Abstract
Context:
Compensatory increases in fibroblast growth factor 23 (FGF23) with increasing phosphate intake may adversely impact health. However, population and clinical studies examining the link between phosphate intake and FGF23 levels have focused mainly on populations living in highly industrialized societies in which phosphate exposure may be homogenous.
Objective:
The objective of the study was to contrast dietary phosphate intake, urinary measures of phosphate excretion, and FGF23 levels across populations that differ by the level of industrialization.
Design:
This was a cross-sectional analysis of three populations.
Setting:
The study was conducted in Maywood, Illinois; Mahé Island, Seychelles; and Kumasi, Ghana.
Participants:
Adults with African ancestry aged 25–45 years participated in the study.
Main Outcome:
FGF23 levels were measured.
Results:
The mean age was 35.1 (6.3) years and 47.9% were male. Mean phosphate intake and fractional excretion of phosphate were significantly higher in the United States vs Ghana, whereas no significant difference in phosphate intake or fractional excretion of phosphate was noted between the United States and Seychelles for men or women. Overall, median FGF23 values were 57.41 RU/mL (interquartile range [IQR] 43.42, 75.09) in the United States, 42.49 RU/mL (IQR 33.06, 55.39) in Seychelles, and 33.32 RU/mL (IQR 24.83, 47.36) in Ghana. In the pooled sample, FGF23 levels were significantly and positively correlated with dietary phosphate intake (r = 0.11; P < .001) and the fractional excretion of phosphate (r = 0.13; P < .001) but not with plasma phosphate levels (r = −0.001; P = .8). Dietary phosphate intake was significantly and positively associated with the fractional excretion of phosphate (r = 0.23; P < .001).
Conclusion:
The distribution of FGF23 levels in a given population may be influenced by the level of industrialization, likely due to differences in access to foods preserved with phosphate additives.
The distribution of FGF23 levels in a given population may be influenced by the level of industrialization, likely due to differences in access to foods preserved with phosphate additives.
Adequate phosphate intake is essential for bone health, but increasing phosphate intake may promote compensatory increases in fibroblast growth factor-23 (FGF23), a phosphaturic polypeptide (1). In the setting of normal glomerular filtration rate (GFR), FGF23 holds serum phosphate levels within a normal range, regardless of phosphate intake via the down-regulation of sodium phosphate cotransporters in the proximal tubule, which inhibits phosphate reabsorption (1). FGF23 also inhibits renal 1α-hydroxylase activity and reduces conversion of 25-hydroxyvitamin D to the active form of vitamin D (1,25-dihydroxyvitamin D), which up-regulates gastrointestinal phosphate absorption (2–4). Higher FGF23 levels are associated with a heightened risk of adverse cardiovascular outcomes (5, 6). Thus, compensatory increases in FGF23 with increasing phosphate intake may adversely impact health (7).
Population and clinical studies examining the link between phosphate intake and FGF23 levels have mainly focused on populations living in highly industrialized societies. The lack of consistent associations noted between phosphate intake and FGF23 levels (8–13) may be a function of the ubiquitous exposure to phosphate additives, which may add up to 1000 mg/d of dietary phosphate intake (14, 15). In addition, standard dietary measures poorly quantify dietary phosphate intake due to the hidden sources of inorganic phosphate in many foods (16, 17). Contrasting urinary measures of phosphate excretion and FGF23 levels across populations that differ by level of economic transition provides a model to explore associations between sustained increases in phosphate intake and FGF23 levels. We have previously demonstrated that adults living in a rural area of Nigeria have significantly lower levels of net phosphate absorption, as reflected by higher urinary phosphate excretion, and lower FGF23 levels compared with adults living in an urban area of the United States (18). However, dietary data were not available, and this study could not confirm lower phosphate intake in the adults with lower FGF23 levels. We now extend the findings of the previous study by examining FGF23 levels, dietary phosphate intake, and fractional excretion of phosphate in three populations of young adults with African ancestry living in environments that differ markedly by level of industrialization: urban United States (Maywood, IL), Mahé, Seychelles (upper middle income economy), and Kumasi, Ghana (lower middle income economy) (19). We hypothesized that adults living in more industrialized environments will have higher phosphate intake and net phosphate absorption as reflected by higher urinary excretion of phosphate and higher FGF23 levels compared with adults living in less industrialized environments.
