Abstract
Context:
Chronic sex steroid deficiency has effects on adipose fatty acid (FA) storage mechanisms and fat oxidation, but the chronology of events are not well understood.
Objective:
The objective of the study was to examine the acute effects of female sex steroid suppression on cellular mechanisms affecting abdominal and femoral subcutaneous adipose tissue FA storage.
Design:
This study had a randomized, longitudinal, parallel study design.
Setting:
The study was conducted at the Mayo Clinic Clinical Research Unit.
Participants:
Thirty-eight nonsmoking premenopausal women aged 18–50 years participated in the study.
Intervention:
The intervention included randomization to receive one of the following: 1) no treatment (control), 2) 3.75 mg of Lupron, or 3) 3.75 mg of Lupron and estrogen, but not progesterone, replacement for 49 days, resulting in at least 4 weeks of sex steroid suppression.
Main Outcome Measures:
Body composition, fat cell size, postprandial chylomicron and nonchylomicron triglyceride concentrations, adipose tissue meal FA storage, direct free fatty acid storage, lipoprotein lipase, acyl CoA synthetase, and diacylglycerol acyltransferase activities, and CD36 content were measured.
Results:
Compared with the control group, the fed state femoral lipoprotein lipase activity was reduced in women taking Lupron and those taking Lupron and estrogen replacement. In addition, we observed significantly greater postprandial chylomicronemia in the Lupron group than in the other two groups. There were no differences in overall fat storage and oxidation. Depending on the mode of data expression (per unit lipid vs per 1000 adipocytes), there were modest changes in acyl CoA synthetase, diacylglycerol acyltransferase, and CD36 in response to acute sex hormone suppression.
Conclusions:
Our results suggest estrogen and progesterone may have different effects on the regulation of FA metabolism and that acute sex steroid deficiency in women does not alter fat storage and oxidation.
Acute female sex steroid deficiency (Lupron) or progesterone deficiency (Lupron + estrogen) blunted the postprandial rise in femoral adipose tissue lipoprotein lipase and exaggerated chylomicronemia.
After menopause the cessation of female sex steroid production is associated with an increase in upper body fat, resulting in a greater trunk to leg fat ratio (1, 2). The roles that estrogen and progesterone play are emphasized by studies showing that hormone replacement in early menopause decreases central adiposity (3). We found that postmenopausal women had up-regulated adipose tissue fatty acid (FA) storage mechanisms and decreased FA oxidation compared with well-matched premenopausal women (4). Because the chronology of these changes is unknown, we investigated the effects of acute female sex steroid suppression on storage of dietary and circulating free fatty acids (FFAs) as well as proteins and enzymes involved in adipocyte FA storage.
Chylomicron and very low-density lipoprotein-triglycerides contribute most FA stored in adipose tissue, whereas the direct FFA reuptake pathway appears to play a role in FA redistribution between adipose depots (5, 6). Lipoprotein lipase (LPL) is required to hydrolyze very low-density lipoprotein and chylomicron-triglyceride into glycerol and FA; the FA can enter the adipocyte via passive (flip-flop) or protein facilitated diffusion (7). A number of proteins and enzymes within the adipocyte are needed to traffic intracellular FA to triglycerides. There is little information regarding the initial effects of loss of female sex steroids on these steps of adipose FA storage.
In this study, we quantitate and integrate measures of meal-derived FA and direct FFA storage with factors regulating adipose FA storage at different tiers: LPL, FA transport protein (CD36), acyl-CoA synthetase (ACS), and diacylglycerol acyltransferase (DGAT). CD36 is a cell-surface protein that enhances cellular FA uptake into cells when concentrations are suppressed (8). Once inside the cell, the ACS enzymes activate FA to long chain acyl-CoA (9). DGAT catalyzes the final step in conversions of FA to triglycerides (10, 11). By examining these proteins in the context of FA storage, we sought to gain insights into the initial changes that occur with estrogen and/or progesterone deficiency in women.
Research Design and Methods
Subjects
Healthy nonsmoking, premenopausal female volunteers aged 18–50 years were randomized to one of three groups: a nonintervention control group, a group administered leuprolide acetate (Lupron [AbbVie]) to suppress sex steroids (Lupron) (12), and a group administered Lupron and estrogen (Climara 0.1 mg transdermal patch) (L+E). All participants were healthy and weight stable (±1.0 kg) for longer than 2 months before the study. Exclusion criteria included diabetes, anemia, taking antidepressants, or taking medications known to affect FA metabolism. Written, informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the Mayo Clinic.
Materials
[l-14C]palmitate and [9,10-3H]triolein were purchased from NEN Life Science Products (PerkinElmer), and 2H2O and [U-13C]palmitate (both 99 atom percentage pure) from Isotec.
Study design
Study visits took place at the Mayo Clinical Research Unit. Participants in the Lupron or L+E groups received treatment for 49 days, which included 3.75 mg of Lupron by im injections on days 1 and 21 of the study. Blood estradiol concentrations were measured on days 1, 21, and 49. Participants randomized to the L+E group were instructed to change the estrogen patch twice a week throughout the treatment period to maintain constant estrogen concentrations. The volunteers filled out a food frequency questionnaire (Viocare Inc) before and at the end of the treatment period and wore a pedometer (Omron HJ-112; Omron Healthcare Inc) throughout the treatment period to monitor physical activity. Body weight was measured weekly during the 49-day treatment period, after which participants underwent the inpatient study. The control group participants underwent the inpatient study immediately.
