Abstract
Context:
Primary hyperparathyroidism with hypercalciuria has not been described in the newborn period.
Objective:
Our objectives are to identify the genetic basis for neonatal primary hyperparathyroidism in a family with 2 affected children.
Subjects:
An African American boy presenting with mild neonatal primary hyperparathyroidism and hypercalciuria was evaluated at The Children's Hospital of Philadelphia. His older brother with neonatal primary hyperparathyroidism had died in infancy of multiple organ failure.
Methods:
We collected clinical and biochemical data and performed exome sequencing analysis on DNA from the patient and his unaffected mother after negative genetic testing for known causes of primary hyperparathyroidism.
Results:
Exome sequencing followed by Sanger sequencing disclosed 2 heterozygous mutations, c.1883C>A, p.(A628D) and c.2786_2787insC, p.(T931fsX10), in the SLC12A1 gene, which was previously implicated in antenatal type 1 Bartter syndrome. Sanger sequencing confirmed the 2 mutations in the proband and his deceased brother; both parents were heterozygous for different mutations and an unaffected sister was homozygous for wild-type alleles.
Conclusions:
These results demonstrate a previously unrecognized association between neonatal primary hyperparathyroidism and mutation of SLC12A1, the cause of antenatal Bartter syndrome type 1, and suggest that the loss of sodium-potassium-chloride cotransporter-2 cotransporter activity influences parathyroid gland function.
We identified biallelic mutations in the SLC12A1 gene, which was previously implicated in antenatal type 1 Bartter syndrome, in DNA from a boy and his deceased brother, both of whom had neonatal primary hyperparathyroidism.
Neonatal hypercalcemia is an uncommon metabolic disorder that is often an early manifestation of familial hypocalciuric hypercalcemia (FHH). In some cases, there is severe hypercalcemia with markedly elevated serum levels of PTH, with skeletal demineralization, hypotonia, and failure to thrive, associated with a low fractional excretion of urinary calcium. This life-threatening form of FHH is termed neonatal severe hyperparathyroidism (NSHPT) and, if untreated, is associated with very high morbidity and mortality (1, 2).
Loss of function mutations in the CASR gene (3) located at 3p13.3 are the most common cause of type 1 FHH and NSHPT. The CASR gene encodes the calcium-sensing receptor (CaSR), a G protein-coupled receptor that is highly expressed in the parathyroid and kidney. In most cases of FHH and NSHPT, these mutations reduce the sensitivity of the CaSR to extracellular calcium, with consequent increased parathyroid secretion of PTH and decreased renal excretion of calcium. FHH (Online Mendelian Inheritance in Man [OMIM] 145980) typically results from heterozygous CASR mutations and in some cases biallelic mutations (3) and is generally asymptomatic. By contrast, NSHPT (OMIM 239200) typically results from homozygous CASR mutations, although in some cases, only a single abnormal allele is present (1, 4–9). Recently, heterozygous loss of function mutations in GNA11 on chromosome 19p13.3 (10) and AP2S1 on chromosome 19q13.3 (11) have been identified as the bases for type 2 and type 3 FHH, respectively. Both of these defects also reduce calcium signaling; the GNA11 gene encodes the α-subunit of the G protein (Gq) that couples CASR to activation of intracellular signaling programs, whereas the AP2S1 gene encodes the σ-subunit of adaptor protein complex 2, which is necessary for clathrin-mediated endocytosis and the recycling of various cell surface proteins including G protein-coupled receptors (12).
By contrast to FHH and NSHPT, primary hyperparathyroidism with hypercalciuria has not been described in the newborn period. Here, we report the identification of compound heterozygous mutations of SLC12A1, the gene encoding the sodium-potassium-chloride cotransporter-2 (NKCC2) that is responsible for antenatal type 1 Bartter syndrome (OMIM 601678), in 2 brothers with neonatal primary hyperparathyroidism, and suggest a relationship between these 2 disorders.
