Abstract
Context:
Recent reports have proposed that sporadic or familial germline Xq26.3 microduplications involving the GPR101 gene are associated with early-onset X-linked acrogigantism (XLAG) with a female preponderance.
Case Description:
A 4-year-old boy presented with rapid growth over the previous 2 years. He complained of sporadic headaches and had coarse facial features. His height Z-score was +4.89, and weight Z-score was +5.57. Laboratory testing revealed elevated serum prolactin (185 μg/L; normal, <18 μg/L), IGF-1 (745 μg/L; normal, 64–369 μg/L), and fasting GH > 35.0 μg/L. Magnetic resonance imaging demonstrated a homogenous bulky pituitary gland (18 × 15 × 13 mm) without obvious adenoma. A pituitary biopsy showed hyperplastic pituitary tissue with enlarged cords of GH and prolactin cells. Germline PRKAR1A, MEN1, AIP, DICER1, CDKN1B, and somatic GNAS mutations were negative. Medical management was challenging until institution of continuous sc infusion of short-acting octreotide combined with sc pegvisomant and oral cabergoline. The patient remains well controlled with minimal side effects 7 years after presentation. His phenotype suggested XLAG, but his peripheral leukocyte-, saliva-, and buccal cell-derived DNA tested negative for microduplication in Xq26.3 or GPR101. However, DNA isolated from the pituitary tissue and forearm skin showed duplicated dosage of GPR101, suggesting that he is mosaic for this genetic abnormality.
Conclusions:
Our patient is the first to be described with somatic microduplication leading to typical XLAG phenotype. This patient demonstrates that a negative test for Xq26.3 microduplication or GPR101 duplication on peripheral blood DNA does not exclude the diagnosis of XLAG because it can result from a mosaic mutation affecting the pituitary.
XLAG can result from somatic mutation resulting in mosaicism. Combination of sc octreotide infusion, pegvisomant and cabergoline resulted in well controlled IGF-1 and height velocity.
X-linked acrogigantism (XLAG) is a recently described clinical syndrome of early-onset gigantism with typical onset in the first few months of life and more often affecting females (1–3). The disease is associated with microduplications at Xq26.3 involving the GPR101 gene, which encodes a G protein-coupled orphan receptor. Most of the patients present with a GH and prolactin (PRL)-secreting pituitary adenoma or less commonly GH and PRL cell hyperplasia. The vast majority of the previously described patients required extensive anterior pituitary resection or radiotherapy, resulting in hypopituitarism to control exuberant hormone secretion and growth velocity, because medical management with somatostatin analogs has not been sufficient (3).
Case Report and Methods
A 4-year and 8-month-old Caucasian boy presented to the Montreal Children's Hospital with rapid growth over the preceding 2 years. Born at term with a weight of 4.7 kg (>95th percentile), he had grown steadily along the 90th percentile for length up to 24 months of age and was above the median for weight and head circumference. He had undergone a tonsillectomy and adenoidectomy at the age of 3 years and 10 months. He complained of intermittent headaches over the preceding 8 months, was otherwise asymptomatic, and took no medications. His family history was unremarkable for any endocrinopathies; his midparental height was 174.5 cm. On examination, he appeared older than his stated age, was prepubertal, and had coarse facial features, which had developed over the previous 3 years. His height was 129.7 cm (Z = +4.89), and his weight was 35.5 kg (Z = +5.57) (WHO Growth Charts for Canada; Ref. 4) (Figure 1A).
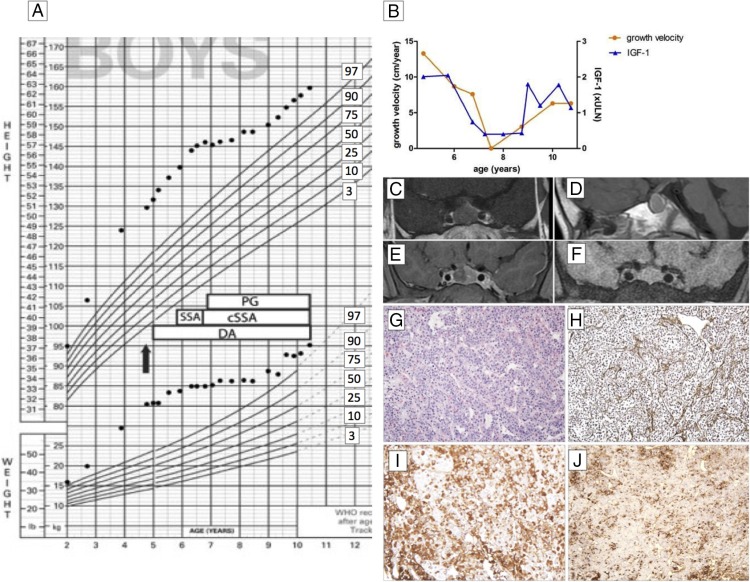
Figure 1.
