Abstract
Context:
Alterations in bile acid (BA) synthesis and transport have the potential to affect multiple metabolic pathways in the pathophysiology of obesity.
Objective:
The objective of the study was to investigate the effects of obesity on serum fluctuations of BAs and markers of BA synthesis.
Design:
We measured BA fluctuations in 11 nonobese and 32 obese subjects and BA transporter expression in liver specimens from 42 individuals and specimens of duodenum, jejunum, ileum, colon, and pancreas from nine individuals.
Main Outcome Measures:
We analyzed serum BAs and markers of BA synthesis after overnight fasting, during a hyperinsulinemic-euglycemic clamp, or a mixed-meal tolerance test and the association of BA transporter expression with body mass index.
Results:
BA synthesis markers were 2-fold higher (P < .01) and preferentially 12α-hydroxylated (P < .05) in obese subjects, and both measures were correlated with clamp-derived insulin sensitivity (r = −0.62, P < .0001, and r = −0.39, P = .01, respectively). Insulin infusion acutely reduced serum BAs in nonobese subjects, but this effect was blunted in obese subjects (δBAs −44.2% vs −4.2%, P < .05). The rise in serum BAs postprandially was also relatively blunted in obese subjects (δBAs +402% vs +133%, P < .01). Liver expression of the Na+-taurocholate cotransporting polypeptide and the bile salt export pump were negatively correlated with body mass index (r = −0.37, P = .02, and r = −0.48, P = .001, respectively).
Conclusions:
Obesity is associated with increased BA synthesis, preferential 12α-hydroxylation, and impaired serum BA fluctuations. The findings reveal new pathophysiological aspects of BA action in obesity that may lend themselves to therapeutic targeting in metabolic disease.
We measured plasma bile acids, markers of bile acid synthesis, and expression of bile acid transporters in obese and nonobese subjects. We found that obesity was associated with increased bile acid synthesis and 12-hydroxylation, blunted response of plasma bile acids to insulin infusion or a mixed meal, and decreased expression of liver bile acid transporters.
Bile acids (BAs) are cholesterol metabolites that have emerged as regulators of lipid and glucose metabolism (1, 2). Recent work from our group and others has shown that individuals with insulin resistance and type 2 diabetes have increases in fasting levels of plasma BAs (3, 4), in particular increases in the subset of BAs that are hydroxylated at the 12α position (4–6). Others have also observed increases in the subset of BAs conjugated with taurine (7). The cause of these alterations is unknown, but these findings have raised the possibility that BA defects contribute to the metabolic abnormalities associated with insulin resistance.
Potential causes for elevated plasma levels of total or 12α-hydroxy BAs in insulin resistant individuals include increased synthesis or defective transport (including input from intestine into plasma or sinusoidal uptake into hepatocytes). BA synthesis occurs only in hepatocytes and is controlled by negative feedback loops in the liver and intestine. The daily rate of BA synthesis is low, and new synthesis contributes only a small fraction of the BA pool (8). In humans, two primary BAs are synthesized-chenodeoxycholic acid and cholic acid, the latter of which is 12α-hydroxylated. In the intestine, BAs can be further modified by gut flora in secondary and tertiary species. Most BAs are efficiently reabsorbed by enterocytes and returned to the liver via the portal vein.
The transport of BAs from the intestine to liver occurs via active and passive mechanisms. Passive diffusion of BAs across cell membranes is limited to unconjugated BA species and occurs only in a gradient-dependent fashion (9). Therefore, most BAs are actively transported. The bile salt export pump (BSEP) transports BAs from hepatocytes into bile canaliculi. BAs cross the intestinal epithelium through the actions of three components (8, 10, 11): the apical sodium-dependent bile salt transporter (ASBT); the intracellular ileal bile acid binding protein; and the basolateral heteromeric transporter, the organic solute transporter (OST)-α-OSTβ. Once in the portal vein, most BAs are taken up into the liver through the sodium-dependent transporter, Na+-taurocholate cotransporting polypeptide (NTCP), and a smaller portion through the sodium-independent organic anion transporting polypeptides (OATPs) (8). A fraction of BAs bypasses liver sinusoidal uptake and is present in the systemic circulation (12, 13).
Alterations in the BA levels have the potential to affect multiple aspects of energy metabolism (14, 15). Changes in BA transport may also have effects, due to tissue-specific alterations in BA receptor signaling. An example of this is the effect of BA sequestrants, which block BA reabsorption in the gut, cause a compensatory increase in BA synthesis, and improve glycemia (16, 17). Similar mechanisms may explain improvements in glycemia due to ASBT inhibition (18, 19).
In this work, we investigated whether obesity and insulin resistance affect BA synthesis or transport. We measured the fluctuations of serum BAs and markers of BA synthesis during two different interventions: a hyperinsulinemic-euglycemic clamp and a mixed-meal tolerance test. Furthermore, we examined the expression of BA transporters in liver biopsies from 42 subjects and intestinal samples from an independent group of nine subjects.
Materials and Methods
Subjects
We recruited 43 nondiabetic, Caucasian subjects (four men and 28 women) from persons attending our clinic; of them, 32 were obese (body mass index [BMI] >30 kg/m2, range 31.1–60.7 kg/m2) and 11 were nonobese (BMI <30 kg/m2, range 20.8–28.7 kg/m2). At the time of the study, all patients were weight stable and ambulatory. The metabolic study consisted of a mixed-meal test and, on a different day, a euglycemic hyperinsulinemic clamp.