Materials and Methods
Study population
The source population for this study was the Vitamin D Ancillary Study (VIDA), an associated study of the Modeling the Epidemiological Transition Study (METS). In the METS study, a cohort study of 2500 young adults with African ancestry aged 25–45 years, subjects were enrolled between January 2010 and September 2011. VIDA extended the METS study by using the framework of ecological contrasts to investigate vitamin D and bone health. The design and recruitment procedures for this study have been described previously (20). Participants were enrolled in each of five study sites: Maywood, Illinois; Kingston, Jamaica; Kumasi, Ghana; Cape Town, South Africa; and Mahé Island, Seychelles. FGF23 was measured in participants from three sites: Maywood, Illinois (n = 502), Mahé Island; Seychelles (n = 500); and Kumasi, Ghana (n = 500). We excluded participants with missing FGF23 values (two in the United States, six in Seychelles, and eight in Ghana) or FGF3 levels greater than 200 RU/mL (44 in the United States [41 women], 21 in Seychelles [20 women], and 23 in Ghana [16 women]) due to potential issues of iron deficiency (21, 22). We also excluded individuals with an estimated GFR less than 80 mL/min per 1.73 m2 (none in Maywood, one in Seychelles, and one in Kumasi) based on the Chronic Kidney Disease-Epidemiology Collaboration equation (23). This left a total of 456, 472, and 468 participants from the United States, Seychelles, and Ghana, respectively, included in the analyses. The METS and VIDA studies were approved by the institutional review boards at all sites, and written content was obtained from all participants in their native language.
Laboratory measurements
Participants fasted for 10 hours prior to the collection of blood samples. Specimens were immediately processed and shipped frozen to the coordinating center in Chicago and maintained in a −80°C freezer. FGF23 levels were measured in plasma at the University of Miami School of Medicine (Miami, Florida) using an ELISA that uses two antibodies directed against different epitopes within the carboxyl-terminal portion of FGF23 and thus captures both the intact hormone and its carboxyl-terminal fragments (Immutopics) with coefficients of variation of less than 5%. The lower limit of detection was 12 RU/mL. Plasma and urine phosphate were measured via spectrophotometry at the University of Miami School of Medicine, and serum and urine creatinine were measured using nephelometry. Fractional excretion of phosphate was calculated as the ratio of (urine phosphate × plasma creatinine)/(plasma phosphate × urine creatinine) and then multiplied by 100%. High fractional excretion of phosphate was defined as values greater than 20%. Total 25-hydroxyvitamin D was measured using a liquid chromatography-tandem mass spectrometric assay at the University of Washington (Seattle, Washington). The calibration of the assay was verified using the National Institute of Standards and Technology standard reference material SRM 972. Interassay variability was 6.0% at 11.5 ng/mL and 5.6% at 12.3 ng/mL for 25-hydroxyvitamins D2 and D3, respectively.
Demographic and dietary data
Demographic and anthropometric data and basic health history were collected by trained staff using standardized questionnaires in the participants' native language. Dietary intakes were collect by two 24-hour recalls using the multiple-pass method. All 24-hour recalls were collected by centrally trained interviewers and sent to the Coordinating Center at Loyola University Chicago (Chicago, Illinois) where the data were entered and analyzed using the Nutrient Data System for Research (University of Minneapolis, Minneapolis, Minnesota). The Nutrient Data System for Research was modified to accommodate foods and recipes unique to each site using the current available site-specific nutrient databases and previously collected 24-hour recall data (21).
