Abstract
Context:
The spectrum of lipid-induced changes in the secretion of hormones important in energy homeostasis has not yet been fully elucidated.
Objective:
To identify potential incretin-like effects in response to lipid administration, we examined the short-term effect of iv vs oral lipids on key molecules regulating energy homeostasis.
Design, Intervention, and Participants:
After a 10-hour overnight fast, 26 subjects were randomized to receive an oral lipid load, a 10% iv lipid emulsion, a 20% iv lipid emulsion, or an iv saline infusion. We obtained blood samples at 30-minute intervals for the first 2 hours and hourly thereafter for a total of 6 hours.
Main Outcome Measures:
Circulating levels of insulin, glucose, c-peptide, free fatty acids, incretins (glucagon-like peptide-1, gastric inhibitory polypeptide), glucagon, peptide YY, ghrelin, fibroblast growth factor 21, fetuin A, irisin, omentin, and adiponectin were measured.
Results:
Oral lipid ingestion resulted in higher glucagon-like peptide-1, gastric inhibitory polypeptide, glucagon, and peptide YY levels, compared with the other three groups (incremental area under the curve P = .003, P < .001, P < .001, P < .001, respectively). The 20% lipid emulsion, leading to higher free fatty acid levels, resulted in greater insulin, c-peptide, and fibroblast growth factor 21 responses compared with placebo and the other two groups (incremental area under the curve P = .002, P = .005, P < .001, P < .001, respectively). Omentin, adiponectin, fetuin A, and irisin levels were not affected by either mode of lipid administration.
Conclusions:
Metabolic responses to lipids depend on the route of administration. Only iv lipids trigger a dose-dependent fibroblast growth factor 21 secretion, which is nonglucagon mediated. Intravenous lipids also induce hyperinsulinemia without concurrent decreases in glucose, a phenomenon observed in insulin-resistant states. Orally administered lipids mostly affect gastrointestinal tract-secreted molecules important in glucose and energy homeostasis such as glucagon, incretins, and peptide YY.
Our study demonstrates that metabolic responses to lipids depend on the route of administration. Only iv lipids trigger a dose-dependent FGF-21 secretion whereas orally administered lipids mostly affect gastrointestinal tract-secreted molecules such as glucagon, incretins, and peptide YY.
Obesity is closely linked to hyperlipidemia, and both are major risk factors for the development of insulin resistance and type 2 diabetes mellitus. Whereas acute exposure to free fatty acids (FFAs) enhances glucose- and nonglucose-induced insulin secretion, chronic exposure to high levels of FFAs is associated with impaired glucose-induced insulin response (1, 2). Hyperlipidemia also leads to the accumulation of unoxidized long-chain fatty acids and ectopic lipid deposition in liver, muscle, and pancreatic islets that further impairs overall metabolism and health (3). Our knowledge about the impacts of hyperlipidemia on human metabolism largely derives from studies on molecular responses to exogenous lipid administration. More recently it has been suggested that the pattern of molecular responses to lipids depends largely on the route of administration (4). For instance, Lindgren et al showed a direct effect of lipid ingestion on incretins, such as gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), and the lack of an incretin response when lipids were administered iv (4). Apart from this study, which compared only the incretin response after oral and iv lipid administration, there is lack of placebo-controlled studies on the impact of exogenous lipids (iv and oral) on molecules important in energy homeostasis (5–9).
The way lipid ingestion vs lipid infusion may impact novel molecules secreted by tissues commonly affected in insulin-resistant states such as liver and muscle have not yet been studied. For instance, whereas the hepatokine fibroblast growth factor 21 (FGF-21) has been largely examined in association with FFAs (10, 11), little is known about the effect of lipids on the novel hepatokine fetuin A. Similarly, it has not yet been elucidated how hyperlipidemia induced by oral as compared with iv lipids alters the physiological regulation of muscle-secreted molecules such as the myokine irisin (12) and fat-secreted molecules such as omentin, a promising cardiovascular marker closely related to lipoprotein profile (13).
This is a placebo-controlled study to differentiate the effects of oral vs iv lipid administration on molecular parameters related to insulin metabolism and diabetes (insulin, c-peptide, glucagon, GLP-1, and GIP) and obesity (appetite regulators such as peptide YY [PYY], ghrelin, and leptin). We also investigate potential effects on novel molecules secreted by tissues affected by insulin resistance (ie, muscle irisin, liver FGF-21, and fetuin A and adipose tissue leptin, adiponectin, and omentin). Furthermore, we examined whether there might be a dose-dependent effect of lipids by comparing two concentrations of iv lipid emulsions as well as by administering them for a longer period of time as compared to previously published studies (4).