Subjects and Methods
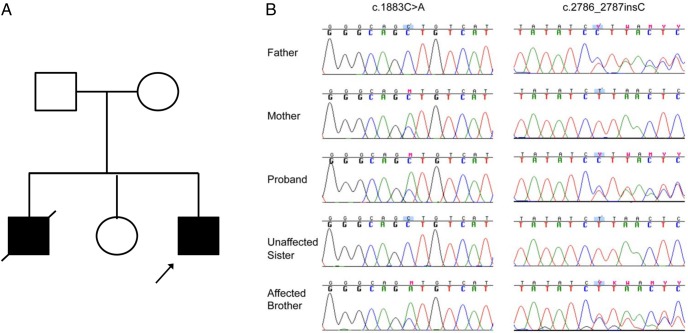
This study included the proband and his deceased affected brother and their unaffected sister and parents (Figure 1A), all of whom provided written informed consent/assent for participation in the study. The study was approved by the institutional review board at The Children's Hospital of Philadelphia. We isolated genomic DNA from blood obtained from the living subjects and from a sample of liver tissue obtained at autopsy of the deceased older brother.
Figure 1.
SLC12A1 mutations with Sanger sequencing in neonatal primary hyperparathyroidism patients. A, Pedigree of the studied family. B, Sanger sequencing confirmed the identified mutations and revealed complete cosegregation with the phenotype.
Case report
The proband is a 12-year-old male who was born to nonconsanguineous parents. The mother had severe polyhydramnios during pregnancy, which led to a premature delivery at 33.5 weeks with birthweight of 2082 g. He was admitted to the neonatal intensive care unit, where he was found to have mild hypercalcemia. Over the next 10 months, he continued to have elevated serum calcium levels (10.6–11.3 mg/dL) with PTH levels that ranged from 4.4 to 18.9 pmol/L (0.7–5.6 pmol/L) and elevated urinary calcium to creatinine ratio of 0.89 to 1.03. His medical history was further complicated by poor feeding, gastroesophageal reflux disease, recurrent upper respiratory tract infections, polyuria, and nephrocalcinosis. By 40 months of age, his serum sodium (136 mmol/L), potassium (2.6 mmol/L), and chloride (96 mmol/L) were low and serum bicarbonate (29 mmol/L) level was elevated. Serum magnesium levels (1.4 mEq/L) were slightly reduced. He had developed radio opaque kidney stones in both kidneys, presumably calcium containing. Table 1 shows relevant laboratory studies. He had been treated with cinacalcet and potassium citrate, with subsequent reduction in PTH and serum calcium. When evaluated at age 9 years, his electrolytes were notable for mildly depressed sodium of 133 mmol/L, slightly increased serum creatinine and mild primary hyperparathyroidism. Subsequent studies over the next several years showed continuous hypercalciuria and elevated serum PTH levels with normal bone mineral density. Plasma renin activity, vasopressin, and urinary prostaglandin E2 levels were elevated, suggesting Bartter syndrome. Neck ultrasound (2008) and parathyroid sestamibi (2009) scans did not reveal a parathyroid adenoma.
Table 1.
Patient Data
| 2009 (Age 6 y) | 2010 (Age 7 y) | 2012 (Age 9 y) | |
|---|---|---|---|
| S Ca (8.8–10.1 mg/dL) | 10.6–11 mg/dL | 9.9 mg/dL | 9–10.4 mg/dL |
| S PTH (9–52 pg/mL) | 115–219 pg/mL | 177 pg/mL | 84–242 pg/mL |
| S Cr (0.1–0.4 mg/dL) | 0.7 mg/dL | 0.7 mg/dL | 0.8 mg/dL |
| U Ca/Cr (<0.25 mg/mg) | 0.51–1.02 mg/mg | 0.51 mg/mg | 0.4–0.5 mg/mg |
| 25(OH)D | 21 ng/mL | 7 ng/mL | 26.9 ng/mL |
| 1,25(OH)2D | 162 pg/mL | 137 pg/mL | 74 pg/mL |
| Arginine vasopressin (0–6.9 pg/mL) | 70 pg/ml | 10.4 pg/mL | |
| Aldosterone (<16 ng/dL) | 9.8 ng/dL | ||
| Plasma renin activity (50–585 ng/dL · h) | 1153 ng/dL · h | ||
| Urinary prostaglandin E2 (400–620 ng/24 h) | 590–735 ng/24 h | ||
| Weight | 19.5 kg (35%tile) | 40.9 kg (96%tile) | |
| Height | 107.2 cm (6%tile) | 124 cm (7%tile) | |
| Bone density L1–4 | |||
| BMD (gm/m2) | 0.47 | 0.6 | |
| Z score (not Ht Z adjusted) | −0.8 | −0.04 |
Abbreviations: S, serum; U, urine; Ca, calcium; Cr, creatinine; BMD, bone mineral density; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
His older brother had also been born prematurely of a pregnancy complicated by polyhydramnios. This child died of multiorgan failure at age of 9 weeks and had hypercalcemia, elevated serum levels of PTH, and renal calcification with multigland parathyroid hyperplasia at autopsy. The parents and a healthy older sister have normal levels of serum calcium and intact PTH. The family history was negative for hyperparathyroidism, hypercalciuria, or kidney stones.
