus, we applied the triple tracer technique (4) with the oral minimal model (19–21) in individuals with T1D on continuous sc insulin infusion (CSII), using pramlintide as a probe, to estimate glucose fluxes, and liver insulin sensitivity (SIL), which is an index to measure insulin action on hepatic glucose production (22) and SI. We also simultaneously measured plasma somatostatin, PP, GLP-1, and ghrelin to explore the possible mechanisms involved in pramlintide mediated effects on glucose metabolism and changes in plasma insulin and glucagon concentrations.
Materials and Methods
After approval of the Mayo Institutional Review Board, 14 subjects with T1D consented and were screened for eligibility. Inclusion criteria were: age 18–70 years, body mass index 19–40 kg/m2, glycated hemoglobin lower than 10.0% (85.8 mmol/mol), c-peptide less than 33 pmol/L with a simultaneous glucose greater than 5 mM, on insulin pump therapy, creatinine at or below 1.5 mg/dL and normal GE for solids and liquids measured with scintigraphy (6). Exclusion criteria were: significant GI symptoms by Bowel Symptom Questionnaire (23), active GI disorders, medications affecting GE (eg, erythromycin) or glucose metabolism (eg, corticosteroids), pregnancy, breast feeding and any active systemic disease.
Screen visit 1.
After an overnight fast, subjects reported to the Clinical Research Trials Unit of Mayo Clinic, Rochester, MN, in the morning. Subject eligibility was confirmed with relevant medical history and physical examination. Pregnancy testing was performed in female subjects with child-bearing capacity with a negative pregnancy test result required for eligibility. Dietary review by a registered dietician ensured that subjects were on a weight maintaining diet that met American Diabetes Association standards of macronutrient and calorie compositions. Body composition was measured by dual energy X-ray absorptiometry (24).
Screen visit 2.
Eligible subjects were scheduled for a gastric emptying test (GET) using scintigraphy as previously described (6) with gastric emptying rates reported separately as T1/2 (50% clearance) for liquids and solids (6). Subjects with normal GE were scheduled for inpatient studies.
Inpatient study visits.
Each subject was studied on two occasions in random order: pramlintide and no pramlintide. Patients administered all insulin sc using their usual CSII system and usual insulin analog.
Day 1.
Subjects reported to the Mayo Clinic Clinical Research Trials Unit at 4 pm. A standard mixed meal (10 kcal/kg, 55% carbohydrates, 15% protein, and 30% fat) was consumed at approximately 5 pm, with subjects providing their customary premeal insulin bolus sc by CSII. No other food was provided until the following morning. An IV cannula was inserted into a forearm vein at approximately 8 pm for infusions during the study. Sips of water were permitted ad lib; subjects continued usual overnight basal insulin through CSII.
Day 2.
A triple tracer mixed meal procedure was performed on day 2 as previously reported (4). At approximately 4 am, a primed continuous infusion of [6, 6-2H2] glucose was started. At approximately 6 am, an 18-gauge cannula was inserted in a retrograde fashion into a hand vein of the contralateral arm and the hand placed in a heated box, whose temperature was regulated at approximately 55°C, for withdrawing arterialized venous blood samples throughout the study for glucose, glucose tracer, insulin, and glucagon, at baseline and time intervals 0, 5, 10, 20, 30, 60, 90, 120, 150, 180, 240, 300, and 360 minutes after the meal. Assays for GLP-1, somatostatin, total and active ghrelin, and PP concentrations were performed at the time intervals stated previously, but ceasing at 180 minutes postprandial. At approximately 7 am (T0), 30 μg of pramlintide was administrated sc, with the first bite of the meal containing 75 g of dextrose labeled with [1-13C] glucose; premeal insulin bolus was reduced by approximately 50% on pramlintide study visit according to the US Food and Drug Administration package insert. On the other occasion, no pramlintide was provided and subjects administered their customary premeal insulin bolus. With the first bite of the meal, the [6, 6-2H2] glucose infusion rate was varied to mimic the anticipated changes in rates of EGP for the next 6 hours. Additionally, an infusion of [6-3H] glucose was started at 7 am and the rate varied to mimic the anticipated systemic rate of meal appearance. These infusion rates were further adjusted after examining the data obtained from the first two subjects, to minimize changes in tracer-tracee ratios for accurate estimation of postprandial glucose turnover. All infusions were discontinued at approximately 1 pm, the cannulae were removed, and the subjects were provided lunch and dismissed thereafter.
GET with pramlintide.
Within a week after completion of the second study visit, subjects underwent a repeat GET as described in screen visit 2. However, during this visit, 30 μg of pramlintide was administered sc at the start of the test to determine the effects of pramlintide on liquid and solid gastric emptying rates.
Analytical methods.