Materials and Methods
Study protocol, participants, and ethics
Participants were recruited from clinics at Beth Israel Deaconess Medical Center through the use of an institutional review board-approved flyer and web-based advertisement. Subjects were chosen without regard for race or socioeconomic status. Males and females between 18 and 65 years of age with a wide range of body mass indices (BMIs; BMI > 18 kg/m2) were included. Written informed consent was obtained at a screening visit.
One hundred subjects were screened; 33 passed the in-person screen and 26 subjects returned for the study visit. All menstruating females participated during the early follicular phase (d 4–6) of their menstrual cycle. Based on a registered dietitian's assessment at screening, subjects were provided with weight-maintaining take-home meals, which they consumed during the 48 hours prior to the main study visit to ensure stable dietary intake. During the main study visit, which consisted of an overnight stay at the clinical research center, registered nurses took vital signs and anthropometric measurements. After a 10-hour overnight fast, subjects were randomized to one of four groups: 1) oral lipid group (n = 6), 2) 20% intralipid iv lipid emulsion group (n = 6), 3) 10% intralipid iv lipid emulsion group (n = 5) and 4) control group (saline infusion) (n = 9) (see Supplemental Materials and Methods for more information). Blood samples were obtained at baseline and at 30-minute intervals for the first 2 hours and then hourly thereafter. Collected blood was centrifuged immediately, and plasma was stored in tubes containing dipeptidyl peptidase IV inhibitors.
Subjects with history of conditions, other than obesity, that could affect insulin sensitivity and lipid metabolism were excluded from the study. Exclusion criteria are presented in detail as Supplemental Materials and Methods.
Laboratory methods
FGF-21 and glucagon levels were determined using an ELISA from R&D Systems (14). Assays from Millipore were used to measure levels of GLP-1, leptin, and adiponectin (RIA) (15) as well as GIP, PYY, and ghrelin (ELISA). Fetuin A and omentin-1 levels were determined with an ELISA from BioVendor (16, 17), whereas irisin was tested with an ELISA from Phoenix Pharmaceutical (18). FFA levels were measured using an ELISA from WAKO Diagnostics (19). A Siemens Immulite1000 (Siemens Healthcare Diagnostics) was used to measure insulin and c-peptide. Glucose was tested with a Cobas c311 clinical chemistry analyzer (Roche Diagnostics). All samples were analyzed in duplicate. Samples that yielded a coefficient of variability above 15% were excluded from our analysis.
Statistical analysis
Because no prior randomized studies had examined the hypothesis addressed herein in an in vivo human model after iv and oral lipid administration, we performed this study and determined our sample size on the basis of a priori assumptions about any effects of lipids from prior nonrandomized studies (20, 21). The statistical analysis was done in an unblinded manner. Statistical Package for the Social Science (SPSS), version 17 (IBM) was used for analysis. Data are presented as means ± SD if normally distributed or as medians (with first and third quartile) if nonnormally distributed. Graphs show percentage changes from baseline. Normality was assessed with graphical methods (pp plots and histograms). Nonnormally distributed data were log transformed or ranked. Comparisons of the response curves of the molecules after the four interventions were performed using the repeated-measures analysis of covariance, testing for a group effect. Post hoc comparisons were performed using Bonferroni tests.
Because of the broad BMI range of the participants, we also checked for a molecule × trial × BMI category interaction, in which the BMI category was defined as a BMI less or more than 25 kg/m2 and a BMI less or more than 27 kg/m2. No significant interactions were observed and further analysis per BMI category was not followed. Total and incremental areas under the curve (AUC and iAUC, respectively) were calculated as well. For the calculation of total AUC, the trapezoid rule was used (sum of the areas under and over the baseline), whereas iAUC was defined as AUC − (baseline × 360 min)/2. The AUC and iAUC were compared by a univariate ANOVA, and Bonferroni post hoc tests were used to compare groups. Data were analyzed using both total AUC and iAUC. We, however, chose to present our data using iAUCs to depict the effect of the intervention excluding differences of baseline values between the groups (22). Results using the AUC are summarized in Supplemental Table 1. Additional analyses were performed with adjustments for FFA levels. Results are reported as significant if the value was P < .05.
Results
Baseline characteristics
The baseline clinical and laboratory characteristics of our 26 participants are summarized in Table 1.
Table 1.