For all groups, total body water and body composition were measured just prior to the inpatient study. In the Lupron and L+E groups, body composition was also measured prior to and at the end of their 49-day treatment. For the 5 days before the inpatient study, participants were provided with all meals to ensure comparable and consistent macronutrient intake as well as weight stability (13). We used our previously described (13) protocol to measure fatty acid metabolism. Briefly, at 8:00 am the morning after admission, participants consumed an experimental meal containing [3H]triolein. Blood samples were collected and indirect calorimetry (DeltaTrac) measurements were performed hourly for 6 hours. Urine was collected over a 24-hour period for nitrogen and 3H2O excretion to measure protein and meal fat oxidation, respectively. The morning after the second night, direct FFA storage rates were measured via iv-administered FFA tracers. Abdominal and femoral adipose tissue biopsies were performed on the first day at 2:00 pm (1 h after lunch) and on the second day at 30 minutes after the bolus infusion of [1-14C]palmitate. Blood samples for the plasma catecholamine concentrations were collected at 0, 300, and 1440 minutes during the study day.
Assays and methods
Plasma triglyceride concentrations and 3H content in chylomicron and nonchylomicron fractions were measured as previously described (14). Urinary nitrogen was measured using an Analox GM7 (Analox Instruments). Plasma catecholamines were measured by HPLC (15), triglycerides with a microfluorometric assay (16), insulin using a chemiluminescent assay on an automated immunoassay system (Assay and DxI; Beckman Instruments), and estrogen via liquid chromatography/mass spectrometry.
Body composition
Fat-free mass (FFM), total body fat, and leg fat mass were measured via dual-energy x-ray absorptiometry (DXA) (Lunar iDXA; GE Healthcare) (17). Visceral fat mass was measured using a single-slice abdominal computed tomography scan at the L2–3 level combined with DXA-measured total abdominal fat content (18).
Substrate oxidation
Participants underwent a 12-hour fast prior to measuring resting energy expenditure (REE) (13). Indirect calorimetry measurements were collected as described above. Carbohydrate and fat oxidation at each time point were calculated at each time point, and total oxidation was determined as the area under the curve (AUC) (19).
Fatty acid metabolism studies
Meal FA storage and oxidation were determined as previously described (13). Briefly, at 8:00 am, participants consumed an Ensure Plus meal that provided 40% of individually measured REE and contained 50 μCi [9,10-3H]triolein (20). Lunch (1:00 pm) and supper (6:00 pm) meals had the same macronutrient composition as those provided during the week before admission. The 3H2O concentration in body water was measured in urine collected after a 24-hour void (13). A continuous infusion of [U-13C]palmitate was used to measure FFA flux (21) and combined with a bolus infusion of [1-14C]palmitate followed by adipose biopsies to measure direct adipose tissue FFA storage rates (22). After lipid extraction, adipose 3H and 14C lipid-specific activity (disintegrations per minute per gram of lipid) were measured as previously described (23).
Adipose tissue analysis
Fat cell size was measured using photomicrographs (24), LPL activity (25), ACS (26), and DGAT (11) activities were determined using enzyme activity assays and CD36 by a sandwich ELISA (27) of whole-tissue extract. On average, less than 5% of ACS, DGAT, and CD36 are found in the stromovascular fraction (28). Thus, the whole-tissue data are representative of adipocyte activity.
Calculations, data analysis, and statistics
Because the way the data are expressed can affect the data interpretation, we express our data in two distinct ways. The per-unit lipid expression allows us to understand whether one body fat depot competes better for fatty acid storage than another depot. The data expressed per 1000 adipocytes are used to examine the factors that regulate adipose tissue FA storage rates at the level of the cell. The calculations have been described in detail elsewhere (13, 29). The Shapiro-Wilk test was performed for goodness of fit and data that were not normally distributed were log transformed. A one-way ANOVA was used for between-group comparisons. A mixed-model 2 and 3 level ANOVA with post hoc least squares means tests was used to determine group differences using time and depot as within-group variables. Relationships between FFA storage and adipogenic proteins were determined using Pearson correlations. Mixed multivariate regressions were used with group as a factor to further examine whether acute estrogen suppression affected the relationship between variables. All data are presented as mean ± SEM and were analyzed using JMP 9.0 (SAS Institute). Statistical significance was defined as P < .05.
Results
Subject characteristics
Participant characteristics are provided in Table 1. A total of 39 women were randomized into the study, but one woman in the Lupron group was excluded due to noncompliance. The food frequency questionnaire data showed no group × time interactions. Women in the Lupron group took more steps per day (8360 ± 724 vs 6171 ± 723; P = .04) than those in the L+E group at week 1, and there was a group × time interaction (P = .047) such that those in the L+E group increased their number of steps at week 7 (6171 ± 723 vs 6908 ± 693), whereas those in the Lupron group (8360 ± 724 vs 7856 ± 701) had decreased their number of steps per day. Visceral fat decreased in the L+E group by 0.13 ± 0.07 kg and increased in the Lupron group by 0.14 ± 0.08 kg (group × time interaction, P = .03). There was also a slight decrease (P = .03) in FFM in the Lupron group. The decrease in FFM was not significant in the L+E group and there was no group × time interaction.
Table 1.