Plasma samples were placed on ice, centrifuged, separated, and stored at –80 C until analyzed. Plasma glucose concentration was measured using a glucose oxidase method (YSI, Inc.). Insulin was measured with a two-site electrochemiluminescence immunoenzymatic assay by DxI automated system (Beckman Instruments). Glucagon was measured by a direct, double antibody RIA (Linco Research). Plasma enrichment of [1-13C] glucose and [6, 6-2H2] glucose was measured using gas chromatography mass spectrometry (Thermoquest). Plasma [6-3H] glucose specific activity was measured by liquid scintillation counting as described (24). Total GLP-1 was measured by RIA for all forms of GLP-1: GLP-1 (7–36) amide, GLP-1 (7–37), GLP-1 (9–36) amide, GLP-1 (9–37), GLP-1 (1–36) amide, and GLP-1 (1–37) in plasma. Ghrelin total and active were measured by competitive RIA (Linco/Millipore Research). Somatostatin was measured by competitive RIA (ALPCO RIA, EURO-IAGNOSTICA AB). PP was measured by the Human PP double antibody RIA kit (Linco Research, Inc.).
Calculations
Glucose kinetics.
Fasting and postprandial rates of glucose turnover were calculated as previously described (4). Briefly, the systemically infused [6-3H] glucose was used to trace the systemic rate of appearance of [1-13C glucose] contained in the meal, and [6, 6-2H2] glucose was used to trace the rate of appearance of EGP. The ratio of plasma concentration of [6-3H] glucose to [1-13C] glucose was used to calculate the MRa. Glucose turnover was then calculated as previously described (4, 5). Urine was collected during 6 hours of inpatient studies for measurement of urinary glucose losses (25).
Whole body and hepatic insulin sensitivity.
The net effect of insulin to stimulate whole body glucose disposal and inhibit EGP, SI, was assessed by the oral minimal model (19, 21). SIL, which is an index to measure insulin action on hepatic glucose production (22), was calculated using an integral formula for SIL based on glucose fluxes.
Statistical analysis
A sample size of 12 was chosen based on the minimum required to achieve stable variance estimates for estimation purposes (26, 27). The a priori statistical plan included a focus on estimation of the change in MRa and measures of glucose turnover. Difference between the crossover treatments were to be summarized using 95% confidence intervals (CI). In this report, we have added P values to the CIs to aid in interpretation of the findings, but we note that the sample size was not determined on power considerations. There may be type II errors (false negatives) as a result.
Descriptive summaries were expressed as mean ± SD unless otherwise stated. Incremental area under the curve (iAUC) was calculated for each measure from baseline (0 minutes) to 120, and 180 or 360 minutes. The spacing in time between study visits and the standardized protocol for data collection was such that we assumed there would be no carryover or period effects as a result of the crossover design (28). Accordingly, we tested our primary working hypothesis that pramlintide would delay MRa, comparisons between visits with and without pramlintide using a paired t test, and estimated 95% CIs on the difference. Secondary outcomes were tested similarly. Nonparametric tests (eg, Wilcoxon signed rank tests) were used as appropriate. In addition, some GI and islet hormones known to affect glucose metabolism were analyzed to explore potential mechanistic actions for pramlintide. Differences were considered as statistically significant if the 95% CI excluded zero (ie, a P < .05 [two-sided]). No adjustment to the level of significance was made for multiple testing. Models were estimated using SAS (version 9.4).
Results
Subject characteristics.
Of the 14 subjects screened for the study, one subject failed the first screen visit because of high plasma c-peptide concentration. Of 13 subjects enrolled, one had hypoglycemia during the pramlintide visit and was withdrawn during the first study visit. Data from 12 subjects who completed all study visits were analyzed. Demographics for subjects are shown in Table 1. Briefly, the mean (SD) age and body mass index were 44.4 (14.7) years and 28.9 (5.9) kg/m2, respectively. Diabetes duration was 27.5 (14.6) years. The mixed meal composition, provided as an online appendix (Supplemental Table 1), was comparable between visits (P > .05 for all meal contents).
Table 1.