Baseline Characteristics of Four Groups (10% and 20% iv Lipid Emulsion, Oral Lipid Group, and Placebo Group)
| Characteristics | 10% iv Intralipids (n = 5) | 20% iv Intralipids (n = 6) | Oral Fat (n = 6) | Placebo (n = 9) |
|---|---|---|---|---|
| Age, y | 48.6 (44.4, 54.9) | 29.1 (24.2, 38.7) | 35.3 (25.0, 47.6) | 27.6 (24.2, 46.9) |
| Gender, % female | 20.0 | 16.7 | 50.0 | 22.2 |
| Weight, kg | 74.0 (66.2, 98.9) | 78.4 (62.0, 91.0) | 78.0 (63.2, 91.4) | 67.0 (63.6, 78.1) |
| BMI, m/kg2 | 27.4 ± 6.3 | 25.3 ± 3.6 | 26.4 ± 4.7 | 23.8 ± 3.6 |
| Glucose, mg/dL | 90.3 ± 3.1 | 83.0 ± 12.0 | 92.4 ± 6.4 | 83.6 ± 8.6 |
| Insulin, mU/mL | 2.08 (2.03, 14.19) | 5.10 (2.10, 7.10) | 7.12 (4.86, 11.95) | 4.14 (3.56, 6.76) |
| C-peptide, ng/mL | 1.03 (0.96, 2.14) | 1.43 (0.80, 1.73) | 1.57 (1.23, 1.61) | 1.17 (0.96, 1.49) |
| HDL, mg/dL | 56.2 ± 21.5 | 65.7 ± 21.3 | 54.3 ± 18.5 | 54.2 ± 14.7 |
| LDL, mg/dL | 103.0 (86.5, 111.5) | 84.5 (80.5, 112.3) | 103.5 (70.0, 145.3) | 87.0 (62.5, 103.5) |
| Total cholesterol, mg/dL | 184.0 ± 15.4 | 171.0 ± 13.2 | 186.7 ± 36.5 | 154.2 ± 24.1 |
| TGs, mg/dL | 95.0 (67.5, 235.5) | 62.0 (49.8, 74.8) | 70.5 (61.8, 157.0) | 53.0 (46.0, 94.5) |
| FFAs, mEq/L | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.3 |
| Leptin, ng/mL | 11.5 (3.8, 33.3) | 6.4 (1.4, 20.8) | 8.2 (4.4, 14.9) | 2.92 (1.87, 14.0) |
| Adiponectin, ng/mL | 5178.8 ± 2408.2 | 6447.0 ± 2896.6 | 5586.3 ± 2940.2 | 4752.9 ± 1603.1 |
| Irisin, ng/mL | 262.4 (247.4, 337,6) | 253.0 (221.1, 333.9) | 258.5 (236.0, 304.3) | 231.8 (215.4, 310.1) |
| GLP-1, pM | 28.8 ± 17.5 | 27.4 ± 13.3 | 21.7 ± 8.2 | 15.3 ± 9.7 |
| GIP, pg/mL | 28.2 (18.7, 149.5) | 35.3 (26.2, 41.7) | 24.4 (21.6, 54.7) | 32.6 (26.1, 45.9) |
| Glucagon, pg/mL | 44.9 (19.8, 83.5) | 59.4 (15.0, 72.5) | 55.9 (13.5, 93.1) | 40.7 (26.7, 89.3) |
| Omenti, ng/mL | 351.4 ± 177.2 | 334.2 ± 154.3 | 281.4 ± 86.6 | 309.0 ± 76.9 |
| Fetuin-A, ng/mL | 250 570.0 (233 045.0, 255 025.0) | 247 945.0 (191 692.5, 280 800.0) | 240 260.0 (170 735.0, 270 287.5) | 242 285.0 (219 560.0, 281 897.5) |
| Ghrelin, pg/mL | 629.2 (336.8, 850.1) | 406.2 (287.8, 562.9) | 706.8 (324.0, 1084.7) | 370.5 (233.8, 668.4) |
| PYY, pg/mL | 272.9 (191.0, 362.8) | 254.5 (189.2, 269.1) | 239.6 (204.3, 262.6) | 276.2 (221.6, 393.2) |
| FGF-21, pg/mL | 132.0 (61.2, 434.9) | 129.4 (78.9, 170.0) | 102.3 (67.5, 166.4) | 35.5 (30.9, 86.0) |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride. Data are presented as mean ± SD if normally distributed or as median (first and third quartile) if not normally distributed.
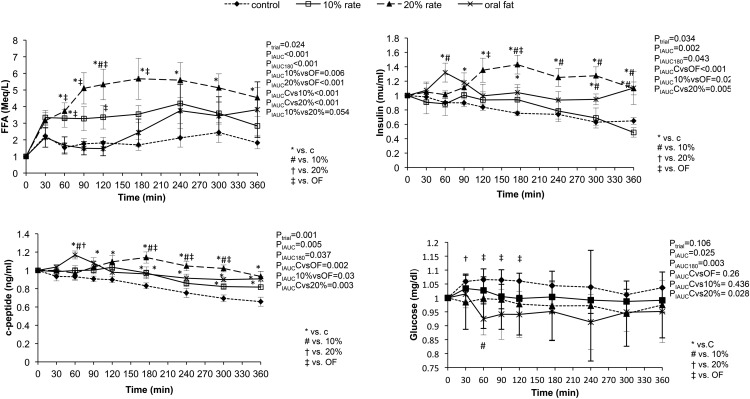
Response of molecular parameters related to insulin resistance in the four groups (Figure 1)
Figure 1.