Mixed-meal test
After an overnight (12 h) fast, the subjects were admitted to the University of Pisa Clinical Research Unit at 8:00 am, and a polyethylene cannula was inserted into an antecubital vein for the infusion of all test substances. A second catheter was inserted retrogradely into an ipsilateral wrist vein on the dorsum of the hand for blood sampling, and the hand was kept in a heated box at 60°C ± 5°C to achieve the arterialization of venous blood. Baseline blood samples were drawn to measure plasma glucose, insulin, and bile acids. The meal consisted of 75 g of glucose in water, 40 g of parmesan cheese, and one 50-g egg (509 kcal, 16% protein, 28% fat, 56% carbohydrate). The meal was consumed over approximately10 minutes. Plasma samples for the determination of plasma glucose, insulin, C-peptide, and bile acids were obtained at 60, 120, and 180 minutes after the meal ingestion.
Euglycemic hyperinsulinemic clamp
Clamps were carried out at the University of Pisa. After an overnight (12 h) fast, two catheters were inserted into an antecubital vein for infusion of all test substances and retrogradely into a vein on the dorsum of the hand for blood drawing. The hand was heated at 60°C ± 5°C to achieve the arterialization of venous blood. At 9:00 am, baseline blood samples were drawn. At time −20, −10, and 0 minutes, blood samples were obtained from the arterialized vein for the measurement of glucose and insulin. At time 0, a primed-continuous insulin (Humulin R; Eli Lilly & Co) infusion (at a rate of 240 pmol · min−1 · m−2) was started and continued for 120 minutes; plasma glucose levels were measured every 5 minutes throughout the clamp. Plasma insulin and free fatty acid concentrations were measured every 20 minutes between the time 80 and 120 minutes after the start of insulin infusion; bile acids were measured at time 0 and 120 minutes.
Liver biopsies
In 42 Caucasian subjects, a liver sample was collected during bariatric surgery at the University of Pisa in RNA-Later (Ambion Inc, Applied Biosystems) and stored at −20°C for total RNA extraction.
Intestinal specimens
Samples of human intestine and pancreas were collected from nine organ donors at Columbia University Medical Center (20) and stored in liquid nitrogen. Ethnicity of donors was not available.
Gene expression
RNA was isolated using Trizol (Life Technologies), cDNA was synthesized using reverse transcriptase (Applied Biosystems), and a quantitative PCR was performed using SyBR Green (Bio-Rad Laboratories). Relative expression was calculated using δδcycle threshold method, and absolute values were determined using quantitative standard curves. (See primer sequences available in Supplemental Table 1.)
Analytical procedures
Fat-free mass (FFM) was estimated with the use of electric bioimpedance on a Tanita scale. Plasma glucose was measured by the glucose oxidase technique on a Beckman glucose analyzer (Beckman). Plasma insulin was assayed by a specific RIA (Linco Research and MYRIA Technogenetics, respectively).
Bile acid measurements
A total of 0.1 mL aliquots of plasma collected after an overnight fast was spiked with an internal standard (10 μL cholate-d4, 25 μM) mixed with 1 mL ice-cold acetonitrile, vortexed, and centrifuged for 10 minutes at 11 000 × g. The supernatant was dried under nitrogen at 45°C and resuspended in 100 μL 55/45 (vol/vol) MeOH/H2O, containing 5 mM ammonium formate. Ten microliters were injected into liquid chromatography-mass spectrometry (Waters Quattro Micro with Waters 2795 Alliance HPLC). Quantitative standard curves were used, and deuterated internal standards were used to measure recovery. Hydrophobicity index was calculated using the published method (21). We measured indirect markers of BA synthesis by liquid chromatography-mass spectrometry as described previously (6).
Data analysis
Insulin-stimulated glucose disposal (M, micromoles per minute) was calculated as the mean exogenous glucose infusion rate during the last 40 minutes of the clamp corrected for changes in glucose concentration within a distribution volume of 200 mL per kilogram of body weight. M values were expressed per kilograms of FFM. Furthermore, to account for differences in achieved plasma insulin concentrations during the clamp, an index of peripheral insulin sensitivity (M/I; in units of micromoles per minute per kilogramFFM per picomole) was calculated as the ratio of M to the steady-state plasma insulin concentrations (22).
Statistics
Data are given as mean ± SD or median and (interquartile range) for normally or nonnormally distributed variables, respectively. Group differences were analyzed by a Mann-Whitney U test. Regression analyses were done by standard methods. A value of P ≤ .05 was considered statistically significant. All statistical analyses were performed using JMP7.0 (SAS Institute).
Study approval
All participants provided written informed consent. This study received institutional ethics committee approval, under study protocols number 245 (EudraCT 2010-018708-99; and number 317 (RF-2011-02348446). The intestinal and pancreatic samples were exempt from institutional review board review after institutional review board review.
Results
Overnight fasting
As reflected by their M/I ratio, obese subjects were markedly insulin resistant as compared with the nonobese group (median value 33 [26] vs 103 [63] μmol · min−1 · kgffm−1 · nM−1 for obese and nonobese, respectively) (Table 1). We measured serum BAs and two markers of BA synthesis: 1) 7α-hydroxy-4-cholesten-3-one (7-HCO), a marker of CYP7A1 activity and total BA synthesis rate; and 2) 7α,12α-dihydroxy-4-cholesten-3-one (7,12-diHCO), the product of CYP8B1 and a marker of the cholic acid (12α-hydroxylated) branch of synthesis. A schematic of BA synthesis is shown in Figure 1A. We calculated the ratio of 7,12-diHCO to 7-HCO to determine the portion of new BA synthesis that is 12α-hydroxylated. Full BA data sets are shown in Supplemental Tables 2 and 3.