Baseline Characteristics of the Subjects Completing the Two-Meal Study (n = 12)
| Variable | Mean | sd | Range |
|---|---|---|---|
| Age, y | 44.4 | 14.7 | 24.5–70.0 |
| Gender, M/F | 6/6 | ||
| Diabetes duration, y | 27.5 | 14.6 | 11.0–54.0 |
| Weight, kg | 86.0 | 20.4 | 56.0–116.7 |
| Body mass index, kg/m2 | 28.9 | 5.9 | 21.7–39.0 |
| Fat free mass, kg | 55.5 | 11.2 | 36.8–72.7 |
| Percent body fat, % | 34.8 | 9.4 | 22.3–51.4 |
| Laboratory values | |||
| Fasting blood glucose, mM | 8.9 | 4.0 | 2.1–15.3 |
| HBA1c % | 7.5 | 1.1 | 5.7–9.3 |
| mmol/mol | 58.9 | 12.0 | 38.8–78.1 |
| Hemoglobin (g/dl) | 14.0 | 1.5 | 11.0–15.8 |
| Creatinine (mg/dl) | 0.9 | 0.1 | 0.6–1.0 |
| BUN | 13.2 | 4.7 | 6.0–21.0 |
| TSH (IU/liter) | 2.4 | 1.8 | 0.6–6.5 |
Abbreviations: BUN, blood urea nitrogen; F, female; HBA1c, glycated hemoglobin; M, male.
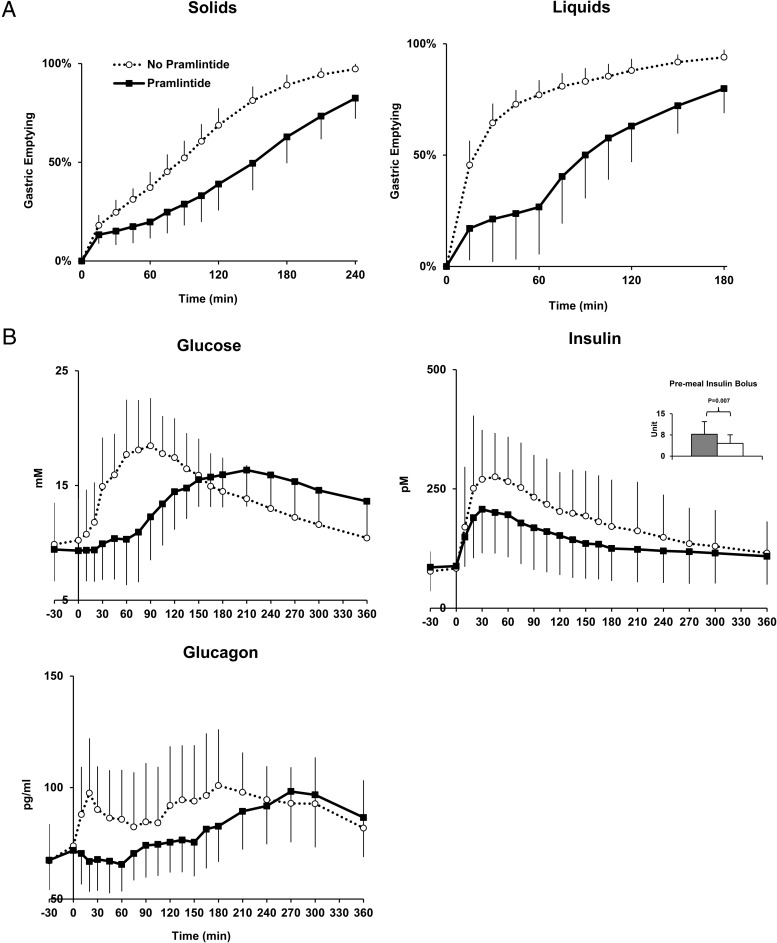
GE rates for solids and liquids (Table 2, Figure 1A).
Table 2.
Outcome Measures of Two Study Visits Without and With Pramlintide in T1D (n = 12)
| Measure | No Pramlintide |
Pramlintide |
Diff (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | |||
| Rate of GE, minutes to 50% clearance | ||||||
| Liquid | 22.5 | 7.8 | 102.5 | 40.9 | −80.0 (−104.5, −55.5) | <.0001 |
| Solid | 92.5 | 14.1 | 162.5 | 39.3 | −70.0 (−93.1, −46.9) | <.0001 |
| Glucose, mmmin | ||||||
| iAUC 120 | 659.4 | 252.6 | 215.5 | 251.9 | 444.0 (230.4, 657.5) | .0003 |
| iAUC 360 | 1382.0 | 1125.3 | 1592.7 | 731.6 | −210.7 (−1014.2, 592.8) | .5921 |
| Peak measure, mm | 19.2 | 4.2 | 17.6 | 2.8 | 1.6 (−1.3, 4.5) | .2720 |
| Time to peak, min | 87.7 | 18.2 | 198.5 | 71.7 | −110.8 (−154.9, −66.6) | .0001 |
| Insulin, pMmin | ||||||
| iAUC 120 | 17 309.6 | 7557.7 | 9667.6 | 4973.7 | 7642 (2036.4, 13 247.5) | .0099 |
| iAUC 360 | 30 283.7 | 11 019.9 | 16 201.1 | 11 106.8 | 14 082.6 (335.3, 24 809.9) | .0131 |
| Peak measure, pM | 315.9 | 131.2 | 221.4 | 90.1 | 94.5 (16.9, 172.1) | .0214 |
| Time to peak, min | 51.7 | 26.1 | 64.6 | 94.0 | −12.9 (−73.9, 48.1) | .6543 |
| Glucagon, pg/mlmin | ||||||
| iAUC 120 | 1527.9 | 1904.6 | −202.7 | 1223.1 | 1730.6 (375.6, 3085.7) | .0147 |
| iAUC 36 | 6232.3 | 4550.0 | 3814.8 | 3984.2 | 2417.5 (−1203.2, 6038.2) | .1800 |
| Peak measure, pg/ml | 113.7 | 25.7 | 103.3 | 20.1 | 10.3 (−9.2, 29.9) | .0662 |
| Time to peak, min | 135.8 | 97.8 | 260.0 | 57.3 | −124.2 (−192.0, −56.3) | .0061 |
| Rate of endogenous glucose production, μM/kg FFMmin | ||||||
| iAUC 120 | −1324.5 | 536.1 | −1219.0 | 381.7 | −105.5 (−499.5, 288.4) | .5842 |
| iAUC 360 | −4320.2 | 1870.3 | −3786.7 | 1339.6 | −533.5 (−1910.7, 843.8) | .4304 |
| Peak measure, μM/kg FFM | 1.6 | 1.9 | 2.0 | 2.4 | −0.5 (−2.3, 1.4) | .6170 |