Changes in glucose, insulin, c-peptide, and FFA levels after administration of oral, iv oral, and iv lipids (10% and 20% intralipids) and placebo. Statistical analysis was performed using the 180- and 360-minute iAUC.
We found an initial increase in insulin levels for the oral lipid group that peaked 60 minutes after lipid ingestion. Insulin levels then decreased and reached a plateau after 120 minutes. The high iv lipid group exhibited a significant increase in insulin levels after 120 minutes until the end of the 360-minute experimental period as compared with the other groups (PiAUC < .05 for all). In addition, the 360-minute iAUC was statistically higher after the administration of the high iv lipid emulsion as compared with low (10%) iv lipid emulsion and placebo (PiAUC < .002). The c-peptide levels showed the same pattern as insulin (PiAUC < .005). Glucose levels showed a more subtle change in the four groups (PiAUC < .11). The change was more pronounced in the first 180 minutes during which glucose significantly decreased in the oral lipid group as compared with the two iv lipid groups (PiAUC-180 < .04 in both cases). Comparing the two iv lipid groups, the 180-minute iAUC for glucose was statistically lower in the high iv lipid group as compared with the low iv lipid group (PiAUC-180 ≤ .001). The same pattern was observed when comparing the high iv lipid group with the placebo group (PiAUC-180 = .01). Glucagon levels continuously rose in the oral lipid group and were significantly increased as compared with all other groups (PiAUC ≤ .0001) (Figure 2).
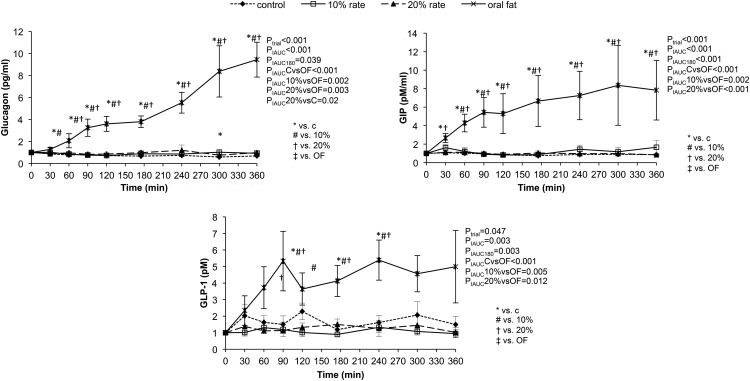
Figure 2.
Response to oral and iv lipids (10% and 20% intralipids) and placebo administration of glucagon, GLP-1, and GIP. Statistical analysis was performed using the 180- and 360-minute iAUC.
FFA levels in the four groups (Figure 1)
FFAs were significantly elevated in both iv lipid groups as compared with the oral lipid and placebo groups (20% iv lipid vs oral, PiAUC ≤ .001; 20% iv lipid vs placebo, PiAUC ≤ .001; 10% iv lipid vs oral lipid, PiAUC < .006; 10% iv lipid vs placebo, PIauc ≤ .001). Comparing the two iv lipid groups, there was a statistically significant dose-dependent increase in achieved FFA levels, with the high iv group achieving higher FFA levels (20% iv lipid vs 10% IV lipid, PiAUC < .05). After 240 minutes, the FFA levels from oral lipid ingestion and 10% iv lipid emulsion reached approximately the same levels, as per the study design.
Response of incretins to lipid administration (Figure 2)
We observed a statistically significant increase of both GIP and GLP-1 levels in the oral lipid group as compared with both the iv and placebo groups. GIP levels began increasing immediately after oral ingestion and continued rising for the rest of the study, with the 360-minute iAUC being significantly higher than the other three groups (oral lipid vs placebo, PiAUC ≤ .001; oral lipid vs 10% iv lipid, PiAUC < .002; oral lipid vs 20% iv lipid, PiAUC ≤ .001). The GIP levels remained unchanged over time in the other three groups. The GLP-1 levels also increased significantly after oral ingestion, but the pattern of response was different from that of GIP. There were two peaks in GLP-1 circulating levels observed at 90 and 240 minutes after the first ingestion of the oral lipids. Conversely, the infusion of high or low concentration iv lipids did not lead to changes in GLP-1 levels. The 360-minute iAUC of GLP-1 in the oral group was significantly higher than the other three groups (oral lipid vs placebo, PiAUC ≤ .001; oral lipid vs 10% iv lipid, PiAUC < .005; oral lipid vs 20% iv lipid, PiAUC < .01).