Table 1.
Anthropometrics and Metabolic Dataa
| Whole Group | Nonobese | Obese | |
|---|---|---|---|
| N | 43 | 11 | 32 |
| Age, y | 38 ± 2 | 37 ± 3 | 39 ± 2 |
| Weight, kg | 106 ± 5 | 66 ± 3 | 120 ± 5b |
| BMI, kg/m2 | 38.7 ± 1.8 | 23.9 ± 0.7 | 44.0 ± 1.6b |
| Clamp, n | 41 | 10 | 31 |
| Fasting glucose, mmol/L | 5.2 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Steady-state glucose, mmol/L | 5.1 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Fasting insulin, pmol/L | 89 [89] | 44 [21] | 104 [76]b |
| Steady-state insulin, pmol/L | 736 [310] | 576 [108] | 811 [259]b |
| M, μmol · min−1 · kgffm−1 | 30 [24] | 61 [31] | 28 [19]b |
| M/I, μmol · min−1 · kgffm−1 · nM−1 | 45 [51] | 103 [63] | 33 [26]b |
| Mixed meal, n | 37 | 10 | 27 |
| Fasting glucose, mmol/L | 5.1 ± 0.1 | 5.0 ± 0.1 | 5.1 ± 0.1 |
| 60-min glucose, mmol/L | 6.9 ± 0.2 | 6.9 ± 0.5 | 6.9 ± 0.2 |
| 120-min glucose, mmol/L | 6.3 ± 0.1 | 6.4 ± 0.2 | 6.2 ± 0.2 |
| 180-min glucose, mmol/L | 6.0 ± 0.1 | 6.2 ± 0.3 | 5.9 ± 0.2 |
| Fasting insulin, pmol/L | 79 [56] | 54 [30] | 100 [73]b |
| 60-min insulin, pmol/L | 432 [304] | 338 [196] | 512 [391]c |
| 120-min insulin, pmol/L | 301 [342] | 205 [134] | 400 [552]d |
| 180-min insulin, pmol/L | 200 [283] | 187 [141] | 238 [324] |
| δInsulin AUC, nmol/L | 209 [264] | 169 [103] | 276 [282]c |
Abbreviation: δAUC, incremental area under the curve.
Entries are mean ± SEM or median [interquartile range].
P ≤ .0001 for the comparison of nonobese vs obese by Mann-Whitney test.
P ≤ .05.
P ≤ .01.
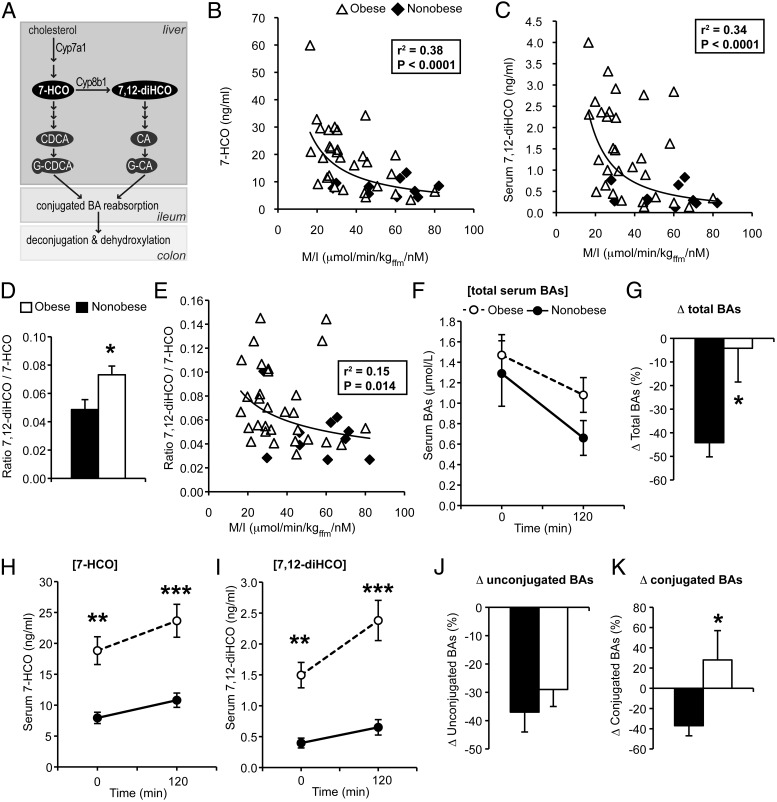
Figure 1.
Serum BAs and markers of BA synthesis after overnight fast and during the hyperinsulinemic-euglycemic clamp. A, Schematic of BA synthesis and modification. B and C, Correlation of clamp-derived insulin sensitivity (M/I) with 7-HCO and 7,12-diHCO at baseline. D, Ratio of 7,12-diHCO to 7-HCO. E, Correlation of M/I with the ratio of 7,12-diHCO to 7-HCO at baseline. F, Total serum BAs during the clamp. G, Percentage change in total BAs during the clamp. H and I, Serum 7-HCO and 7,12-HCO during the clamp. J and K, Percentage change in unconjugated and conjugated BAs during the clamp. *, P < .05, **, P < .01, ***, P < .001 between obese and nonobese by Mann-Whitney test. CA, cholic acid; CDCA, chenodeoxycholic acid; G-CA, glycocholic acid,; G-CDCA, glycochenodeoxycholic acid.