| Time to peak, min | 121.3 | 79.7 | 127.9 | 62.0 | −6.7 (−67.2, 53.8) | .8213 |
| Rate of meal glucose appearance, μM/kg FFMmin | ||||||
| iAUC 120 | 4217.3 | 1130.6 | 3021.1 | 1219.4 | 1196.2 (117.4, 2275.0) | .0316 |
| iAUC 360 | 6406.3 | 1655.4 | 6011.3 | 3885.5 | 395.0 (−2205.7, 2995.7) | .7505 |
| Peak measure, μM/kg FFM | 76.7 | 19.1 | 57.8 | 15.7 | 18.9 (4.1, 33.7) | .0147 |
| Time to peak, min | 43.8 | 18.2 | 116.3 | 27.2 | −72.5 (−92.1, −52.9) | <.0001 |
| Rate of Rd, μM/kg FFMmin | ||||||
| iAUC 120 | 2897.0 | 1145.7 | 1402.8 | 1037.8 | 1494.2 (448.0, 2540.4) | .0083 |
| iAUC 360 | 3529.4 | 2286.2 | 4220.5 | 2191.6 | −691.2 (−2848.7, 1466.3) | .5095 |
| Peak measure, μM/kg FFM | 62.9 | 16.4 | 50.5 | 14.4 | 12.4 (−0.7, 25.4) | .0619 |
| Time to peak, min | 53.8 | 11.9 | 133.8 | 25.9 | −80.0 (−97.5, −62.5) | <.0001 |
| Indices of glucose action, 10−4 dL/kg/min per μU/ml | ||||||
| SI | 5.8 | 3.6 | 6.9 | 4.6 | −1.1 (−4.6, 2.4) | .4238 |
| SIL | 2.9 | 2.3 | 4.7 | 3.7 | −1.8 (−3.5, 0.0) | .1100 |
Figure 1.
A, Gastric emptying for solids and liquids during triple tracer studies without pramlintide (dashed-open circle) and with pramlintide (solid-closed square) in subjects with type 1 diabetes (T50 gastric emptying for liquids and solids emptying was delayed with pramlintide, P < .001). B, Plasma glucose, insulin, and glucagon concentrations during triple tracer meal studies without pramlintide (dashed-open circle) and with pramlintide (solid-closed square) in subjects with type 1 diabetes. Error bars represent SD.
With pramlintide, the T1/2 for liquids and solid emptying were delayed. For liquids, 80 additional minutes (95% CI, 55.5–104.5 minutes; P < .0001) were required to reach 50% clearance during the GE study. Similarly, solids required an additional 70 minutes (95% CI, 46.9–93.1 minutes; P < .0001).
Plasma glucose, insulin, and glucagon concentrations
The peak postprandial glucose was delayed on average 110.8 minutes (95% CI, 66.6–154.9 minutes, P = .0001) with pramlintide (Table 2, Figure 1B). This delay did not affect the total postprandial glucose excursion over 360 minutes (difference in iAUC, 210.7 [95% CI, –1014.2 to 592.8] mMmin, P = .5921), but did have an impact on the 2-hour postprandial glucose excursions immediately following the meal (difference in iAUC, 444.0 [95% CI, 230.4–657.4] mMmin, P = .0003). Plasma insulin concentrations were lower with pramlintide during 120 minutes per study design (difference in iAUC, 6457.5 [95% CI, 2036.4–13 247.5] pMmin; P = .0099). Early postprandial excursions (120 minutes) of glucagon were lower with pramlintide (difference in iAUC, 1730.6 [95% CI, 375.6–3085.7]) pg/mlmin; P = .0147).