Subject Characteristics
| Control (n = 13) | L+E (n = 13) |
Lupron (n = 12) |
|||
|---|---|---|---|---|---|
| Week 1 | Week 7 | Week 1 | Week 7 | ||
| Age | 34 ± 3 | 34 ± 3 | 33 ± 3 | ||
| Weight, kg | 77.0 ± 3.6 | 70.7 ± 3.5 | 70.5 ± 3.7 | 69.5 ± 1.9 | 69.2 ± 2.0 |
| BMI, kg/m2 | 26.5 ± 1.2 | 25.7 ± 1.2 | 25.6 ± 1.3 | 25.8 ± 0.9 | 25.7 ± 0.9 |
| Fat, % | 36.7 ± 1.8 | 35.6 ± 1.7 | 35.1 ± 1.8 | 37.0 ± 1.9 | 36.8 ± 1.9 |
| Fat, kg | 28.1 ± 2.6 | 25.2 ± 2.5 | 24.8 ± 2.6 | 25.0 ± 1.8 | 25.0 ± 1.8 |
| UBSQ, kg | 14.7 ± 1.6a | 13.4 ± 1.6 | 13.2 ± 1.8a | 13.5 ± 1.3 | 13.4 ± 1.3a |
| LBSQ, kg | 11.3 ± 0.8 | 10.1 ± 0.8 | 10.1 ± 0.8 | 9.8 ± 0.5 | 9.7 ± 0.5 |
| Visceral fat, kg | 2.1 ± 0.4 | 1.7 ± 0.2b | 1.6 ± 0.2 | 1.7 ± 0.3 | 1.8 ± 0.3 |
| Abdominal fat cell size, μg lipid/cell | 0.64 ± 0.11a | 0.51 ± 0.07a | 0.53 ± 0.06a | ||
| Femoral fat cell size, μg lipid/cell | 0.98 ± 0.12 | 0.82 ± 0.07 | 0.82 ± 0.05 | ||
| Estradiol, pg/mL | 145 ± 73 | 95 ± 25 | 181 ± 58 | 119 ± 25 | Undetectable |
| Week 3 | N/A | 147 ± 15 | Undetectable | ||
| Fasting insulin, μU/L | 4.6 ± 0.7 | 4.5 ± 0.9 | 5.4 ± 0.6 | ||
| Fasting plasma glucose, mg/dL | 90 ± 2c | 93 ± 2c,d | 96 ± 2d | ||
| Plasma epinephrine, pg/mL | 34 ± 10 | 33 ± 7 | 30 ± 6 | ||
| Plasma norepinephrine, pg/mL | 167 ± 11 | 163 ± 20 | 192 ± 11 | ||
| Plasma palmitate, μmol/L | 101 ± 5 | 107 ± 9 | 123 ± 9 | ||
| Palmitate flux, μmol/min | 83 ± 8 | 72 ± 6 | 74 ± 5 | ||
Abbreviation: BMI, body mass index. Values are mean ± SEM. Estrogen concentrations were not random variables and therefore not subject to statistical testing.
P < .05 between upper and lower body within groups.
P < .05 for group × time interaction in L+E and Lupron groups.
P < .05 between groups.
The three groups of women were well matched for age, body mass index, and body composition (Table 1) for the inpatient study days. By design, serum estrogen concentrations were greater in the L+E and control groups than the Lupron group. Baseline insulin was not different between groups, whereas fasting plasma glucose was greater (P = .02) in the Lupron group than in the control group. Plasma AUC insulin concentrations, the average plasma catecholamine concentrations, and overnight postabsorptive plasma palmitate concentrations and flux were not different between groups (Table 1).
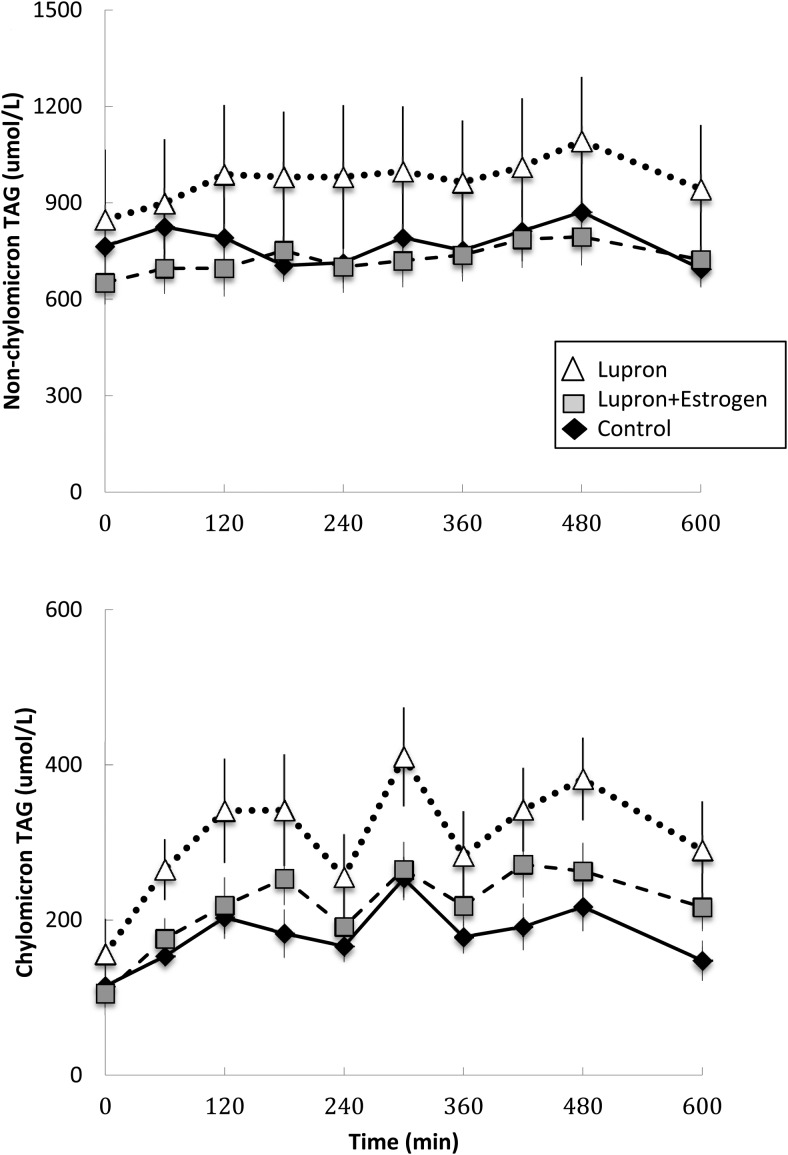
The AUC of plasma chylomicron triglyceride concentrations was greater (P = .02) in the Lupron than the control group and marginally (P = .14) greater than the L+E group. Nonchylomicron triglyceride concentrations did not differ between the groups (Figure 1).