After the overnight fast, 7-HCO levels in obese subjects were doubled compared with nonobese subjects (18.83 ± 2.25 ng/mL vs 7.93 ± 0.92 ng/mL, P = .007). This finding confirms prior observations that BMI is positively associated with BA synthesis and fecal BA levels (23–25). We also found that 7,12-diHCO levels were nearly quadrupled in obese subjects, compared with nonobese subjects (1.50 ± 0.21 vs 0.40 ± 0.08 ng/mL, P = .003). Both of these metabolites were positively correlated with BMI (Supplemental Figure 1, A and B) and even more strongly negatively correlated with clamp-derived insulin sensitivity, as reflected by M/I (r = −0.62, P < .0001, and r = −0.59, P < .0001, for 7-HCO and 7,12-diHCO, respectively) (Figure 1, B and C). The ratio of 7,12-diHCO to 7-HCO was significantly elevated in obese subjects and was negatively correlated with M/I (r = −0.39, P = .014) (Figure 1, D and E). However, this ratio was not significantly correlated with BMI (Supplemental Figure 1C). These findings indicate the following: 1) obese subjects have elevated BA synthesis; 2) a greater proportion of new BA synthesis is 12α-hydroxylated; and 3) these abnormalities are associated with insulin resistance.
Euglycemic-hyperinsulinemic clamp
Next, we examined serum BAs during the clamp, allowing us to determine the isolated effects of insulin on serum BA dynamics. During the clamp, serum BAs unexpectedly decreased by nearly half in nonobese subjects (Figure 1, F and G). In contrast, in obese subjects, insulin's ability to reduce serum BAs was markedly blunted (Figure 1, F and G). The decrease of BAs by insulin was unlikely to be due to acute inhibition of BA synthesis because new BA synthesis contributes a minor fraction of the BA pool (8). Indeed, there was no decrease, or a slight increase, in markers of BA synthesis during the clamp in both groups (Figure 1, H and I). This suggests that insulin decreases serum BAs by affecting BA efflux into plasma or clearance out of plasma (ie, BA transport) and that obese subjects have a defect in this pathway.
Next, we analyzed individual and subsets of BAs to determine which were affected by insulin. In nonobese subjects, most BA species were reduced by insulin (Supplemental Tables 2 and 3). In obese subjects, unconjugated BA species were reduced similarly to nonobese subjects (Figure 1J). However, conjugated BAs were unaffected, or even slightly increased, by insulin in the obese group (Figure 1K). Thus, in the obese group, the clamp-induced reduction in conjugated BAs relative to unconjugated BAs was directly related to insulin sensitivity (r = 0.47, P = .008). These data suggest that obese, insulin-resistant subjects have a defect in insulin-regulated transport of conjugated BAs.
Mixed-meal tolerance test
Fasting glucose and glucose excursions during the meal were similar between groups. However, obese subjects showed relative hyperinsulinemia at baseline and at all time points throughout the meal, consistent with their insulin resistance (Table 1).
The high intra- and interindividual variability in serum BAs is well documented (23). However, we found that the differences in BA synthesis markers between obese and nonobese subjects were highly reproducible, demonstrating the robustness of these phenotypes (Supplemental Figure 2). These data support our observation that obese subjects have an increased BA synthesis and a preferential synthesis of 12α-hydroxylated BAs.
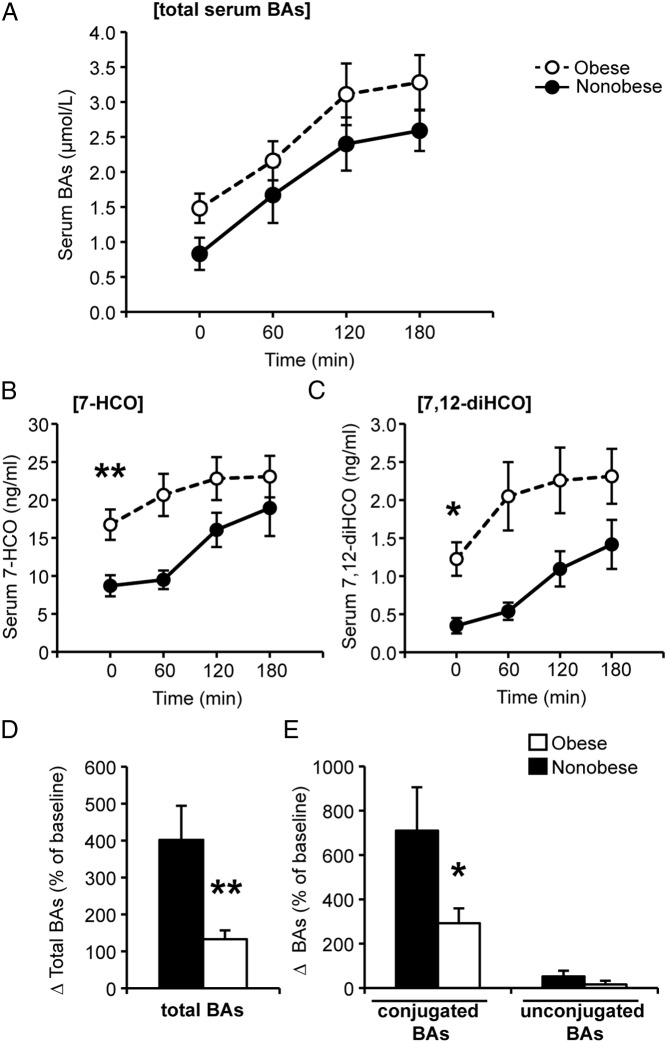
During the meal, serum BAs increased in all subjects (Figure 2A), as expected (12, 13, 26). We also detected increases in BA synthesis markers during the meal (Figure 2, B and C). Prior studies have also indicated that plasma 7-HCO may increase postprandially in addition to diurnally (23, 27). In absolute amounts, obese subjects tended to have slightly higher postprandial serum BAs (Figure 2A), but the change in BAs from baseline to 180 minutes was slightly smaller than that in the nonobese subjects (an increase of 1.23 ± 0.34 μmol/L vs 1.66 ± 0.39 μmol/L). One might have expected obese subjects to show a proportionally larger flux through the enterohepatic cycle, based on their higher fasting serum BAs. On the contrary, when expressed as a percentage of the baseline, the increase of serum BAs during the meal was significantly blunted in obese subjects (Figure 2D).