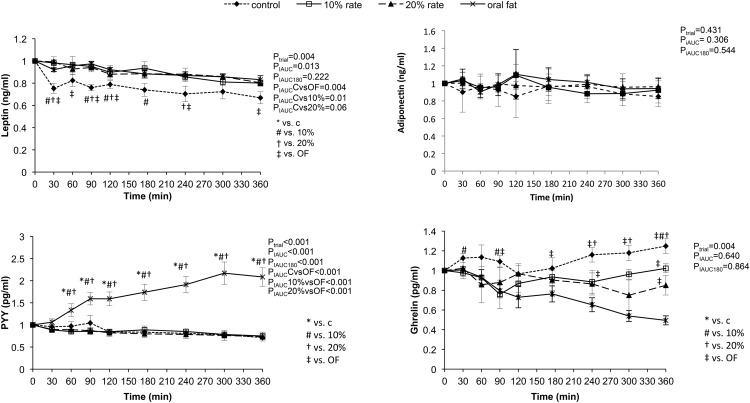
Response of appetite regulators (Figure 3)
Figure 3.
Response of appetite regulators PYY, ghrelin, adiponectin, and leptin to administration of oral and iv lipid as well as placebo. Statistical analysis was performed using the 180- and 360-minute iAUC.
Oral lipid ingestion led to an increase in PYY levels (PiAUC < .001), but there was a lack of PYY response after iv lipids or placebo. Ghrelin was increased in the placebo group and remained significantly elevated as compared with the other groups (PiAUC < .05 for all post hoc comparisons indicated in Figure 2). Ghrelin responses were similar across the oral and iv lipid groups. Leptin levels decreased significantly in the placebo group as compared with the oral lipid and iv lipid groups (PiAUC = .013).
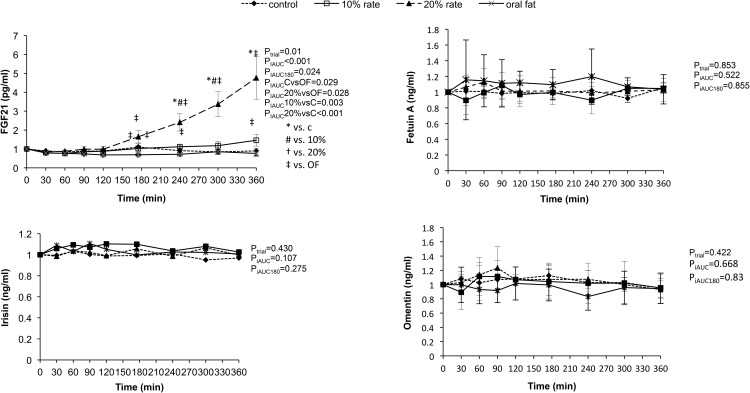
Response of novel hepatokines, myokines, and adipokines (Figure 4)
Figure 4.
Response of FGF-21, fetuin A, omentin, and irisin to oral and iv lipid administration as well as placebo. Statistical analysis was performed using the 180- and 360-minute iAUC.
FGF-21 increased significantly in the high iv lipid group as compared with the other groups (PiAUC ≤ .001), but this difference disappeared when we adjusted for FFA levels (PiAUC < .172, data not shown). There was a modest increase of FGF-21 in the low iv lipid group, but it was not as prominent as in the high iv lipid group, suggesting a dose-dependent response of FGF-21. The levels of the hepatokine fetuin A did not change with oral or iv-induced hyperlipidemia. We also report that levels of the myokine irisin did not change with oral or iv lipids. The novel adipokines adiponectin and omentin also remained unaffected by lipid administration.
Discussion
This is a placebo-controlled study that systematically examined the response of molecules important in energy homeostasis to exogenous lipids administered either orally or iv. We found that the metabolic response was dependent on the route of lipid administration. Intravenous lipids had a direct, dose-dependent effect on liver-secreted FGF-21 but not fetuin A as well as on pancreatic β-cells, causing insulin secretion that was not mediated by incretins and without a concomitant decrease in glucose levels, a pattern commonly encountered in insulin-resistant states (23). Conversely, oral lipids triggered an incretin-mediated insulin response, resulting in a nonsignificant decrease in glucose levels and an appropriate increase in the anorexigenic molecule PYY and leptin. Both of these physiological responses have beneficial effects on metabolic equilibrium. Well-known appetite modulators such as leptin and ghrelin displayed appropriate responses to lipid ingestion without differing responses to route or dose. Other novel myokines, such as irisin, and adipokines, such as omentin and adiponectin, remained unaffected by lipid administration, and this lack of response persisted, even with the administration of a high concentration of iv lipids.