Figure 1.
Daytime plasma triglyceride concentrations. Plasma nonchylomicron (top panel) and chylomicron triglyceride concentrations (lower panel) during the first 10 hours of the experimental meal day. Open triangles, Lupron women; black diamonds, control women; gray squares, L+E women. The AUC of plasma chylomicron triglyceride concentrations in the Lupron group was greater (P = .02) than the control group and tended to be greater (P = .14) than the L+E group. There were no significant between-group differences in the nonchylomicron triglyceride concentrations.
Substrate and meal FA oxidation
Total fatty acid oxidation for 6 hours after breakfast (indirect calorimetry) and 24-hour meal fatty acid oxidation (3H2O generation) were not different between the three groups (Table 2). Overnight basal metabolic rate and respiratory exchange ratio was also not different between groups. Thus, from an energy/substrate oxidation perspective, the three groups were metabolically similar on the study days.
Table 2.
Energy and FA Metabolism
| Control (n = 13) | L+E (n = 13) | Lupron (n = 12) | |
|---|---|---|---|
| REE, kcal/d | 1657 ± 27 | 1628 ± 44 | 1579 ± 46 |
| Baseline respiratory exchange ratio | 0.83 ± 0.01 | 0.83 ± 0.01 | 0.82 ± 0.01 |
| 6-Hour substrate oxidation | |||
| Carbohydrate, g | 69 ± 4 | 64 ± 4 | 62 ± 3 |
| Fat, g | 14 ± 2 | 14 ± 1 | 15 ± 1 |
| Protein, g | 22 ± 1 | 22 ± 2 | 21 ± 1 |
| 24-Hour meal FA oxidation | |||
| Grams | 7.8 ± 0.5 | 8.6 ± 0.5 | 8.5 ± 0.5 |
| Percentage | 40 ± 2 | 45 ± 3 | 46 ± 1 |
| Abdominal | Femoral | Abdominal | Femoral | Abdominal | Femoral | |
|---|---|---|---|---|---|---|
| 24-Hour meal FA storage, mg/g lipid | 0.48 ± 0.05 | 0.47 ± 0.06 | 0.51 ± 0.09 | 0.38 ± 0.05 | 0.44 ± 0.05 | 0.33 ± 0.05 |
| Rate of direct FFA storage | ||||||
| micromoles/kg adipose tissue/min | 0.26 ± 0.03 | 0.30 ± 0.03 | 0.25 ± 0.02 | 0.24 ± 0.02 | 0.25 ± 0.04 | 0.26 ± 0.04 |
| × 10−4, pmol/1000 cells/min | 1.62 ± 0.35a | 2.99 ± 0.54 | 1.27 ± 0.24a | 2.02 ± 0.26 | 1.24 ± 0.16a | 2.09 ± 0.26 |
| Adipose tissue FA storage, % | UBSQ | LBSQ | UBSQ | LBSQ | UBSQ | LBSQ |
|---|---|---|---|---|---|---|
| Meal FA | 31 ± 2 | 25 ± 3 | 28 ± 3a | 19 ± 3 | 28 ± 3a | 17 ± 3 |
| Direct FFA | 4.5 ± 0.7 | 4.2 ± 0.5 | 4.7 ± 0.6a | 3.5 ± 0.3 | 4.3 ± 0.4 | 3.4 ± 0.4 |
Values are mean ± SEM. Six-hour substrate oxidation refers to the indirect calorimetry data collected, beginning just before and for 6 hours after the experimental breakfast meal. Twenty-four-hour meal FA storage and oxidation refers to isotope-measured 3H-triolein disposal into adipose tissue (as assessed by biopsies and generation of 3H2O) 24 hours after consumption of the experimental breakfast meal. Direct adipose FFA storage rates are based on isotope dilution measures using adipose biopsies and palmitate kinetics.
P < .05 between depots within a group.
Regional meal and direct FA (palmitate) storage
There was a trend (P = .056) for meal FA storage (milligrams per gram of lipid) to be greater in the abdominal than femoral region in the L+E group (Table 2). The proportion of meal FA stored in upper body sc fat (UBSQ) was greater (P = .003 and P =.0009, respectively) than in the lower body sc fat (LBSQ) in the L+E and Lupron groups. In the control group, there was only a trend (P = .06) for percentage meal FA storage to be greater in UBSQ than LBSQ.
Rates of FFA palmitate storage are presented as micromoles per kilogram of adipose tissue per minute for physiological interpretation and picomoles per 1000 cells per minute to examine mechanisms at the cellular level. There was effect (P = .04) of depot on the rates of direct palmitate fatty acid storage (picomoles per 1000 cells per minute), with femoral greater than abdominal storage in all three groups (Table 2). There was no effect of group, depot, or group × depot on the rates of direct FFA storage at the physiological level (micromoles per kilogram of adipose tissue per minute).
Adipose specific effectors of FA storage
LPL activity
Fasting LPL activity was greater (P < .05) in femoral than abdominal fat in all three groups (Table 3). In the fed state, femoral LPL was greater (P < .0001 and P = .04, respectively) than abdominal LPL activity in the control and Lupron groups and there was a trend (P = .07) for fed LPL activity to be greater in femoral than abdominal fat in the L+E group. Fed LPL activity in the femoral region was greater (P < .05) in the control than in the Lupron and the L+E groups. There were no differences between the Lupron and the L+E groups. Abdominal LPL activity increased (P < .05) from the fasted to the fed state in all groups and in the femoral fat depot in the control group only.