Statistical analysis
STATA/IC 13.1 (StataCorp LP) was used to perform all statistical analysis. Summary statistics for key baseline characteristics were compared by site and sex. After stratifying by sex, continuous variables were compared across the three sites using an ANOVA, and categorical variables were compared using the Fisher's exact test. The level of statistical significance was set as P < .01 to account for multiple comparisons. Scatterplots of FGF23 levels by plasma phosphate, dietary phosphate intake, and fractional excretion of phosphate were examined. Spearman rank correlation coefficients were calculated to quantify the correlation between plasma phosphate, dietary phosphate intake, fractional excretion of phosphate, and FGF23 levels after pooling all three sites. Histograms of variables were examined and FGF23 demonstrated a right skewed distribution. The optimal transformation of FGF23 to normality, as determined by the ladder of powers approach, was a log transformation and the histogram of log transformed FGF23 showed a normal distribution.
To examine differences in FGF23 levels across sites, unadjusted and adjusted geometric mean values of log-transformed FGF23 levels were calculated for each site. SEs were calculated using the δ-method (24), and adjusted geometric mean values were adjusted for age, sex, and body mass index (BMI). Separate standardized linear regression models were constructed to examine the association between log-transformed FGF23 as the dependent variable and plasma phosphate levels, dietary phosphate intake, and the fractional excretion of phosphate as independent variables with adjustment for age, sex, BMI, and total 25-hydroxyvitamin D levels in the pooled sample. Interaction terms were fitted in the fully adjusted models to explore effect modification by sex. Standardized regression coefficients were obtained by standardizing ([value − mean]/SD) values for plasma phosphate, dietary phosphate intake, and fractional excretion of phosphate. These coefficients resemble partial correlation coefficients and can be interpreted as the correlation between a given variable after accounting for the effect of other variables in the model. Furthermore, the standardized regression coefficient associated with a variable represents the anticipated change in SD units of the dependent variable for every SD change in the variable of interest.
Results
Table 1 shows the characteristics of the study participants. Mean BMI and both systolic and diastolic blood pressures were highest among men and women in Maywood compared with men and women in Seychelles or Ghana. The distribution of 25-hydroxyvitamin D levels has previously been described (25), and overall, total 25-hydroxyvitamin D levels were significantly lower among men and women in the United States compared with men and women in Seychelles or Ghana. Plasma phosphate levels were highest in both men and women in Ghana (3.36 [0.72] and 3.23 [0.45], respectively) and lowest among men and women in Seychelles (2.87 [0.50] and 2.84 [0.41]), respectively. The fractional excretion of phosphate was significantly lower among men and women in Ghana compared with US men and women, respectively, but no significant difference in the fractional excretion of phosphate was noted between the United States and Ghana for both men and women. Table 2 shows the dietary characteristics of the three populations by sex. Among men, average energy intake was approximately 500 kcal/d higher in the United States compared with Seychelles or Ghana. Among women, energy intake in the United States was approximately 300 kcal/d higher than Seychelles or Ghana. Mean phosphate intake was significantly higher among men (1280.91 [537.43] and women (1053.73 [411.79] in the United States compared with men (1015.51 [381.41]) and women (802.89 [279.67] in Ghana [P < .001 for both comparisons]), whereas no significant difference in phosphate intake was noted between the United States and Seychelles for men or women (Table 2).
Table 1.