The role of lipids in regulating insulin/glucose
The role of lipids in insulin physiology is complex, with chronic exposure to elevated levels of FFAs having detrimental effects on β-cell function (24). Insulin secretion after lipid ingestion occurs both through the direct actions of lipids on β-cells and through the indirect actions of incretins (4). Here we report an incretin (GLP-1 and GIP) mediated initial nonsustained increase in insulin levels with a concomitant decrease in glucose levels in the oral lipid group. Conversely, there was a late but sustained nonincretin-mediated increase in insulin levels to high iv lipid infusion. This late insulin response was not accompanied by a decrease in glucose levels, a phenomenon commonly observed in insulin-resistant conditions. Furthermore, this response was not observed with the low iv lipid infusion, which suggests a dose-dependent response. The increase of glucagon in the oral fat group is an unexpected finding, given the known glucagon-suppressing actions of GLP-1 (25). This response of glucagon could be interpreted as a counterregulatory response to the decreasing levels of glucose and/or as a dual effect of lipids on glucagon secretion, a hypothesis that requires further study. A study by Christensen et al (26) has shown that glucagon secretion can be triggered by GIP during fasting or hypoglycemic conditions with no effect on insulin secretion, and hence, the glucagon response may be induced by GIP. In contrast to the oral lipid group and in agreement with the findings of Lindgren et al (4), we reported no change in GLP-1 and GIP levels after the administration of iv lipids. This was an expected finding, given the fact that the gastrointestinal tract was bypassed with the more direct iv route. In addition to lipids, Lindgren et al studied the incretin effect of oral and iv amino acids and reported GIP but not GLP-1 response to oral amino acids alone, and this response was specific to orally administered lipids only (27).
Impacts of lipids on appetite regulators/energy homeostasis
We then examined the effects of oral and iv lipids on appetite regulators. The anorexigenic molecule PYY is cosecreted with GLP-1 from enteroendocrine L cells, and, similar to GLP-1, it is released postprandially after glucose, amino, and/or lipid ingestion (28). Its secretion is blunted under dyslipidemic conditions such as obesity and type 2 diabetes (29). Although the effects of oral lipid ingestion on PYY have been previously reported (6), we are the first to show unaffected PYY levels with iv lipid administration. Insulin resistance and diabetes have also been associated with low levels of the orexigenic molecule of ghrelin (30). We observed a decline in ghrelin levels in the lipid groups, but these differences were not statistically significant. The adipose tissue-secreted molecule of leptin decreased in the control group. This may be due to the lack of FFA response in this group and/or to prolonged fasting because they ingested and received saline only during the 6-hour blood collection period (31).
Effects of lipids on hepatokines: FGF-21 and fetuin A
Hyperlipidemia is associated with excessive ectopic fat accumulation in tissues such as the liver, which in turn exacerbates insulin resistance (32). The underlying mechanisms of this correlation between liver pathology, hyperlipidemia, and insulin resistance remain to be fully elucidated. Newly identified hepatokines seem to be significant metabolic regulators (33). Insulin-resistant or fasting states in which elevated FFAs are commonly encountered are characterized by elevated FGF-21 levels (10, 11). In our placebo-controlled study, we compared FGF-21 levels after oral, iv lipid and iv saline administration and reported an elevation of FGF-21 in the groups that received iv lipids and achieved higher FFA levels. As noted above, lipid infusions were accompanied by hyperinsulinemia, and hence, the effects of hyperinsulinemia and hyperlipidemia on FGF-21 secretion are difficult to distinguish. Mai et al (10) studied FGF-21 levels administering either iv lipids or placebo on healthy subjects as well as type 1 persons with diabetes, and their findings support the notion that insulin is not required for the FFA-induced increase of FGF-21. Our findings also support this notion, given that the statistically significant change in FGF-21 levels between the iv groups disappeared after we adjusted for FFA (data not shown). According to a study by Arafat et al (34), FGF-21 secretion in healthy and persons with type 1 diabetes while fasting is regulated by glucagon independently of endogenous insulin levels. Based on this physiological observation, we would expect to find an increase in FGF-21 after oral lipid administration because there is a significant increase in glucagon levels. We, however, did not observe an FGF-21 increase in this group, implying that the impacts of circulating glucagon on FGF-21 levels may not be direct and may be more complex than previously suggested. Conversely, FGF-21 levels increased in a nonglucagon-dependent fashion in the iv lipid groups. FGF-21 remained unchanged in the oral lipid group. A study by Matikainen et al (35) showed that the fluctuation of FGF-21 levels after an oral fat load varies, depending on the presence of adipose tissue in the liver and circulating triglyceride levels but not FFA levels. They also reported an initial decrease of FGF-21 levels (35). In our study we excluded subjects with significant hypertriglyceremia, and this may explain the different findings in FGF-21 levels after the oral lipid load.
In addition to FGF-21, we studied the physiological regulation of fetuin A, a molecule secreted from both the liver and adipose tissue and linked with obesity, type 2 diabetes, and nonalcoholic fatty liver disease (36) and report the lack of fetuin A response to elevated FFAs. This may imply that its role in the development of insulin resistance is not mediated by lipids.