Prior laboratory testing and mutation screening
The proband underwent cytogenetic evaluation by genomic microarray that showed normal male karyotype (46 XY). No evidence of clinically significant numerical or structural chromosome abnormalities was found by fluorescence in situ hybridization analysis on chromosomes 7 and 22, which contain the loci of Williams syndrome and DiGeorge syndrome (13, 14). Initial genetic evaluation included sequencing of CASR, MEN1, CLDN16, and KCNJ1 genes and failed to identify any rare deleterious mutation indicative of hyperparathyroidism, familial hypomagnesemia with hypercalciuria and nephrocalcinosis, or Bartter syndrome type 2 (15–18).
Exome sequencing (ES) and bioinformatic analysis
We performed exome capture and sequencing, as well as read processing, mapping to human genome reference (GRCh37-derived alignment set used in 1000 Genomes Project), variant calling, annotations, and filtering for rare variants affecting the coding sequence and/or consensus splice sites, as previously described. The family pedigree suggests an autosomal recessive mode of inheritance, so we only considered nonsynonymous, splice-altering variants, and frameshift variants cosegregating with the disease in the family with a minor allele frequency less than or equal to 1% in public databases (ie, 1000 Genomes Project and NHLBI ESP6500SI). Subsequent gene prioritization was on basis of deleterious predication, biological and clinical relevance by referring to existing databases (ie, OMIM and Human Gene Mutation Database).
Sanger sequencing
Validation of the mutation candidates detected by ES was performed by Sanger sequencing in all the members of this family whose DNAs were available using standard PCR amplicons with the following primer sets: 1) 5′-AGGATGGAGACCTGCGTAT-3′ and 5′-CATTATCTTACCTGGCTTCTTA-3′ and 2) 5′-GCTATTCATTTCCTTCCATT-3′ and 5′-CCAGATAACCAAGTAAACACG-3′.
Results
We conducted exome sequencing on the proband and his unaffected mother (Figure 1A). We focused our analyses primarily on a gene harboring a rare homozygous or compound heterozygous missense, nonsense, splice-altering variants, and coding indels that are more likely to be pathogenic mutations given the fact there are 2 affected children in this kindred. After standard multiple filtering steps described elsewhere (19) and followed by Sanger sequencing with both parents, we identifies a known substitution (c.1883C>A, p.(A628D)) (20) and a novel frameshift insertion (c.2786_2787insC, p.(T931fsX10)) in SLC12A1 (encoding a protein known as NKCC2) as the most likely fitting disease-causing candidate. An analysis of public databases (1000 Genomes Project, 6503 exomes from the Exome Sequencing Project (ESP6500SI), and Exome Aggregation Consortium dataset; ExAC v0.3) and existing sequencing data from more than 2000 ES samples in our own local database did not disclose an occurrence of either mutation. Sanger sequencing of 5 family members demonstrated cosegregation of the mutation and phenotype (Figure 1B).
Discussion
We describe 2 brothers with neonatal-onset primary hyperparathyroidism that was associated with compound heterozygous mutations in SLC12A1, the gene responsible for antenatal Bartter syndrome. Antenatal Bartter syndrome is a severe and early-onset form of Bartter syndrome that is characterized by maternal polyhydramnios with preterm birth and subsequently polyuria, recurrent vomiting, dehydration, and developmental delay (21, 22). In addition, there is increased production of prostaglandin E2 in affected infants that has led to the alternative term as the hyperprostaglandin E syndrome (23). By contrast, classical Bartter syndrome tends to be less severe and of later onset, presenting from infancy to adolescence. Hypokalemic metabolic alkalosis and hyperreninemia are important biochemical features that are common to all variants, however (24, 25).