Table 3.
Adipose Tissue Characteristics
| Control (n = 13) |
L+E (n = 13) |
Lupron (n = 12) |
||||
|---|---|---|---|---|---|---|
| Abdominal | Femoral | Abdominal | Femoral | Abdominal | Femoral | |
| LPL activity, μmol/g tissue/h | ||||||
| Fasted | 0.78 ± 0.21a,b | 1.95 ± 0.38b | 0.71 ± 0.19a,b | 1.40 ± 0.25 | 0.79 ± 0.16a,b | 2.13 ± 0.38 |
| Fed | 1.85 ± 0.19a | 3.46 ± 0.38c | 1.31 ± 0.25 | 1.89 ± 0.24 | 1.71 ± 0.21a | 2.41 ± 0.34 |
| Adipocyte factors | ||||||
| ACS, pmol/mg lipid/min | 74.6 ± 8.4 | 82.8 ± 7.1 | 78.6 ± 12.3 | 74.2 ± 5.5 | 73.9 ± 8.6 | 74.8 ± 6.6 |
| DGAT, pmol/mg lipid/min | 7.1 ± 0.8 | 6.4 ± 0.6 | 8.2 ± 1.5a | 6.4 ± 0.6 | 7.3 ± 1.1 | 5.8 ± 0.7 |
| CD36, relative units/mg lipid | 10.7 ± 1.7 | 11.2 ± 1.0 | 8.8 ± 0.83a | 11.4 ± 1.0 | 8.8 ± 0.8a | 12.3 ± 1.4 |
| ACS, pmol/1000 cells/min | 40.8 ± 4.3a | 77.7 ± 8.7c | 31.9 ± 3.5a | 59.0 ± 4.9 | 34.0 ± 2.5a | 59.2 ± 4.8 |
| DGAT, pmol/1000 cells/min | 3.8 ± 0.3a | 6.0 ± 0.8 | 3.2 ± 0.3a | 5.1 ± 0.5 | 3.3 ± 0.3a | 4.5 ± 0.4 |
| CD36, relative units/1000 cells | 6.0 ± 0.9a | 10.8 ± 1.5 | 4.4 ± 0.9a | 9.5 ± 1.3 | 4.5 ± 0.5a | 9.6 ± 1.0 |
Values are mean ± SEM.
P < .05 between depots within a group.
P < .05 across time (ie, fasted vs fed).
P < .05 within depot in control group vs Lupron and L+E groups.
LPL activity and meal FA storage
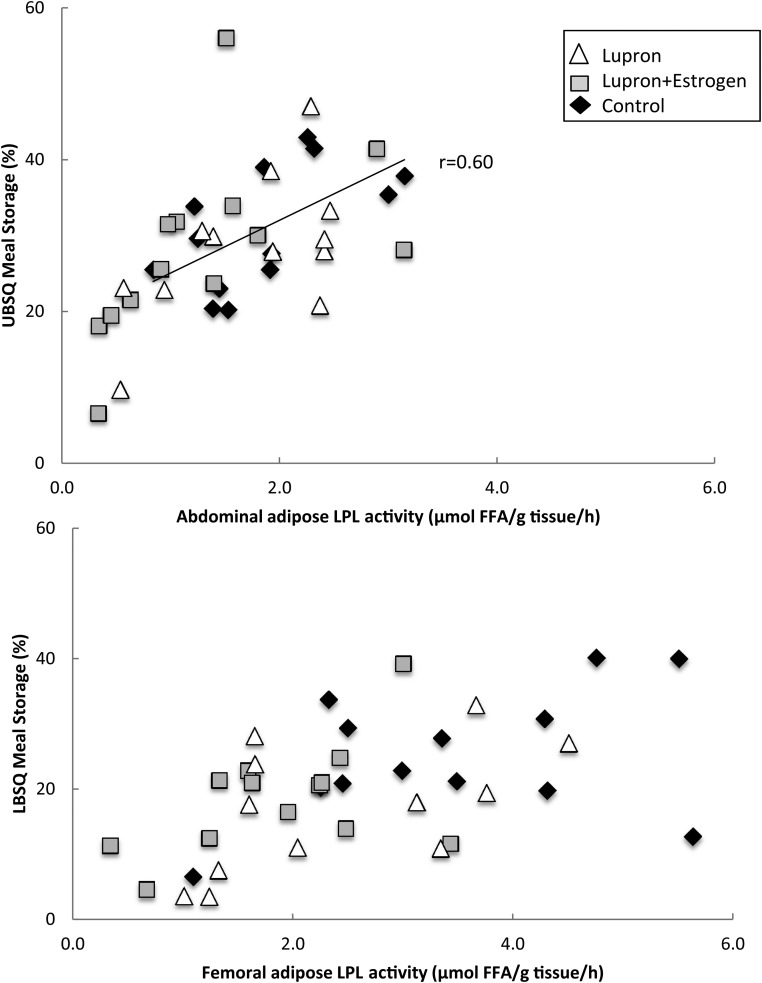
For the three groups combined, there were significant correlations between the fed LPL activity and the proportion of meal FA stored in both the abdominal and femoral depot (Figure 2; r = 0.56, P = .0002, and r = 0.54, P = .0005, respectively). When subdivided into the individual smaller groups, only the fed, abdominal LPL activity in the control group correlated with fractional meal FA storage (r = 0.60, P = .03). However, the trends remained for positive correlations between abdominal LPL activity and fractional meal FA storage in the L+E and Lupron groups (r = 0.54, P = .054, and r = 0.57, P = .055, respectively). When divided into subgroups, none of the correlations between the fed LPL activity and the proportion of meal FA stored in the femoral fat remained statistically significant. Multivariate regression analyses indicated that the fed LPL activity, but not the group, predicted (r2 = 0.33 and r2 = 0.32, P < .01 for both) the meal fatty acid storage in the UBSQ and LBSQ region.