A, Growth chart before and after surgical and medical interventions. Treatment strategies are indicated as follows: arrow, diagnosis and surgery; DA, dopamine agonist (initially bromocriptine then followed by cabergoline); SSA, somatostatin analog (initially Sandostatin LAR and Somatuline Autogel, then continuous sc infusion [cSSA] administered at 120–320 μg/d dose); and PG, pegvisomant dose, 30–105 mg/wk. B, Growth velocity in centimeters per year (left y-axis, circles) and IGF-1 concentrations (right y-axis, triangles; fold above 95% upper reference limit for age). C—F, Magnetic resonance imaging of the pituitary gland, coronal (C) and sagittal (D) view before surgery, and 5 weeks (E) and 24 months (F) after surgery. G and H, Histological examination of the pituitary tissue demonstrates normal architecture of the gland with preservation of the reticulin network but markedly enlarged cell cords, suggesting diffuse hyperplasia: hematoxylin-eosin staining, ×10 (G); and Gömöri's reticulin stain, ×10 (H). I and J, The gland shows an increase in GH-positive (immunoperoxidase, ×10; I) and PRL-positive cells (immunoperoxidase, ×10; J).
Laboratory investigations revealed elevated serum concentrations of PRL (185 μg/L; normal, <18 μg/L [8043 pmol/L; normal, <788 pmol/L]; Access II; Beckman Coulter), IGF-1 (74.5 μg/L; normal for age, 6.4–36.9 μg/L [9.78 nmol/L, 0.8–4.8]; Immulite), and a fasting GH of > 35.0 μg/L (Access II; Beckman Coulter) (Figure 1B). GH suppressed only to 29.0 μg/L after standard oral glucose load (normal, <1.0). His clinical and biochemical diagnosis was pituitary gigantism with hyperprolactinemia. Magnetic resonance imaging demonstrated a homogeneous sellar mass measuring 18 × 15 × 13 mm with mild suprasellar extension without optic chiasm or cavernous sinus invasion (Figure 1, C and D). Surgical debulking was performed via transfrontal craniotomy. Histological examination of the tissue confirmed the absence of adenoma and demonstrated a hyperplastic pituitary with enlarged cords of GH and PRL cells (Figure 1, G–J). Cells with ultrastructural features resembling mammosomatotrophs were also identified. Genetic analysis was negative for germline AIP, PRKAR1A, DICER1, MEN1, and CDKN1B mutations and for GNAS mutations in both leukocyte and pituitary tissue-derived DNA. Plasma GHRH (Inter Science Institute) levels were normal on two separate samples. His bone age was 5 years.
Postoperatively, his hormone levels remained elevated; the hyperplastic tissue re-expanded rapidly in the fossa (Figure 1, E and F), and his growth continued at an accelerated pace (Figure 1, A and B). His management was challenging. Dopamine agonists proved unsuccessful in suppressing GH hypersecretion; bromocriptine was initiated for the first 3 months but was later replaced with cabergoline (0.25–0.5 mg/wk) to control PRL levels. Long-acting somatostatin analog therapy induced a slight drop in GH but not in IGF-1 levels. Because the patient refused monthly injections (initially Sandostatin LAR and then Somatuline Autogel), after about 9 months, treatment was switched to continuous sc infusion of short-acting octreotide (5) at doses ranging from 120–320 μg/d. The combination of somatostatin analog and cabergoline was insufficient to normalize GH or IGF-1 levels. The addition of the GH receptor antagonist pegvisomant (30–105 mg/wk) normalized IGF-1 and induced a plateau in his growth; the pituitary volume remained stable with this treatment combination (Figure 1F). Around the age of 7.5 years, the parents wished to reduce the dose of pegvisomant and short-acting octreotide therapy to allow controlled growth. They felt that he was destined to be a tall boy; thereafter his growth followed parallel to the 97th height percentile (Figure 1A), and his IGF-1 remained within the normal range.
Currently, at the age of 11 years, he remains well; his growth velocity is normal. He is on oral cabergoline 125 μg thrice weekly, short-acting sc octreotide 222 μg/24 h, and sc pegvisomant 20 mg twice weekly.