A characteristic manifestation of antenatal Bartter syndrome is marked hypercalciuria, and as a secondary consequence, affected infants develop nephrocalcinosis and osteopenia (26). Levels of PTH and 1,25(OH)2D have been reported to be elevated in children with antenatal Bartter syndrome (26–28), and serum calcium levels are occasionally elevated (28). By contrast, plasma calcium levels are usually normal in classic Bartter syndrome, with the exception of rare patients with the type 5 variant that is due to activating mutations of CASR, who have hypocalcemia and very low PTH in addition to the usual biochemical features (16).
The basis for hyperparathyroidism, and in some patients, hypercalcemia, in antenatal Bartter syndrome is uncertain. Although this work was in progress, a patient with overlapping features with SLC12A1 variants was reported (29). The reported patient was a 5-month-old male with nephrocalcinosis at birth, who had diarrhea and vomiting at 3 months with normal levels of sodium, potassium, calcium, and magnesium. At 5 months, he had mildly elevated serum levels of calcium and PTH with an increased urine calcium to creatinine ratio, but hypercalcemia and hypercalciuria were well controlled with iv fluids and oral potassium citrate. The history and progression of hyperparathyroidism described in this patient differs in onset and severity to the patient we report here, however, because hypercalcemia was not present in the newborn period and appeared to be more responsive to conventional treatment. Nevertheless, our work and this previously published report confirm the association between loss of function mutations of SLC12A1 and primary hyperparathyroidism.
The NKCC2 protein is a Na+/K+/2Cl− cotransporter and essential for normal kidney function, which works with other transport proteins to regulate the movement of ions into and out of kidney cells. Although the related NKCC1 cotransporter is widely distributed, the NKCC2 protein is not expressed in the parathyroid gland, however (Roizen J, Levine MA.; submitted for publication). Hence, it is unlikely that loss of NKCC2 function affects parathyroid function directly. It is conceivable, however, that the elevated levels of prostaglandin E2 that are a hallmark of antenatal Bartter syndrome are the basis for increased PTH secretion (30), which accounts for the elevated serum levels of 1,25(OH)2D and, at least in some patients, hypercalcemia. This mechanism would explain the lack of parathyroid adenoma formation in antenatal Bartter syndrome and is consistent with multigland parathyroid hyperplasia seen at autopsy of the proband's brother. Our patient showed improvement in hypercalcemia with age, and a significant decrease in serum levels of PTH and 1,25(OH)2D while taking the calcimimetic cinacalcet, but this appeared to have only a partial effect on urinary calcium excretion (Table 1). It is possible that the very high urinary calcium excretion in antenatal Bartter syndrome provides an important compensatory mechanism that ameliorates hypercalcemia as the child ages.
So far, 63 mutations in SLC12A1 have been described in antenatal Bartter syndrome (31), but to our knowledge, this is the first report of the mutation (c.2786_2787insC, p.T931fsX10) in SLC12A1. Our report refines the molecular and biochemical characterization of antenatal Bartter syndrome and extends the differential diagnosis of neonatal primary hyperparathyroidism.
Acknowledgments
We thank Dr Stuart Chalew (Louisiana State University Health, New Orleans) for providing biochemical and endocrinological data for this patient.
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Disease Grant R01DK079970 (to M.A.L.) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant UL1TR000003 (to M.A.L.). Additional funding for this study was provided by the Institutional Development Fund to the Center for Applied Genomics from The Children's Hospital of Philadelphia (H.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CaSR
- calcium-sensing receptor
- ES
- exome sequencing
- FHH
- familial hypocalciuric hypercalcemia
- NKCC2
- sodium-potassium-chloride cotransporter-2
- NSHPT
- neonatal severe hyperparathyroidism
- OMIM
- Online Mendelian Inheritance in Man.