Figure 2.
Regional LPL activity vs regional meal FA storage. Fed LPL activity in the abdominal (top panel) and femoral (lower panel) is plotted vs the proportion of meal FAs stored in the UBSQ and the LBSQ adipose tissue. Open triangles, Lupron women; black diamonds, control women; gray squares, L+E women. For the three groups combined, there were significant correlations between the fed LPL activity and the proportion of meal FA stored in both the abdominal and femoral depot (r = 0.56, P = .0002, and r = 0.54, P = .0005, respectively). When subdivided into the individual, only fed, abdominal LPL activity in the control group remained significantly correlated with fractional meal FA storage (r = 0.60, P = .03).
Adipocyte FA storage factors
Fasting adipose tissue CD36 content, ACS, and DGAT activity data are provided in Table 3. At the cellular level, ACS and DGAT activity and CD36 content were significantly greater in the femoral than abdominal depot in all groups. Femoral adipose ACS activity per 1000 cells was greater in the control group women than the L+E and Lupron women (P = .01 vs L+E and P = .02 vs Lupron). Physiologically (per milligram of lipid), DGAT activity was greater (P = .04) in the abdominal than femoral region in the L+E group only. Femoral CD36 content was greater than the abdominal CD36 content per milligram of lipid in the L+E and Lupron groups (P = .045 and P = .01, respectively).
Adipocyte proteins vs direct FFA palmitate storage rates
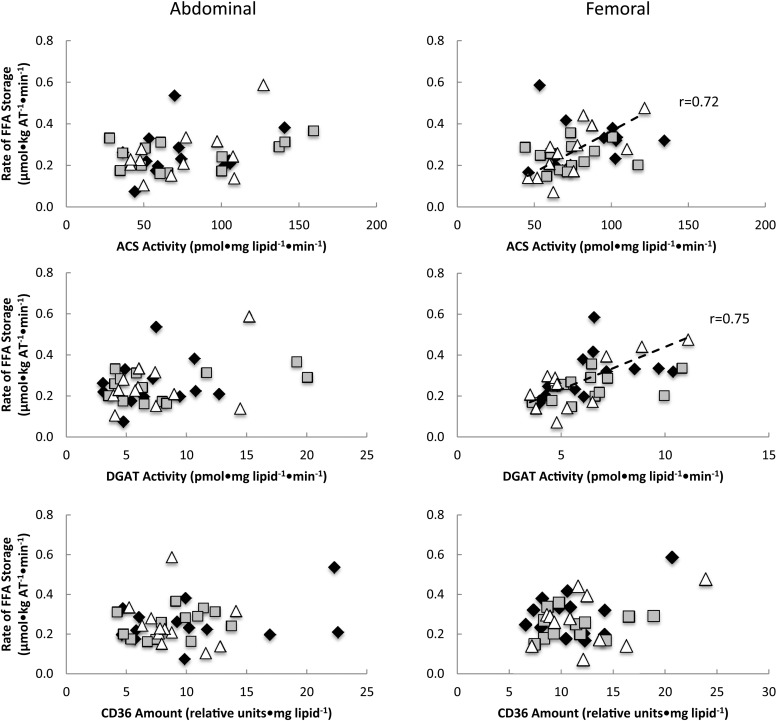
The activities of femoral ACS and DGAT (per milligram of lipid) were correlated with palmitate storage rates (per kilogram of adipose tissue) for women in the Lupron group (r = 0.72, P = .008, and r = 0.75, P = .005, respectively) but not the control or L+E groups (Figure 3). There were no significant associations between abdominal adipocyte proteins (per milligram of lipid) and palmitate storage rates (per kilogram of adipose tissue) in any group.
Figure 3.
Relationships between abdominal and femoral adipose tissue FA storage factors vs direct FFA storage rates expressed relative to adipocytes. Regional rates of FFA storage (micromoles per kilogram of fat per minute) vs lipogenic protein activity expressed per milligram of lipid per minute. Open triangles, Lupron women; black diamonds, control women; gray squares, L+E women. The dashed line represents a relationship between activity and rate of FFA storage in Lupron women. The activity of ACS and DGAT was significantly correlated with the rate of FA storage in the femoral region of women in the Lupron (r = 0.72, P = .008, and r = 0.75, P = .005, respectively) but not the control or L+E groups. No associations were found between adipocyte proteins (per milligram of lipid) and the rate of FA storage (per kilogram of adipose tissue) in the abdominal region in any group.
Multivariate regression analysis using palmitate concentrations and FA storage factors as independent variables revealed that palmitate concentrations and abdominal ACS activity (per milligram of lipid) predicted (r2 = 0.24, P = .03 and P =.01 for palmitate and ACS, respectively) the rates of adipose FFA-palmitate storage (per kilogram of adipose tissue). Abdominal DGAT activity and palmitate concentrations also predicted (r2 = 0.24, P = .02 and P =.01 for palmitate and DGAT activity) direct FFA-palmitate storage. Femoral adipose ACS activity and palmitate concentrations predicted direct palmitate FA storage in thigh fat (r2 = 0.22, P = .046, for palmitate and P = .01 for ACS). Rates of femoral adipose FFA storage were also predicted by DGAT activity and palmitate concentrations (r2 = 0.39, P = .02 and P =.0001, respectively). The predictive variables remained significant when group was included as a factor.