Figure 2.
Serum BAs during the mixed-meal tolerance test. A, Total serum BAs. B and C, Serum 7-HCO and 7,12-diHCO during the meal. D and E, Percentage change in total, conjugated, and unconjugated BAs during the meal. *, P < .05, **, P < .01 between obese and nonobese by Mann-Whitney test.
Next, we analyzed individual and subsets of BAs (Supplemental Tables 2 and 3). We found that in nonobese subjects, conjugated BA species accounted for most of the increase during the meal (Figure 2E). This was as expected, due to the high efficiency of conjugated BA transport into the portal vein by ileal BA transporters (8, 23). In contrast, in obese subjects, relative excursions of conjugated BAs were substantially blunted (Figure 2E). Overall, these data are consistent with obese subjects having reduced postprandial excursions of conjugated BAs relative to their serum levels.
Tissue-specific expression of BA transporters and BA-responsive genes
To identify possible contributors to BA transport defects, we examined gene expression. Liver biopsies were collected from 42 obese subjects. In a separate group of nine subjects, samples were obtained from segments of intestine and pancreas. Subject data are shown in Table 2.
Table 2.
Tissue Sample Patient Data
| Liver Samples | Intestine and Pancreas Samples | |
|---|---|---|
| n (F/M)a | 42 (21/21) | 9 (4/4) |
| Type 2 diabetes | 28 (67%) | 2 (22%) |
| Age, y | 47.4 ± 1.4 | 32.6 ± 3.7 |
| BMI, kg/m2 | 42.2 ± 1.6 | 33.2 ± 2.9 |
| Fasting plasma glucose, mmol/L | 8.76 ± 0.62 | n.a. |
| Fasting plasma insulin, pmol/L | 124.2 [73.1] | n.a. |
| HbA1C, %b | 7.95 ± 0.36 | 6.66 ± 1.05 |
Abbreviations: F, female; M, male; n.a., not available.
Sex was not available for one of the intestinal samples.
Glycated hemoglobin was available for 29 of 42 liver samples and five of nine intestinal samples.
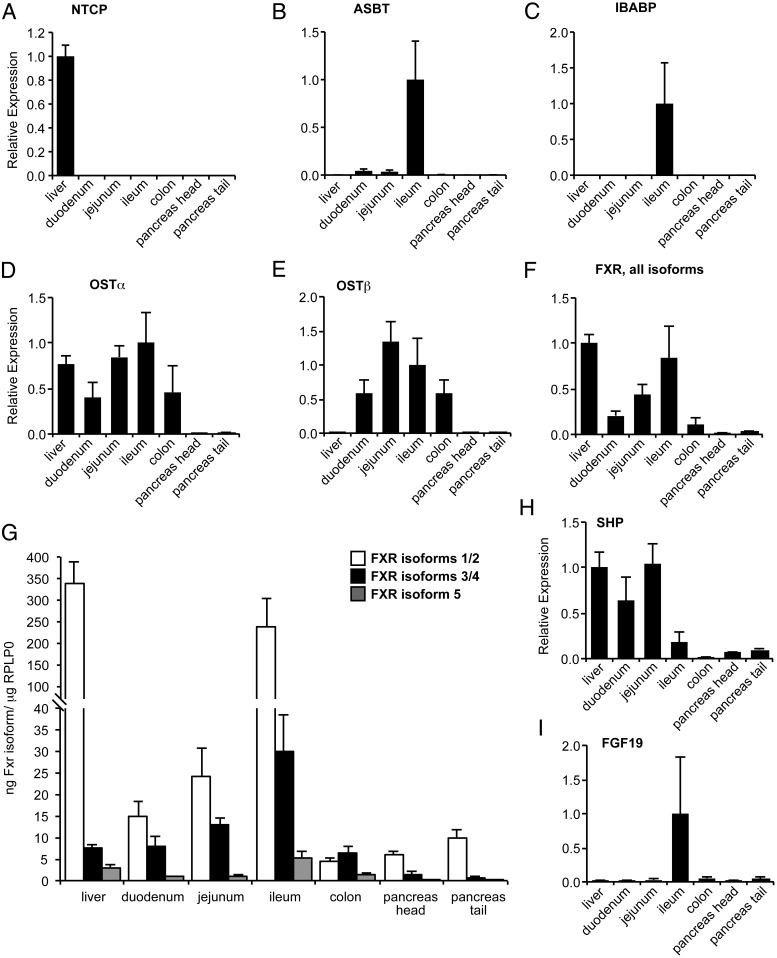
We first established the tissue-specific expression of BA transporters. We found NTCP (encoded by SLC10A1) was expressed exclusively in liver, as expected (Figure 3A). ASBT and ileal bile acid binding protein (encoded by SLC10A2 and FABP6) were expressed nearly exclusively in ileum (Figure 3, B and C). OSTα (encoded by SLC51A) was highly expressed in the ileum, jejunum, liver, duodenum, and colon, whereas OSTβ (encoded by SLC51B) was high in the intestine and 50- to 100-fold lower in the liver (Figure 3, D and E).