Effects of hyperlipidemia on novel myokines and adipokines
Several mechanisms have been proposed for the lipid-induced reduction of insulin activity at the muscle level, but many aspects of the muscle pathophysiology need to be further clarified (37). Irisin, a newly identified myokine responsible for browning of white or beige adipocytes, has been reported to circulate at reduced levels in individuals with type 2 diabetes mellitus and to be associated with obesity, serum triglyceride levels, and intrahepatic triglyceride levels (37). We examined the effect of iv and oral lipid load on irisin and report an absence of irisin response after lipid administration. This is in agreement with the findings of in vitro studies by Sanchez et al (12), who reported a lack of an effect of FFAs on irisin expression in muscle cells.
Last but not least, we examined the potential interplay between adipokines and lipids. Apart from the extensively studied molecule of adiponectin, we examined the novel molecule omentin, a beneficial adipokine with a significant role in the development of insulin resistance and cardiovascular disease (38). Omentin is positively associated with adiponectin and inversely correlated with BMI, triglyceride, and leptin levels (13), whereas animal studies have demonstrated a significant increase in omentin and adiponectin levels with GLP-1 analogs such as exenatide (39). Based on these findings, we were expecting to observe a change in omentin and adiponectin levels after ingestion of oral lipids. We, however, report no changes in the levels of either omentin or adiponectin in any of the groups. Regardless of this finding, their response to a more chronic exposure to lipids may differ from the one observed in our study and will need to be examined with future studies.
Conclusions
In this placebo-controlled study, we examined the physiological response to exogenous lipid administration of novel molecules that are significant in glucose metabolism and energy homeostasis. This is also the first time that the responses to FFAs of novel hepatokines, myokines, and adipokines such as fetuin A, irisin, and omentin, have been studied. The duration of lipid administration was lengthier as compared with previous studies and allowed us to observe the effects of persistent rather than acute exposure to FFAs. However, it remains unclear what the molecular responses would be with even longer durations of sampling/exposure. The sample of our study was relatively small, albeit adequate for the demonstration of statistical significance, and thus, our findings should be confirmed in larger trials. The randomization not infrequently proves to be imperfect for small studies, and thus, some baseline parameters such as age appear to vary herein, but the baseline characteristics in fact matched in the study groups, despite this not being readily obvious from the mean levels provided due to wide variations observed.
In conclusion, metabolic responses to exogenous lipids depend on the route of administration. Prolonged administration of iv lipids triggers hyperinsulinemia without a concurrent decrease in glucose levels, a phenomenon observed in insulin-resistant states. Importantly, only iv lipids trigger a dose-dependent FGF-21 secretion, which is nonglucagon mediated. Orally administered lipids mostly affect gastrotestinal tract-secreted molecules important in glucose and energy homeostasis such as PYY and incretins as well as glucagon. Other novel molecules important in energy homeostasis such as irisin, omentin, and fetuin A remained unaffected by lipids. Furthermore, in vivo and in vitro studies are warranted to fully explore mechanisms underlying the alterations in levels of the molecules reported herein and the potential pathophysiological implications of these findings as well as the role of novel energy regulators in states of hyperlipidemia.
Acknowledgments
The study had a clinical trial registry number of NCT01520454.
Authors contributions included the following: M.T.V. conducted the research, wrote the manuscript, and performed the blood analysis; O.-P.H. conducted the research; A.S.-E. conducted the research; A.G. performed the statistical analysis; F.D. performed the blood test analysis; O.M.F. performed the blood analysis and assisted with the manuscript writing; and C.S.M. designed and supervised the research. All authors read and approved the final manuscript.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
This work was supported by Award 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the Veterans Affairs Office of Research and Development. The project was also supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Center for Research Resources. O.M.F. is supported by Training Grant 5T32HD052961.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- total area under the curve
- BMI
- body mass index
- FFA
- free fatty acid
- FGF-21
- fibroblast growth factor 21
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide-1
- iAUC
- incremental area under the curve
- PYY
- peptide YY.