Relationship between adipocyte FA storage factors
We found relationships between abdominal ACS and DGAT (r2 = 0.75, P < .0001), ACS and CD36 (r2 = 0.13, P = .03), and DGAT and CD36 (r2 = 0.16, P = .02). For thigh adipose, there was a relationship (r2 = 0.50, P < .0001) between ACS and DGAT only. The inclusion of group as a factor did not affect these relationships. For abdominal and thigh fat, ACS and DGAT activity (per milligram of lipid) were correlated in all groups. For the Lupron group only, femoral CD36 was correlated with DGAT (r = 0.80, P = .0003) and tended to correlate with ACS (r = 0.57, P = .068). Abdominal CD36 was also correlated (r = 0.62, P = .02) with DGAT for the control group women.
Discussion
This appears to be the first study examining how acute suppression of female sex steroids affects in vivo fatty acid storage and its underlying mechanisms. Healthy premenopausal women were randomized to a control group, Lupron (acute estrogen and progesterone deficiency), or L+E (isolated progesterone deficiency) to study the effects of sex steroids. We found changes in body composition and factors that regulate adipose FA storage, supporting the concept that female sex steroids modulate adipose tissue function. Visceral fat increased in the Lupron compared with the L+E group, and there was a slight but significant decrease in FFM in the Lupron group. Both the Lupron and L+E women failed to increase femoral LPL in the postprandial state relative to control women, whereas abdominal LPL increased similarly in all three groups. Plasma chylomicron triglyceride concentrations were significantly greater in the Lupron than the control group. Finally, femoral adipose ACS activity per 1000 cells was significantly greater in the control group women than the L+E and Lupron women.
The visceral fat gain in the Lupron group relative to the L+E group is consistent with reports of increased upper body fat as a result of the menopausal transition (30). The differing visceral fat responses in the Lupron vs estrogen-replete L+E group suggest that the loss of estrogen, rather than progesterone, is responsible. Despite the changes in visceral fat mass, body composition parameters were similar between the groups at the time of the fat metabolism study.
The elevated postbreakfast chylomicron triglyceride concentrations in the Lupron group suggest reduced clearance, perhaps related to lesser femoral adipose LPL. This finding is consistent with the chylomicron triglyceride responses in postmenopausal women (4). Our data suggest that abdominal LPL activity is not female sex steroid dependent, whereas femoral LPL activity is. Femoral LPL activity in the fed state was less in both the Lupron and L+E groups than in the control group, suggesting a potential role for progesterone in the increase in femoral adipose tissue responses to meal ingestion. Of note, chylomicron triglyceride concentrations were not as elevated in the L+E group despite similarly reduced fed-state femoral LPL. This may indicate that other factors, such as adipose blood flow, contribute to chylomicron triglyceride clearance. Dysregulation in adipose blood flow has been observed in postmenopausal women (31). However, whether it is progesterone that modulates adipose blood flow is unclear.
In contrast to our observation that postmenopausal women had lower fat oxidation than premenopausal women (4), we found no differences in fat oxidation between the three groups. Previous studies have shown that transdermal estrogen repletion for 6 months does not change body composition or fat oxidation (32), whereas fat oxidation increased after 12 months of transdermal estrogen supplementation (33). Therefore, it appears that chronic female sex steroid deficiency is necessary to alter fat oxidation.
We also examined the effects of acute sex hormone suppression on adipocyte storage factors. At the physiological level, we saw no differences in ACS activity between groups or depots, whereas in our previous study, both pre- and postmenopausal women had greater femoral than abdominal ACS activity (4). These results indicate that chronicity of the condition or age may be factors in determining physiological ACS activity. DGAT activity was greater in the abdominal vs femoral depot only in the L+E group, suggesting that progesterone may affect regional differences in the DGAT activity. We previously noted that DGAT activity was greater in postmenopausal women in both depots (4), whereas with acute progesterone or estrogen + progesterone deficiency, DGAT was not different from control women. Again, this may indicate that the chronicity of the deficiency, or age per se may be playing a role.
Femoral ACS activity per 1000 cells was lower in the Lupron and L+E than the control women, whereas we previously found that abdominal ACS activity was higher in postmenopausal women (4). Perhaps the responses to acute female sex steroid deficiency are to attenuate femoral ACS, whereas longer-term effects are to increase abdominal adipose ACS activity. At the cellular level, femoral ACS and DGAT activity, as well as CD36 content, was greater than in the abdominal adipose tissue, consistent with our previous study in pre- and postmenopausal women (4). This suggests that female sex steroids do not affect the relative regional activity of these adipogenic proteins at the cellular level.
Unlike with chronic female sex steroid deficiency (4), we found no effect of acute sex hormone suppression on regional meal fatty acid storage. With regard to direct FFA storage, although there were no differences at the physiological level, at the cellular level, femoral was greater than abdominal direct FFA storage in every group, again consistent with our previous finding in pre- and postmenopausal women (4). However, at the physiological level, postmenopausal women had greater femoral FFA storage than premenopausal women (4), indicating again that shifts in meal FA and direct FFA storage require a more chronic hypogonadal state.
A major strength of this study is in the study design that allows us to tease out the acute effects of estrogen + progesterone deficiency vs progesterone deficiency on proteins involved in FA storage. Although it would have been ideal to include a Lupron and progesterone group to examine the acute effects of progesterone in absence of estrogen, to some extent, our three groups allowed us to indirectly examine the effects of progesterone. The examination of FA storage and its associated mechanisms in both the thigh and abdomen gave us a better picture of the effects of acute hypogonadism in women. Had we examined only abdominal fat, we would have missed the changes in femoral LPL activity in the Lupron and L+E groups. Some of our findings in acute hypogonadism were not consistent with those in our previous study of chronic hypogonadism (4). One explanation for these differences is that the women in this study had a mean age of 34 years, which was 16 years younger than the pre- and postmenopausal women previously studied, who had a mean age of 50 years.