Figure 3.
Tissue distribution of BA transporters and BA-responsive genes. A–I, Relative gene expression. G, Absolute expression quantitated by standard curve.
We also examined tissue-specific expression of other BA-related genes. Using primers that detect all isoforms, we detected farnesoid X receptor (FXR; encoded by NR1H4) in all examined tissues, with the highest levels in the ileum and liver (Figure 3F). Using isoform-specific primers, we found that FXR mRNA isoforms 1/2 were expressed at higher levels than other isoforms, especially in the liver and ileum (Figure 3G). The FXR target gene small heterodimer partner (SHP; encoded by NR0B2) was highest in the liver, jejunum, and duodenum and unexpectedly much lower in the ileum (Figure 3H). Fibroblast growth factor 19 (FGF19) was found almost exclusively in the ileum and was highly variable between individuals (Figure 3I).
Liver BA transporters in obesity
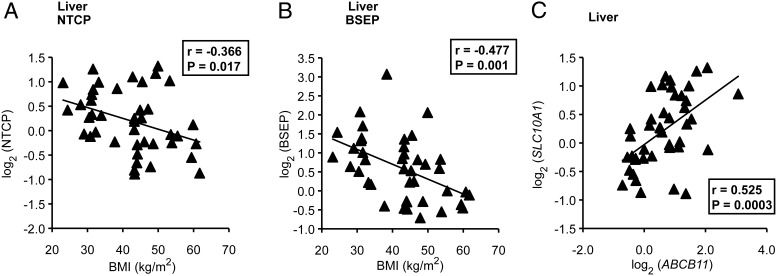
Next we examined the relationship between the expression of these genes and BMI. This analysis was performed only in liver specimens, which were collected during bariatric surgery (n = 42). We found that BMI was negatively correlated with BA transporters NTCP (r = −0.37, P = .017) and BSEP (r = −0.48, P = .001) (Figure 4, A and B). Moreover, expression of NTCP and BSEP were positively correlated with each another (r = 0.53, P = .0003) (Figure 4C). Other BA transporters (including OSTα, OSTβ, and OATP1A2 [SLCO1A2]) and cholesterol transporters (ABCG5 and ABCG8) were not significantly associated with BMI (Supplemental Table 4). This suggests that obesity may be associated with a decreased expression of the major sinusoidal uptake and canalicular efflux BA transporters.
Figure 4.
Relationship of BMI with BA transporter expression. A–C, Correlations of NTCP and BSEP gene expression with BMI (A and B) and each other (C).
We also investigated whether defects in FXR signaling could be involved in altered expression of BA transporters because many of them are FXR targets. BMI was not correlated with FXR (all isoforms) or its canonical target gene SHP (Supplemental Table 4). However, FXR isoforms 1/2 were positively correlated with BMI, whereas other isoforms showed no significant relationship.
Discussion
BAs are now known for their myriad effects on energy metabolism. Defects in BA levels or transport that arise in obesity may adversely affect these processes. The main findings from this work are that in obese, insulin-resistant subjects, the following occurs: 1) BA synthesis markers are higher and 2) preferentially 12α-hydroxylated; moreover, 3) insulin acutely reduces serum BAs in healthy subjects; but in obesity, 4) this effect of insulin is blunted; 5) serum BA changes during a meal are blunted relative to fasting BA levels; and 6) the expression of liver BA transporters is negatively associated with BMI.
These data demonstrate that with the onset of obesity and insulin resistance, multiple BA defects arise. One of the most striking phenotypes we observed is the increase of BA synthesis biomarkers. This demonstrates clear alterations in cholesterol balance during obesity that have not yet been fully appreciated.
The preferential increase of 12α-hydroxylated BA synthesis that we observed in obesity extend previous observations. We have found that insulin resistance in nonobese subjects is associated with progressive increases in 12α-hydroxylated BAs (4) and that obese subjects with type 2 diabetes have a higher synthesis of 12α-hydroxy BAs compared with obese subjects with normal glucose tolerance (6). Earlier another group also reported increased 12α-hydroxy BA synthesis in people with well-controlled type 2 diabetes (5). Could increased synthesis of these BA species be detrimental? Some evidence would suggest this. We found that in healthy subjects, increases in 12α-hydroxy BAs correlate with key features of insulin resistance, including fasting glucose, insulin, triglycerides, and low high-density lipoprotein (4). Moreover, Cyp8b1-deficient mice, which lack 12α-hydroxy BAs, have lower body weight, improved glucose tolerance, increased glucagon like peptide-1, and reduced atherosclerosis (28, 29). These findings highlight the possibility that redressing the excess BA 12α-hydroxylation in insulin resistance may have therapeutic benefit.