Population Characteristics by Site and Sex
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| United States (n = 241) | Seychelles (n = 228) | Ghana (n = 198) | United States (n = 215) | Seychelles (n = 244) | Ghana (n = 270) | |
| Age, y | 35.56 (6.21) | 36.47 (5.11) | 34.62 (6.77) | 34.45 (6.31) | 34.45 (6.31) | 34.13 (6.73) |
| BMI, kg/m2 | 29.75 (7.56) | 26.45 (4.92) | 22.24 (2.68)a | 34.32 (8.84) | 34.32 (8.84) | 25.48 (5.08)a |
| Mean systolic BP, mm Hg | 128.00 (14.48) | 122 (14) | 119 (13)a | 117 (16) | 117 (16.02) | 110 (14)a |
| Mean diastolic BP, mm Hg | 81 (12) | 75 (11) | 68 (11)a,b | 79 (13) | 79 (13) | 66.04 (11)a |
| Plasma phosphate, mg/dL | 3.10 (0.50) | 2.87 (0.50)a | 3.36 (0.72)a | 3.16 (0.51) | 2.84 (0.41)a | 3.23 (0.45)a |
| FEPi, %c | 10.97 (5.75) | 12.34 (6.74) | 6.61 (4.73)a | 9.46 (4.1) | 11.05 (6.47) | 6.28 (7.99)a |
| FEPi ≥20%, % | 7.92 | 9.9 | +1.72 | 4.73 | 9.5 | 2.97 |
| Vitamin D, pg/mL | 17.42 (7.68) | 31.20 (8.65)a | 32.02 (7.35)a | 17.32 (8.41) | 27.52 (6.57)a | 29.24 (6.34)a |
Abbreviations: BP, blood pressure; FEPi, fractional excretion of phosphate.
P < .001 vs US men or women.
P < .01 vs US men or women.
FEPi was calculated as (urine phosphate (milligrams per deciliter) × serum creatinine) divided by (plasma phosphate × urine creatinine) × 100 and reported as a percentage.
Table 2.
Dietary Characteristics by Site and by Sex
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| United States (n = 241) | Seychelles (n = 228) | Ghana (n = 198) | United States (n = 215) | Seychelles (n = 244) | Ghana (n = 270) | |
| Kilocalories | 2539.42 (931.08) | 2065.37 (619.41)a | 2074.97 (515.85)a | 2063.89 (745.37) | 1659.70 (486.21)a | 1707.65 (420.65)a |
| Fat, g/d | 105.84 (42.92) | 64.95 (30.98)a | 55.81 (29.93)a | 87.30 (38.89) | 57.13 (24.11)a | 43.72 (23.05)a |
| Carbohydrates, g/d | 286.09 (116.78) | 264.98 (81.54) | 329.16 (78.64)a | 241.12 (89.91) | 206.75 (65.29)a | 284.55 (73.34)b |
| Total protein, g/d | 95.55 (37.29) | 89.83 (32.95) | 69.10 (30.53)a | 76.81 (31.20) | 76.07 (26.12) | 52.72 (21.02)a |
| Animal protein, g/d | 69.81 (32.57) | 65.15 (30.53) | 34.38 (24.02)a | 57.79 (27.57) | 55.70 (23.74) | 24.71 (16.04)a |
| Vegetable protein, g/d | 25.74 (11.50) | 24.69 (9.15) | 34.80 (14.08)a | 22.03 (9.42) | 20.23 (7.79) | 28.05 (10.49)a |
| Fiber, g/d | 14.45 (7.52) | 14.42 (7.78) | 27.75 (10.83)a | 14.04 (6.60) | 12.70 (5.62) | 23.16 (8.34)a |
| Calcium | 695.41 (388.04) | 573.54 (252.00)a | 361.89 (152.26)a | 605.96 (268.38) | 518.78 (247.24)a | 316.39 (138.68)a |
| Phosphate, mg/d | 1280.91 (537.43) | 1228.9 (406.96) | 1015.51 (381.41)a | 1053.73 (411.79) | 1059.77 (370.38) | 802.89 (279.67)a |
| Iron, mg/d | 15.91 (8.86) | 14.03 (4.67)b | 16.05 (5.25) | 13.10 (5.51) | 12.34 (4.56) | 13.16 (3.93) |
P < .001 vs US men or women.
P < .01 vs US men or women.