In summary, acute hypogonadism changes adipogenic storage factors and increases chylomicronemia prior to any changes in overall fat storage and oxidation. The results of this study help delineate the early mechanisms underlying fat storage alterations with menopause and may lead to strategies to prevent the associated fat gain. We found that femoral rather than abdominal adipogenic factors respond to acute sex hormone suppression, and our results suggest different effects of estrogen and progesterone in regulating fatty acid metabolism.
Acknowledgments
We are indebted to the research volunteers for their participation. We are also grateful to Barbara Norby and Carley Vrieze for the assistance with nursing care and adipose biopsies and Christy Allred, Debra Harteneck, Darlene Lucas, and Lendia Zhou for the assay development and performance. The Lupron used in the study was kindly provided by AbbVie Inc.
Author contributions included the following: S.S. contributed to the study design, performed the studies, researched the data, and wrote the manuscript. S.B. contributed to the summary and the analysis of data. M.D.J. designed and oversaw the study, reviewed and edited the manuscript, and is the guarantor of the work.
This work was supported by National Center for Research Resources Grant 1UL1 RR024150, National Institutes of Health Grants DK45343, DK40484, and DK50456, and American Diabetes Association Grant 7-06-DCS-03. S.S. received support from the Natural Sciences and Engineering Research Council of Canada, and Canadian Diabetes Association incentive funding and is the recipient of a Canada Research Chair, Tier 2 in Clinical Nutrition.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACS
- acyl-CoA synthetase
- AUC
- area under the curve
- DGAT
- diacylglycerol acyltransferase
- DXA
- dual-energy x-ray absorptiometry
- FA
- fatty acid
- FFA
- free fatty acid
- FFM
- fat-free mass
- LBSQ
- lower body sc
- L+E
- Lupron and estrogen
- LPL
- lipoprotein lipase
- REE
- resting energy expenditure
- UBSQ
- upper body sc.
References
- 1. Gambacciani M, Ciaponi M, Cappagli B, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. [Erratum 1997;82(12):4074] 1997;82:414–417. [DOI] [PubMed] [Google Scholar]
- 2. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuksel H, Odabasi AR, Demircan S, Koseoglu K, Kizilkaya K, Onur E. Effects of postmenopausal hormone replacement therapy on body fat composition. Gynecol Endocrinol. 2007;23:99–104. [DOI] [PubMed] [Google Scholar]
- 4. Santosa S, Jensen MD. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes. 2013;62:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santosa S, Jensen MD. Why are we shaped differently, and why does it matter? Am J Physiol. 2008;295:E531–E535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne). 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–2269. [DOI] [PubMed] [Google Scholar]
- 8. Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N. Regulation of FAT/CD36 gene expression: further evidence in support of a role of the protein in fatty acid binding/transport. Prostagland Leukotrienes Essent Fatty Acids. 1997;57:17–21. [DOI] [PubMed] [Google Scholar]
- 9. Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2:465–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity. 2009;17:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. [DOI] [PubMed] [Google Scholar]
- 13. Santosa S, Jensen MD. Effects of male hypogonadism on regional adipose tissue fatty acid storage and lipogenic proteins. PloS One. 2012;7:e31473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in non-obese humans. Am J Physiol. 2000;279:E455–E462. [DOI] [PubMed] [Google Scholar]
- 15. Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem. 1981;116:223–226. [DOI] [PubMed] [Google Scholar]
- 16. Humphreys SM, Fisher RM, Frayn KN. Micromethod for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem. 1990;27:597–598. [DOI] [PubMed] [Google Scholar]
- 17. Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. [DOI] [PubMed] [Google Scholar]
- 18. Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. [DOI] [PubMed] [Google Scholar]
- 19. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. [DOI] [PubMed] [Google Scholar]
- 20. Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in human adipose tissue: technical and experimental design issues. Am J Physiol. 2000;279:E447–E454. [DOI] [PubMed] [Google Scholar]
- 21. Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56:1369–1375. [DOI] [PubMed] [Google Scholar]
- 23. Marin P, Rebuffe-Scrive M, Bjorntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest. 1990;20:158–165. [DOI] [PubMed] [Google Scholar]
- 24. Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res. 2003;44:1795–1801. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17:536–541. [PubMed] [Google Scholar]
- 26. Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278:43008–43013. [DOI] [PubMed] [Google Scholar]
- 27. Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res. 2011;52:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hames KC, Koutsari C, Santosa S, Bush NC, Jensen MD. Adipose tissue fatty acid storage factors: effects of depot, sex and fat cell size. Int J Obes (Lond). 2015;39:884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol. 1949;2:1–15. [DOI] [PubMed] [Google Scholar]
- 30. Santosa S, Jensen MD. Sex and sex steroids: impact on the kinetics of fatty acids underlying body shape. Horm Mol Biol Clin Investig. 2014;20:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson J, Sjostrom LG, Karlsson M, et al. Dysregulation of subcutaneous adipose tissue blood flow in overweight postmenopausal women. Menopause. 2010;17:365–371. [DOI] [PubMed] [Google Scholar]
- 32. O'Sullivan AJ, Crampton LJ, Freund J, Ho KK. The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest. 1998;102:1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. dos Reis CM, de Melo NR, Meirelles ES, Vezozzo DP, Halpern A. Body composition, visceral fat distribution and fat oxidation in postmenopausal women using oral or transdermal oestrogen. Maturitas. 2003;46:59–68. [DOI] [PubMed] [Google Scholar]