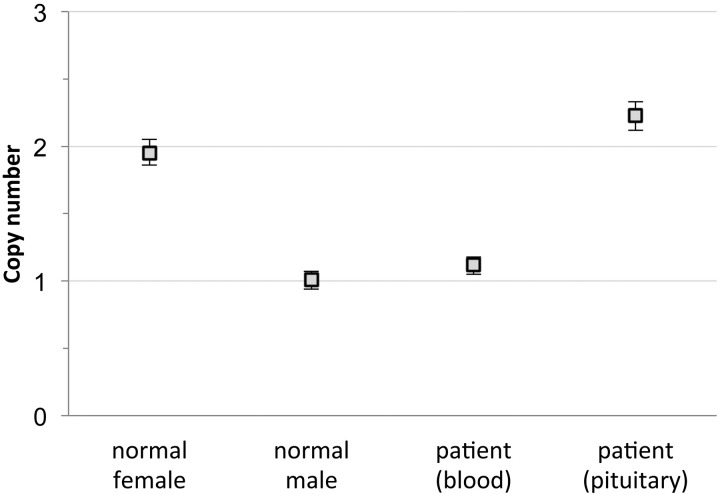
After the recent description of germline Xq26.3 microduplication in young children with acrogigantism (1), likely due to the duplicated GPR101 gene within this region, we tested the patient's leukocyte-, saliva-, and buccal cell-derived DNA using a comparative genomic hybridization array (BlueGnome CytoChip ISCA 8 × 60k v2.0; Illumina) and copy number variation droplet digital PCR for GPR101 (Taqman assays Hs01818174_cn and Hs01730605_cn; Life Technologies) without evidence of Xq26.3 microduplications or GPR101 duplication, although his clinical phenotype was identical to the previously published patients. We therefore tested DNA isolated from the hyperplastic pituitary tissue and found duplicated dosage of GPR101 (Figure 2). DNA extracted from a forearm skin biopsy also showed the microduplication, suggesting that he is mosaic for this mutation.
Figure 2.
GPR101 copy number as measured with copy number variation droplet digital PCR (assay id Hs01818174_cn, Life Technologies), showing two copies of GPR101 for normal female control, one copy for normal male control, one copy for the patient's blood-derived DNA, and two copies for the patient's pituitary tissue-derived DNA. Vertical bars show 95% Poisson confidence limits.
Ethics approval was granted from the Montreal Children's Hospital Research Ethics Board. Informed consent was obtained for all genetic analyses from the parents of this boy and for publication of this case report.
Discussion
XLAG often manifests in the first year of life and is more prevalent in girls. Unlike our case, all previously reported patients have had either sporadic or familial germline Xq26.3 microduplications allowing a definitive diagnosis on leukocyte-derived DNA. Because our case presented with a typical phenotype, we tested the pituitary tissue sample, and this showed GPR101 microduplication. One of the four different genomic DNA samples we tested from various tissues also showed GPR101 duplication, suggesting that this patient has somatic mosaicism as a result of a postzygotic mutation. No cases of somatic mosaicism have been described previously in patients with XLAG syndrome.
Treatment of XLAG is challenging. Previously described cases treated with surgery followed by medical treatment and/or radiotherapy have often developed hypopituitarism and central diabetes insipidus (1–3, 6). Management of our patient with cabergoline, continuous sc infusion of short-acting somatostatin analog, and pegvisomant proved successful in controlling growth without hypopituitarism or other significant side effects.
Conclusion
This is the first report on a patient with documented somatic GPR101 duplication leading to XLAG. The case is instructive because it: 1) demonstrates that a negative genetic test for Xq26.3 microduplication or GPR101 duplication on peripheral blood DNA does not exclude the diagnosis of XLAG because it can result from a somatic mutation affecting the pituitary and possibly other tissues as a result of somatic mosaicism; and 2) proposes an effective combination and route of medical treatment for this condition that can be acceptable for young children.
Acknowledgments
We are grateful to Prof Kalman Kovacs, Toronto, Canada, for advice on the pathology findings, and to Prof Michael Besser, London, UK, for management of the case.
Grant support from the National Institutes of Health Research (NIHR), Wales Gene Park, and Pfizer Ltd is gratefully acknowledged. C.E.S. is supported by a Wellcome Clinical Training Fellowship. S.E. is a Wellcome Trust Senior Investigator.
C.R., M.M., and M.K. cared for the patient. S.A., J.T., and F.R. made the histological diagnosis. All other authors were involved in the genetic testing; all authors contributed to the writing of the manuscript.
Disclosure Summary: M.K. is a member of the Scientific Advisory Board of Pfizer. None of the other authors report any conflict of interest.
Footnotes
- PRL
- prolactin
- XLAG
- X-linked acrogigantism.
References
- 1. Trivellin G, Daly AF, Faucz FR, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371:2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beckers A, Lodish MB, Trivellin G, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22:353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rostomyan L, Daly AF, Petrossians P, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canadian Paediatric Society. WHO Growth Charts. Promoting optimal monitoring of child growth in Canada: using the WHO growth charts. http://www.cps.ca/tools-outils/who-growth-charts Accessed December 27, 2015.
- 5. Näntö-Salonen K, Koskinen P, Sonninen P, Toppari J. Suppression of GH secretion in pituitary gigantism by continuous subcutaneous octreotide infusion in a pubertal boy. Acta Paediatr. 1999;88:29–33. [DOI] [PubMed] [Google Scholar]
- 6. Naves LA, Daly AF, Dias LA, et al. Aggressive tumor growth and clinical evolution in a patient with X-linked acro-gigantism syndrome. Endocrine. 2016;51:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]