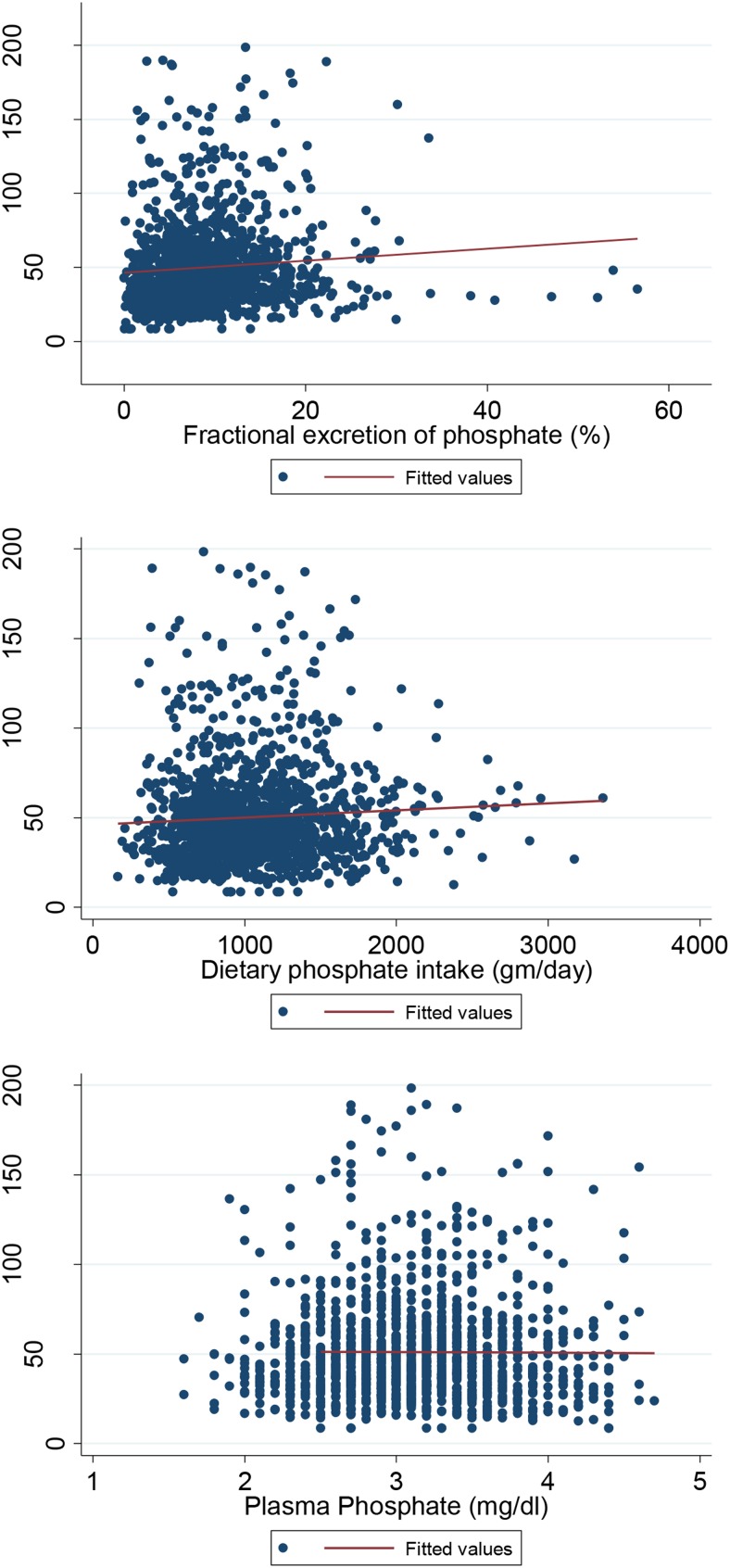
Overall, median FGF23 values were 57.41 RU/mL (interquartile range [IQR] 43.42–75.09) in the United States, 42.49 RU/mL (IQR 33.06–55.39) in Seychelles, and 33.32 RU/mL (IQR 24.83–47.36) in Ghana. Across all sites, females had higher median FGF-23 values compared with men. Median FGF23 levels in men ranged from 53.91 (IQR 41.55–67.20) in the United States, to 42.91 (IQR 33.62–56.27) in Seychelles, to 31.19 (IQR 22.71–41.42) in Ghana. Median FGF23 levels in females ranged from 64.37 (IQ 47.62–96.91) in the United States to 42.75 (IQR 32.99–55.71) in Seychelles to 35.98 (IQR 27.31–55.60) in Ghana. Figure 1 shows the unadjusted geometric mean values of FGF23 and the geometric man values adjusted for age, gender (in pooled samples), and BMI. After adjustment for demographics, FGF23 values were significantly higher in the United States vs Seychelles or Ghana. Figure 2 shows the scatterplots of FGF23 levels by plasma phosphate levels, dietary phosphate intake, and by the fractional excretion of phosphate, and Table 3 shows the Spearman rank correlation coefficients for FGF23 levels and plasma phosphate levels, dietary phosphate intake, and the fractional excretion of phosphate. FGF23 levels were significantly and positively correlated with dietary phosphate intake (r = 0.11; P < .001, and the fractional excretion of phosphate (r = 0.13; P < .001) but not with plasma phosphate levels (−0.001; P = .8). Dietary phosphate intake was significantly and positively associated with the fractional excretion of phosphate (r = 0.23; P < .001). Table 4 shows the results of the standardized linear regression models. Both dietary phosphate intake and the fractional excretion of phosphate were significantly associated with FGF23 levels with adjustment for age, sex, BMI, and total 25-hydroxyvitamin D levels but not plasma phosphate levels. However, after adjustment for site, both dietary phosphate intake and fractional excretion of phosphate were no longer associated with FGF23 levels.