To our knowledge, the acute effect of insulin on serum BAs in healthy subjects has not yet been reported. We were surprised to find that insulin strongly reduces serum BAs. Because BA synthesis markers were not similarly reduced, we expect that insulin's effect occurs through modulation of BA efflux or uptake, rather than synthesis. The two major controllers of serum BA levels are input through the intestine and uptake into the liver. Our study cannot distinguish between these two possible sites of insulin action or other less likely possibilities such as uptake into alternative tissues. However, the flux of BAs through the enterohepatic circulation proceeds overwhelmingly in the direction of gallbladder→intestine→portal vein→liver. Thus, we predict insulin action would more likely promote uptake from blood into liver (ie, promoting the enterohepatic circulation) than to suppress efflux from intestine into blood (ie, blocking the enterohepatic circulation). Interestingly, the phosphatidylinositol 3-kinase-AKT pathway has been shown to induce NTCP localization to the basolateral membrane of hepatocytes (30, 31). This provides a potential explanation for the acute effects we observed in healthy subjects during the clamp. An informative comparison could be made between the effect of insulin alone on serum BA fluctuations, which we find to decrease circulating BAs and the effect of oral glucose, which causes insulin secretion as well as excursions of circulating BAs that peak within 30 minutes after ingestion (32, 33). It is possible that BA excursions after oral glucose reflect a combination of increased BA transport from intestine into plasma (via effects on intestine and/or gallbladder), followed by reuptake in liver. Our data predict the latter effect is enhanced by insulin action.
Obesity was associated with a major defect in serum BA fluctuations in our protocols, although we acknowledge that the absence of isotope tracers is a limitation. The results of the clamp showed a blunted effect of insulin to reduce conjugated serum BAs in obese subjects. The negative correlation of NTCP with BMI suggests an explanation for this effect, again involving insulin resistance, although this finding was limited by a modest sample size (n = 42), the lack of samples from normal controls, and the fact that the liver specimens were from a different cohort of subjects than the serum measurements. A defect of NTCP in obese subjects would also provide a possible explanation for the specific defect in conjugated BA fluctuations because NTCP preferentially transports conjugated BAs, whereas unconjugated BAs are primarily transported by other mechanisms including OATPs and passive diffusion (8, 34). The results of the mixed-meal tolerance test suggest that obese subjects also have relatively slower enterohepatic circulation, which is consistent with prior studies (35, 36) and may be explained by the negative correlation of BSEP with BMI. Together these findings suggest that obese subjects have defective hepatic BA transport, potentially including both uptake and efflux. It is worth noting that BSEP and NTCP are typically thought to be regulated in opposite directions, with FXR activating BSEP and suppressing NTCP (37). However, we observed a significant positive correlation between these genes. Because FXR isoforms can have distinct effects on targets (38, 39), perhaps differential associations of FXR isoforms with BMI explains this unexpected link.
Could defects in hepatic BA transport result in the increased BA synthesis of obesity? The effects of genetic mutations of these transporters may shed some light on this. Genetic mutations in BSEP lead to poor canalicular BA efflux and can cause progressive familial intrahepatic cholestasis or benign recurrent intrahepatic cholestasis (40, 41). The former leads to severe toxicity and liver failure, whereas the latter has much milder effects. Both are expected to cause decreased BA synthesis due to excessive activation of FXR in hepatocytes (40). As yet, only one patient with a genetic mutation in NTCP has been described (42). One might have expected this mutation to cause reduced hepatic FXR signaling and increased BA synthesis. However, this patient presented with high plasma BAs and no change in 7-HCO. Thus, the phenotype of our obese cohort does not mimic even mild forms of these conditions and suggests a unique defect. We can speculate that the combined slight reduction of BA transport on both sinusoidal and canalicular membranes slows the enterohepatic circulation while protecting the liver from the BA toxicity observed in genetic BSEP deficiencies.
How might defects of hepatic BA transport contribute to metabolic abnormalities of obesity? It is possible that transient increases in total plasma BAs due to poor hepatic clearance cause signaling defects. For example, one might imagine slower postprandial BA signaling in liver but excessive signaling in pancreas or fat. Intriguingly, altering tissue-specific BA signaling is already known to affect energy metabolism, as demonstrated by bile acid sequestrants and ASBT inhibitors that improve systemic glucose metabolism (17–19). This raises the possibility that obesity-related changes in BA transport impair endogenous mechanisms of metabolic regulation.
Another important point to consider is the effect of the gut flora on BAs. Bacteria dehydroxylate and deconjugate BAs and thus determine the levels of secondary BAs as well as unconjugated BAs. In this study, we did not find significant differences between obese and nonobese subjects in the serum levels or fluctuations of secondary or unconjugated BAs. Thus, although gut microbiota are altered by obesity (43), such alterations may not be involved in the phenotypes described here. On the other hand, it is possible that obesity-induced changes in BAs (levels, composition, intestinal transit time, etc) may influence the gut microbiome. Additional studies would be required to assess this possibility.
Although the direct consequences of these BA defects on energy metabolism are incompletely understood, these findings provide novel insight into endogenous regulation of BAs and BA signaling in obesity. Interest in therapeutics targeting BA pathways has been growing (1, 14, 15, 44). Understanding the alterations in these pathways that are inherent in the pathophysiology of metabolic disease may aid in our design of these therapies.
Acknowledgments
We gratefully acknowledge Domenico Accili, Mark Erion, Stephen Previs, Martin Brenner, and David Kelley for their support of and input into this manuscript; Ryotaro Bouchi and Francesca Cinti for intestinal samples; and Jessie Lee for technical assistance.
Author contributions include the following: R.A.H. and E.F. conceived the study, designed and supervised the experiments, analyzed the data, and wrote the paper. S.C. performed in vivo experiments, analyzed data, and wrote the paper. B.A. and M.N. performed the in vivo experiments and contributed to the discussions. J.C.-P., D.X., L.W., and M.C. measured the bile acid synthesis markers in a blinded fashion and contributed to the discussion. All authors edited the paper.