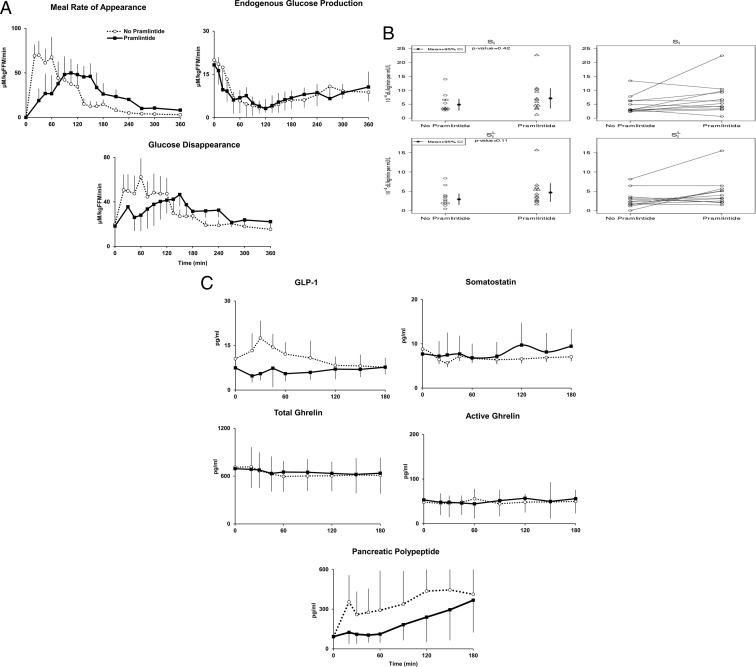
MRa, EGP, and Rd
The iAUC of MRa was lower with pramlintide during 0–120 minutes (difference, 1196.2 [95% CI, 117.4–2275.0] μM/kg fat free mass [FFM]; P = .0316) (Table 2 and Figure 2A). However, the iAUC of MRa during the entire 0–360 minutes did not differ with or without pramlintide (difference, 395.0 [95% CI, –2205.7 to 2995.7] μM/kg FFM; P = .7505). The time to peak of rate of MRa was delayed (difference, 72.5 [95% CI, 52.9–92.1] minutes; P < .0001) with pramlintide.
Figure 2.
A, Rates of meal glucose appearance, endogenous glucose production, and Rd during triple tracer studies without pramlintide (dashed-open circle) and with pramlintide (solid-closed square) in subjects with type 1 diabetes. B, Whole body SI and SIL obtained during triple tracer studies without pramlintide and with pramlintide in subjects with type 1 diabetes (left panels). Individual SI and SIL are shown (right panels). C, Plasma concentrations of GLP-1, somatostatin, total and active Ghrelin, and pancreatic polypeptide obtained during the studies.
In contrast, the iAUC of EGP between the groups did not differ during 0–120 minutes after the meal (difference in iAUC, –105.5 [95% CI, –499.5 to 288.4] μM/kg FFM; P = .5842) or during the entire 0–360 minutes (difference in iAUC, –533.5 [95% CI, –1910.7 to 843.8] μM/kg FFM; P = .4304), and there were no differences in time to nadir of EGP (difference, –6.7 [95% CI, –67.2 to 53.8] minutes; P = .8213) with or without pramlintide.
The iAUC of Rd was lower during 0–120 minutes (difference in iAUC, 1494.2 [95% CI, 434.6–2553.9] μM/kg FFM; P = .0083) with pramlintide. However, the iAUC of Rd during the entire 0–360 minutes did not differ with or without pramlintide (difference in iAUC, 691.2 [95% CI, –2848.7 to 1466.3] μM/kg FFM; P = .5095). The time to peak Rd was delayed (difference, 80.0 [95% CI, 62.5–97.5] min; P < .0001) with pramlintide. Urine glucose excretion with and without pramlintide was not significant (P = .97; median, [interquartile range], 1119.7 [565.6, 2493.9] vs 932.2 [713.9, 2283.4] μmol/kg FFM, respectively). As shown in Supplemental Figure 1, top panel, the tracer-tracee ratio for calculation of EGP was fairly constant apart from a change during the pramlintide study day. As shown in Supplemental Figure 1, bottom panel, the tracer-tracee ratio applied to calculate MRa was also fairly constant for almost the entire duration (20–360 minutes) of the study apart from the initial perturbations (0–20 minutes) that are unavoidable when both the IV infused tracer and orally ingested tracee are entering the systemic circulation. Therefore, fluctuations in tracer-tracee ratios were minimized enabling accurate measurements of postprandial glucose turnover.