References
- 1. Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol. 2008;22:129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearce S, Steinmann B. Casting new light on the clinical spectrum of neonatal severe hyperparathyroidism. Clin Endocrinol (Oxf). 1999;50:691–693. [DOI] [PubMed] [Google Scholar]
- 3. Lietman SA, Tenenbaum-Rakover Y, Jap TS, et al. A novel loss-of-function mutation, Gln459Arg, of the calcium-sensing receptor gene associated with apparent autosomal recessive inheritance of familial hypocalciuric hypercalcemia. J Clin Endocrinol Metab. 2009;94:4372–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:275–282. [DOI] [PubMed] [Google Scholar]
- 5. Gunn IR, Gaffney D. Clinical and laboratory features of calcium-sensing receptor disorders: a systematic review. Ann Clin Biochem. 2004;41:441–458. [DOI] [PubMed] [Google Scholar]
- 6. Bouschet T, Henley JM. Calcium as an extracellular signalling molecule: perspectives on the Calcium Sensing Receptor in the brain. C R Biol. 2005;328:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole De, Forsythe CR, Dooley JM, Grantmyre EB, Salisbury SR. Primary neonatal hyperparathyroidism: a devastating neurodevelopmental disorder if left untreated. J Craniofac Genet Dev Biol. 1990;10:205–214. [PubMed] [Google Scholar]
- 8. Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. [DOI] [PubMed] [Google Scholar]
- 9. Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. [DOI] [PubMed] [Google Scholar]
- 10. Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagener BM, Marjon NA, Revankar CM, Prossnitz ER. Adaptor protein-2 interaction with arrestin regulates GPCR recycling and apoptosis. Traffic. 2009;10:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayés M, Magano LF, Rivera N, Flores R, Pérez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet. 2003;73:131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de la Chapelle A, Herva R, Koivisto M, Aula P. A deletion in chromosome 22 can cause DiGeorge syndrome. Hum Genet. 1981;57:253–256. [DOI] [PubMed] [Google Scholar]
- 15. Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe S, Fukumoto S, Chang H, et al. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. [DOI] [PubMed] [Google Scholar]
- 17. Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet. 1997;6:1169–1175. [DOI] [PubMed] [Google Scholar]
- 18. Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. [DOI] [PubMed] [Google Scholar]
- 19. Li D, Weber DR, Deardorff MA, Hakonarson H, Levine MA. Exome sequencing reveals a nonsense mutation in MMP13 as a new cause of autosomal recessive metaphyseal anadysplasia. Eur J Hum Genet. 2015;23:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brochard K, Boyer O, Blanchard A, et al. Phenotype-genotype correlation in antenatal and neonatal variants of Bartter syndrome. Nephrol Dial Transplant. 2009;24:1455–1464. [DOI] [PubMed] [Google Scholar]
- 21. Ammenti A, Montali S. “Neonatal variant” of Bartter syndrome presenting with acidosis. Pediatr Nephrol. 1996;10:79–80. [DOI] [PubMed] [Google Scholar]
- 22. Pierratos A, Couture RA, Hierlihy PJ, Bell RC, Levine DZ. Bartter's syndrome, nephrocalcinosis and renal insufficiency. CMAJ. 1989;141:1055–1057. [PMC free article] [PubMed] [Google Scholar]
- 23. Seyberth HW, Königer SJ, Rascher W, Kühl PG, Schweer H. Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol. 1987;1:491–497. [DOI] [PubMed] [Google Scholar]
- 24. Landau D. Potassium handling in health and disease: lessons from inherited tubulopathies. Pediatr Endocrinol Rev. 2004;2:203–208. [PubMed] [Google Scholar]
- 25. Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006;104:73–80. [DOI] [PubMed] [Google Scholar]
- 26. Leonhardt A, Timmermanns G, Roth B, Seyberth HW. Calcium homeostasis and hypercalciuria in hyperprostaglandin E syndrome. J Pediatr. 1992;120:546–554. [DOI] [PubMed] [Google Scholar]
- 27. Sann L, David L, Bernheim J, François R. Hypophosphatemia and hyperparathyroidism in a case of Bartter's syndrome. Helv Paediatr Acta. 1978;33:299–310. [PubMed] [Google Scholar]
- 28. Deschenes G, Burguet A, Guyot C, et al. [Antenatal form of Bartter's syndrome]. Ann Pediatr (Paris). 1993;40:95–101. [PubMed] [Google Scholar]
- 29. Gross I, Siedner-Weintraub Y, Simckes A, Gillis D. Antenatal Bartter syndrome presenting as hyperparathyroidism with hypercalcemia and hypercalciuria: a case report and review. J Pediatr Endocrinol Metab. 2015;28:943–946. [DOI] [PubMed] [Google Scholar]
- 30. Gardner DG, Brown EM, Windeck R, Aurbach GD. Prostaglandin E2 stimulation of adenosine 3′,5′-monophosphate accumulation and parathyroid hormone release in dispersed bovine parathyroid cells. Endocrinology. 1978;103:577–582. [DOI] [PubMed] [Google Scholar]
- 31. Stenson PD, Ball EV, Mort M, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21:577–581. [DOI] [PubMed] [Google Scholar]