This work was supported in part by the European Union Grant 115372, the Italian Ministry of University and Research Grant 2010329EKE, the National Institutes of Health Grant HL111206 (to R.A.H.), Clinical and Translational Science Awards Grant UL1 TR000040, and an unrestricted grant from Merck Research Laboratories.
This work was funded in part by an unrestricted grant from Merck Research Laboratories, which had no input into the study design or data analysis.
Disclosure Summary: J.C.-P., D.X., L.W., and M.C. are employees of Merck Research Laboratories. E.F. has been a speaker and consultant for Boehringer Ingelheim, Merck, Sanofi, Eli Lilly and Co, Johnson & Johnson, Astellas, Daiichi Sankyo, Bristol Myers Squibb/AstraZeneca, and Novartis. R.A.H., S.C., M.N., and B.A. have nothing to disclose.
Footnotes
- ASBT
- apical sodium-dependent bile salt transporter
- BA
- bile acid
- BMI
- body mass index
- BSEP
- bile salt export pump
- 7,12-diHCO
- 7α,12α-dihydroxy-4-cholesten-3-one
- FFM
- fat-free mass
- FXR
- farnesoid X receptor
- 7-HCO
- 7α-hydroxy-4-cholesten-3-one
- M
- insulin-stimulated glucose disposal
- M/I
- peripheral insulin sensitivity
- NTCP
- Na+-taurocholate cotransporting polypeptide
- OATP
- organic anion transporting polypeptide
- OST
- organic solute transporter
- SHP
- small heterodimer partner.
References
- 1. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. [DOI] [PubMed] [Google Scholar]
- 2. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cariou B, Chetiveaux M, Zair Y, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nut Metab. 2011;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52(4):1455–1464. [DOI] [PubMed] [Google Scholar]
- 6. Ferrannini E, Camastra S, Astiarraga B, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes 2015;64(10):3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. [DOI] [PubMed] [Google Scholar]
- 9. Dietschy JM. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968;9:297–309. [PubMed] [Google Scholar]
- 10. Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. [DOI] [PubMed] [Google Scholar]
- 11. Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OSTα-OSTβ: a key membrane transporter of bile acids and conjugated steroids. Front Biosci (Landmark edition). 2009;14:2829–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angelin B, Bjorkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LaRusso NF, Hoffman NE, Korman MG, Hofmann AF, Cowen AE. Determinants of fasting and postprandial serum bile acid levels in healthy man. Am J Digest Dis. 1978;23:385–391. [DOI] [PubMed] [Google Scholar]
- 14. Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. [DOI] [PubMed] [Google Scholar]
- 16. Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab. 2010;12:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prawitt J, Caron S, Staels B. Glucose-lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol Metab. 2014;25:235–244. [DOI] [PubMed] [Google Scholar]
- 18. Chen L, Yao X, Young A, et al. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am J Physiol Endocrinol Metab. 2012;302:E68–E76. [DOI] [PubMed] [Google Scholar]
- 19. Wu Y, Aquino CJ, Cowan DJ, et al. Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J Med Chem. 2013;56:5094–5114. [DOI] [PubMed] [Google Scholar]
- 20. Bouchi R, Foo KS, Hua H, et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun. 2014;5:4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 22. Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens. 1998;16:895–906. [DOI] [PubMed] [Google Scholar]
- 23. Steiner C, Othman A, Saely CH, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6:e25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miettinen TA. Cholesterol production in obesity. Circulation. 1971;44:842–850. [DOI] [PubMed] [Google Scholar]
- 25. Stahlberg D, Rudling M, Angelin B, et al. Hepatic cholesterol metabolism in human obesity. Hepatology. 1997;25:1447–1450. [DOI] [PubMed] [Google Scholar]
- 26. van Berge-Henegouwen GP, Hofmann AF. Systemic spill-over of bile acids. Eur J Clin Invest. 1983;13:433–437. [DOI] [PubMed] [Google Scholar]
- 27. Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. [DOI] [PubMed] [Google Scholar]
- 28. Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2014;64(4):1168–1179. [DOI] [PubMed] [Google Scholar]
- 29. Slatis K, Gafvels M, Kannisto K, et al. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res. 2010;51:3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Webster CR, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–G1172. [DOI] [PubMed] [Google Scholar]
- 31. Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem. 2002;277:28578–28583. [DOI] [PubMed] [Google Scholar]
- 32. Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–E393. [DOI] [PubMed] [Google Scholar]
- 34. Meier PJ. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am J Physiol. 1995;269:G801–G812. [DOI] [PubMed] [Google Scholar]
- 35. Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes. (Lond). 2013;37(12):1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glicksman C, Pournaras DJ, Wright M, et al. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010;47:482–484. [DOI] [PubMed] [Google Scholar]
- 37. Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaquero J, Monte MJ, Dominguez M, Muntane J, Marin JJ. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 2013;86:926–939. [DOI] [PubMed] [Google Scholar]
- 39. Boesjes M, Bloks VW, Hageman J, et al. Hepatic farnesoid X-receptor isoforms α2 and α4 differentially modulate bile salt and lipoprotein metabolism in mice. PLoS One. 2014;9:e115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jansen PL, Strautnieks SS, Jacquemin E, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. [DOI] [PubMed] [Google Scholar]
- 41. van Mil SW, van der Woerd WL, van der Brugge G, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379–384. [DOI] [PubMed] [Google Scholar]
- 42. Vaz FM, Paulusma CC, Huidekoper H, et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology. 2015;61(1):260–267. [DOI] [PubMed] [Google Scholar]
- 43. Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. [DOI] [PubMed] [Google Scholar]
- 44. Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]