SI and SIL
SI was not significantly different (difference, 1.1 [95% CI, –4.6 to 2.4] dl/kg/min per μU/ml; P = .4238) between study visits. SIL was also not significantly different (difference, 1.8 [95% CI, –3.5, 0.0] dl/kg/min per μU/ml; P = .11) (Table 2, Figure 2B).
Other islet hormone and GI peptides during 0–120 minutes study period
There were no differences in total GLP-1 (difference in iAUC, 395.9 ± 1613.1 [95% CI, –969.8 to 1761.7] (pg/ml)min; P = .55), peak GLP-1 (difference, 9.8 ± 11.8 [95% CI, –0.2 to 19.7] (pg/ml)min; P = .0545), total and active Ghrelin (difference in iAUC, –4760 ± 7540 [95% CI, –11 143 to 1624] (pg/ml)min; P = .14 and difference in iAUC, –2677 ± 10 721 [95% CI, –12 268 to 6913] (pg/ml)min; P = .55, respectively) and somatostatin (difference in iAUC 0–120: –282.6 ± 501.7 [95% CI: –717.3 to 152.1[ (pg/ml)min; P = .18699) with and without pramlintide (Figure 2C). Furthermore, peak and time to peak of these measures were also not different with/without pramlintide. Pramlintide reduced PP excursions during the first 2 hours after the mixed meal (difference in iAUC 0–120, 19 857 ± 18 628 [95% CI, 3469–36 246] (pg/ml)min; P = .02). Time to peak of PP was delayed 50.8 ± 42.2 minutes with pramlintide.
Discussion
This study confirmed that suppressing postprandial glucagon concentrations and delaying gastric emptying using premeal pramlintide decreased glucose excursions for the first 2 hours after a mixed meal (5, 15, 16). Applying the triple tracer technique to assess postprandial glucose fluxes, we demonstrated that this effect was achieved by reducing meal-derived glucose appearance from 0 to 120 minutes. Despite significantly lower insulin concentrations per study design during the pramlintide study day, plasma glucose, EGP, and Rd excursions were comparable with and without pramlintide during 6 hours after the mixed meal, with improved whole body insulin sensitivity in 8 of 12 subjects, and improved hepatic insulin sensitivity in 9 of 12 subjects. Pramlintide reduced 2-hour plasma glucagon excursions after the mixed meal. EGP is regulated predominantly by prevailing glucose, insulin, and glucagon concentrations (22). Glucagon effect on glucose metabolism is predominantly from its effects on hepatic glycogenolysis. It has been well-established that insulin and glucagon ratio in the portal circulation regulates EGP (29, 30). In this study, despite lower insulin concentrations between 0 and 120 minutes with pramlintide, EGP rates were comparable, suggesting that pramlintide use enhanced insulin effect on liver because of its effects on glucagon suppression. Additionally, because plasma glucose concentrations also influence EGP (25), comparable EGP rates with pramlintide despite lower plasma glucose concentrations also imply enhanced hepatic glucose effectiveness with pramlintide. However, the relative contributions of enhanced hepatic insulin action and hepatic glucose effectiveness cannot be determined from this study.
Pramlintide also decreased PP during this period but did not impact GLP-1, total and active ghrelin, or somatostatin. Pramlintide sends satiety signals to the brain to reduce food intake (7), delays GE (8), and lowers postprandial plasma glucagon concentrations (9). In our study, it delayed GE, confirming prior studies in subjects with T1D (15, 16). This effect is probably mediated through vagal inhibition, as demonstrated by reduced excursion of PP during 120 minutes after the mixed meal. PP is secreted by the pancreas in response to ingestion of food, “sham” feeding or hypoglycemia secondary to vagal nerve stimulation (31) and has effects on GI motility and food intake (32). Secretion of PP can be blocked by vagotomy or atropine (31, 33). Suppression of PP with pramlintide further corroborates the notion that pramlintide likely modifies vagal nerve output. Somatostatin slows GE and inhibits glucagon concentrations (34). We reasoned that pramlintide effect on GE and glucagon concentrations could have been mediated, at least in part, through somatostatin. However, we did not observe any acute effects of pramlintide on postprandial excursions of somatostatin, thus demonstrating that pramlintide effect on these parameters is not mediated via somatostatin. Taken together, our study suggests that the effect of pramlintide on GE and glucagon excursion is likely mediated through the vagus nerve (35). Both pramlintide and GLP-1 have been reported to decrease glucagon concentrations resulting in their antihyperglycemic action. One study in humans investigated the relationship between GLP-1 and IAPP on GE and glucose homeostasis and proposed that GLP-1 mediates its effect on GE and glucagon secretion independent of IAPP (36). However, the effect of the IAPP analog pramlintide on GLP-1 has not been reported. In this study, integrated excursion of GLP-1 in the first 2 postprandial hours was not different with and without pramlintide, although a limited sample size could have accounted for this observation. Ghrelin is secreted mainly by the stomach and stimulates appetite (37). Because active ghrelin is unstable (38), both total and active ghrelin were measured. This study shows that pramlintide had no acute effects on postprandial ghrelin excursions, supporting previous findings that ghrelin release may not be mediated by the vagus nerve (37, 39).