References
- 1. Oprescu AI, Bikopoulos G, Naassan A, et al. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927–2937. [DOI] [PubMed] [Google Scholar]
- 2. Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. [DOI] [PubMed] [Google Scholar]
- 4. Lindgren O, Carr RD, Deacon CF, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96:2519–2524. [DOI] [PubMed] [Google Scholar]
- 5. Robertson MD, Henderson RA, Vist GE, Rumsey RD. Plasma ghrelin response following a period of acute overfeeding in normal weight men. Int J Obes Relat Metab Disord. 2004;28:727–733. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez-Garcia JC, Murri M, Coin-Araguez L, Alcaide J, El Bekay R, Tinahones FJ. GLP-1 and peptide YY secretory response after fat load is impaired by insulin resistance, impaired fasting glucose and type 2 diabetes in morbidly obese subjects. Clin Endocrinol (Oxf). 2014;80:671–676. [DOI] [PubMed] [Google Scholar]
- 7. Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem. 2013;24:1663–1677. [DOI] [PubMed] [Google Scholar]
- 8. Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khoury DE, Hwalla N, Frochot V, Lacorte JM, Chabert M, Kalopissis AD. Postprandial metabolic and hormonal responses of obese dyslipidemic subjects with metabolic syndrome to test meals, rich in carbohydrate, fat or protein. Atherosclerosis. 2010;210:307–313. [DOI] [PubMed] [Google Scholar]
- 10. Mai K, Bobbert T, Groth C, et al. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab. 2010;299:E126–E130. [DOI] [PubMed] [Google Scholar]
- 11. Yu H, Xia F, Lam KS, et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011;57:691–700. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez J, Nozhenko Y, Palou A, Rodriguez AM. Free fatty acid effects on myokine production in combination with exercise mimetics. Mol Nutr Food Res. 2013;57:1456–1467. [DOI] [PubMed] [Google Scholar]
- 13. Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism. 2014;63:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foo JP, Aronis KN, Chamberland JP, Paruthi J, Moon HS, Mantzoros CS. Fibroblast growth factor 21 levels in young healthy females display day and night variations and are increased in response to short-term energy deprivation through a leptin-independent pathway. Diabetes Care. 2013;36:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bluher S, Panagiotou G, Petroff D, et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring). 2014;22:1701–1708. [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Hamnvik OP, Chamberland JP, et al. Circulating alanine transaminase (ALT) and gamma-glutamyl transferase (GGT), but not fetuin-A, are associated with metabolic risk factors, at baseline and at two-year follow-up: the prospective Cyprus Metabolism Study. Metabolism. 2014;63:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamnvik OP, Thakkar B, Chamberland J, Aronis K, Schneider B, Mantzoros CS. Omentin-1 levels are reduced by pharmacologic doses of leptin, but remain unaffected by energy deprivation and display no day-night variation. Int J Obes (Lond). 2015;39:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foo JP, Aronis KN, Chamberland JP, Mantzoros CS. Lack of day/night variation in fibroblast growth factor 21 levels in young healthy men. Int J Obes (Lond). 2015;39:945–948. [DOI] [PubMed] [Google Scholar]
- 20. Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest. 1995;95:2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–2339. [DOI] [PubMed] [Google Scholar]
- 22. Carstensen M, Thomsen C, Hermansen K. Incremental area under response curve more accurately describes the triglyceride response to an oral fat load in both healthy and type 2 diabetic subjects. Metabolism. 2003;52:1034–1037. [DOI] [PubMed] [Google Scholar]
- 23. Saisho Y. β-Cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gravena C, Mathias PC, Ashcroft SJ. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol. 2002;173:73–80. [DOI] [PubMed] [Google Scholar]
- 25. Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297:127–136. [DOI] [PubMed] [Google Scholar]
- 26. Christensen M, Vedtofte L, Holst JJ, Vilsboll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindgren O, Pacini G, Tura A, Holst JJ, Deacon CF, Ahren B. Incretin effect after oral amino acid ingestion in humans. J Clin Endocrinol Metab. 2015;100:1172–1176. [DOI] [PubMed] [Google Scholar]
- 28. Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36): part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. [DOI] [PubMed] [Google Scholar]
- 29. Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring). 2010;18:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Name M, Giannini C, Santoro N, et al. Blunted suppression of acyl-ghrelin in response to fructose ingestion in obese adolescents: the role of insulin resistance. Obesity (Silver Spring). 2015;23:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet. 2008;47:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol Cell Endocrinol. 2015;418(Pt 1):55–65. [DOI] [PubMed] [Google Scholar]
- 33. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144–152. [DOI] [PubMed] [Google Scholar]
- 34. Arafat AM, Kaczmarek P, Skrzypski M, et al. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia. 2013;56:588–597. [DOI] [PubMed] [Google Scholar]
- 35. Matikainen N, Taskinen MR, Stennabb S, et al. Decrease in circulating fibroblast growth factor 21 after an oral fat load is related to postprandial triglyceride-rich lipoproteins and liver fat. Eur J Endocrinol. 2012;166:487–492. [DOI] [PubMed] [Google Scholar]
- 36. Trepanowski JF, Mey J, Varady KA. Fetuin-A: a novel link between obesity and related complications. Int J Obes (Lond). 2015;39:734–741. [DOI] [PubMed] [Google Scholar]
- 37. Eckardt K, Gorgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57:1087–1099. [DOI] [PubMed] [Google Scholar]
- 38. Vu A, Sidhom MS, Bredbeck BC, Kosmiski LA, Aquilante CL. Evaluation of the relationship between circulating omentin-1 concentrations and components of the metabolic syndrome in adults without type 2 diabetes or cardiovascular disease. Diabetol Metab Synd. 2014;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feng WH, Yuan XW, Tong GY, et al. Correlated increase of omentin-1 and adiponectin by exenatide, avandamet and dietary change in diet-induced obese rats. Folia Biol. 2013;59:217–224. [PubMed] [Google Scholar]