As with all studies, this study has limitations. The effects of pramlintide on postprandial glucose metabolism were assessed during a single dose of pramlintide administration before a meal. Longer duration studies are needed to explore the long-term effects of pramlintide on the metabolic parameters and adverse effects. Plasma glucose concentrations did not return to baseline during the pramlintide visit 6 hours after meal ingestion. The lower insulin dose administered with pramlintide, because of subject safety issues (to lower risk of hypoglycemia and as recommended by U.S. Food and Drug Administration) could have contributed to delayed postprandial hyperglycemia such that the rate of Rd (mediated by plasma insulin and to an extent by plasma glucose concentrations) did not compensate for the delayed increase in rates of meal glucose appearance. A longer follow-up time would have (presumably) observed a return to baseline of plasma glucose concentrations. In this context, the dose and timing of insulin in the presence of pramlintide warrants further investigation. Furthermore, as we have mentioned previously (5), the independent contributions of suppressing glucagon and delaying gastric emptying on insulin sensitivity and postprandial glucose fluxes cannot be determined from our study.
To summarize, the IAPP analog pramlintide modulates postprandial glucose homeostasis via its effects on GE and glucagon excursions in T1D subjects, as we have demonstrated previously in healthy controls (5).
Acknowledgments
We thank the research participants and the staff of the Mayo Clinic Center for Translational Science Activities Clinical Research Unit (CRU), Gastrointestinal Motility Core, the CRU Mass Spectroscopy Laboratory, CRU Immunochemical Core Laboratory, and Barbara Norby (RN), Shelly McCrady-Spitzer (research assistant), Michael Slama (technician), and Brent McConahey (technician), all of which are at Endocrine Research Unit, Mayo Clinic, Rochester, MN.
The work was supported by National Institutes of Health (NIH) Grants DK R01 085516 and DK DP3 094331 and Grant UL1 TR000135 from the National Center for Advancing Translational Science, a component of the NIH. C.D.M. and C.C. are partially funded by Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Progetto FIRB 2009).
Author Contributions: L.H., A.M., R.B., Y.K., R.E.C., J.R.G., and A.B. assisted in study conduct, data gathering and analyses, manuscript writing, and editing. M.S., V.D., C.D.M., A.E.B., R.E.C., J.R.G., and C.C. assisted in data analyses, manuscript writing, and editing.
Disclosure Summary: Y.C.K. and A.B. are the guarantors of this work, had full access to all data, and take full responsibility for the integrity of data and the accuracy of data analysis. There are no conflicts of interest to declare for any of the authors.
Footnotes
- CI
- confidence interval
- CSII
- continuous sc insulin infusion
- EGP
- endogenous glucose production
- FFM
- fat free mass
- GE
- gastric emptying
- GET
- gastric emptying test
- GI
- gastrointestinal
- GLP-1
- glucagon like peptide-1
- IAPP
- islet amyloid polypeptide
- iAUC
- incremental area under the curve
- MRa
- meal rate of glucose appearance
- PP
- pancreatic polypeptide
- Rd
- glucose disappearance
- SI
- insulin sensitivity
- SIL
- liver SI
- T1D
- type 1 diabetes.
References
- 1. Kudva YC, Carter RE, Cobelli C, Basu R, Basu A. Closed-loop artificial pancreas systems: physiological input to enhance next-generation devices. Diabetes Care. 2014;37:1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cengiz E, Weinzimer SA, Sherr JL, et al. Acceleration of insulin pharmacodynamic profile by a novel insulin infusion site warming device. Pediatr Diabetes. 2013;14:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hinshaw L, Dalla Man C, Nandy DK, et al. Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes. 2013;62:2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basu R, DiCamillo B, Toffolo G, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol (Endocrinol Metab). 2003;284:55–69. [DOI] [PubMed] [Google Scholar]
- 5. Hinshaw L, Schiavon M, Mallad A, et al. Effects of delayed gastric emptying on postprandial glucose kinetics, insulin sensitivity, and beta-cell function. Am J Physiol Endocrinol Metab. 2014;307:E494–E502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf). 2009;70:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chapman I, Parker B, Doran S, et al. Low-dose pramlintide reduced food intake and meal duration in healthy, normal-weight subjects. Obesity (Silver Spring). 2007;15:1179–1186. [DOI] [PubMed] [Google Scholar]
- 8. Heptulla RA, Rodriguez LM, Mason KJ, Haymond MW. Gastric emptying and postprandial glucose excursions in adolescents with type 1 diabetes. Pediatr Diabetes. 2008;9:561–566. [DOI] [PubMed] [Google Scholar]
- 9. Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes. 2005;54:1100–1107. [DOI] [PubMed] [Google Scholar]
- 10. van Jaarsveld BC, Hackeng WH, Nieuwenhuis MG, Erkelens DW, Geerdink RA, Lips CJ. Islet-amyloid polypeptide in human plasma. Lancet. 1990;335:60. [DOI] [PubMed] [Google Scholar]
- 11. Schmitz O, Brock B, Rungby J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes. 2004;3(53 Suppl):S233–S238. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Ridgway Z, Cao P, Ruzsicska B, Raleigh DP. Analysis of the ability of pramlintide to inhibit amyloid formation by human islet amyloid polypeptide reveals a balance between optimal recognition and reduced amyloidogenicity. Biochemistry. 2015;54:6704–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Abeta1–42 at human amylin receptors. Endocrinology. 2014;155:21–26. [DOI] [PubMed] [Google Scholar]
- 14. Cort JR, Liu Z, Lee GM, et al. Solution state structures of human pancreatic amylin and pramlintide. Protein Eng Des Sel. 2009;22:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab. 2008;294:E103–E109. [DOI] [PubMed] [Google Scholar]
- 16. Woerle HJ, Albrecht M, Linke R, et al. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care. 2008;31:2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- 18. Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr. 2014;34:237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49:419–429. [DOI] [PubMed] [Google Scholar]
- 20. Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. [DOI] [PubMed] [Google Scholar]
- 21. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–E643. [DOI] [PubMed] [Google Scholar]
- 22. Visentin R, Dalla Man C, Basu R, Basu A, Rizza RA, Cobelli C. Hepatic insulin sensitivity in healthy and prediabetic subjects: from a dual- to a single-tracer oral minimal model. Am J Physiol Endocrinol Metab. 2015;309:E161–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. [DOI] [PubMed] [Google Scholar]
- 24. Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness and postprandial glucose metabolism. Diabetes Care. 2009;32:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basu A, Basu R, Shah P, et al. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes. 2001;50:1351–1362. [DOI] [PubMed] [Google Scholar]
- 26. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:5. [Google Scholar]
- 27. van Belle G. Statistical Rules of Thumb. 2nd edition Hoboken, NJ: Wiley-Interscience; 2008;xxx. [Google Scholar]
- 28. Senn SS. Cross-over Trials in Clinical Research. 2nd edition New York, NY: John Wiley Sons; 2002. [Google Scholar]
- 29. Felig P, Gusberg R, Hendler R, Gump FE, Kinney JM, Mulrow PJ. Concentrations of glucagon and the insulin:glucagon ratio in the portal and peripheral circulation. Proc Soc Exp Biol Med. 1974;147:88–90. [DOI] [PubMed] [Google Scholar]
- 30. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;1(13 Suppl):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz TW. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983;85:1411–1425. [PubMed] [Google Scholar]
- 32. Williams JA. 2014. Pancreatic Polypeptide. The Pancreapedia: Exocrine Pancreas Knowledge Base. http://www.pancreapedia.org. doi:10.3998/panc.2014.4.
- 33. Koch MB, Go VL, DiMagno EP. Can plasma human pancreatic polypeptide be used to detect diseases of the exocrine pancreas? Mayo Clin Proc. 1985;60:259–265. [DOI] [PubMed] [Google Scholar]
- 34. Salera M, Pironi L, Giacomoni P, et al. Effect of somatostatin on fasting and glucose-stimulated gastric inhibitory polypeptide release in man. Digestion. 1982;24:126–132. [DOI] [PubMed] [Google Scholar]
- 35. Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol. 2000;278:G946–G951. [DOI] [PubMed] [Google Scholar]
- 36. Asmar M, Bache M, Knop FK, Madsbad S, Holst JJ. Do the actions of glucagon-like peptide-1 on gastric emptying, appetite, and food intake involve release of amylin in humans? J Clin Endocrinol Metab. 2010;95:2367–2375. [DOI] [PubMed] [Google Scholar]
- 37. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. [DOI] [PubMed] [Google Scholar]
- 38. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin–a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. [DOI] [PubMed] [Google Scholar]