Figure 1.
Unadjusted and adjusted (†) geometric mean values of FGF23 by site and by sex.
Figure 2.
Scatterplot of FGF23 by dietary phosphate intake, fractional excretion of phosphate, and plasma phosphate levels in the pooled samples.
Table 3.
Spearman Rank Correlation Coefficients of FGF23, Dietary Phosphate Intake, Plasma Phosphate, and Fractional Excretion of Phosphate in the Pooled Sample
| FGF23 | Phosphate Intake | Plasma Phosphate | Fractional Excretion of Phosphate | BMI | 25(OH)D | |
|---|---|---|---|---|---|---|
| FGF23 | 1.0 | |||||
| Phosphate intake | 0.11a | 1.0 | ||||
| Plasma phosphate | −0.007 | −0.05 | 1.0 | |||
| Fractional excretion of phosphate | 0.13a | 0.23a | −0.20a | |||
| BMI | 0.28a | 0.06 | −0.09 | 0.20a | 1.0 | |
| 25(OH)D | −0.28a | −0.07 | −0.01 | −0.11a | −0.37a | 1.0 |
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
P < .001.
Table 4.
Standardized Linear Regression Analysis for Standardized Log-Transformed FGF23 Levels in the Pooled Sample
| Model 1a Standardized Adjusted Coefficient (95% CI)b | P Value | Model 1 + Site Standardized Adjusted Coefficient (95% CI)b | P Value | |
|---|---|---|---|---|
| Standardized independent variables | ||||
| Plasma phosphate | −0.018 (−0.050, 0.015) | .3 | 0.019 (−0.014, 0.052) | .3 |
| Dietary phosphate intake | 0.045 (0.011, 0.079) | .01 | −0.002 (−0.036, 0.032) | .9 |
| Fractional excretion of phosphate | 0.077 (0.032, 0.123) | .008 | −0.003 (−0.037, 0.0310 | .8 |
Abbreviation: CI, confidence interval. Model 2 adjusts for all variables in model 1 and site; all variables in model are standardized: (value − mean)/(SD).
Model 1 adjusts for age, sex, BMI, and total 25-hydroxyvitamin D levels.
Discussion
We used data from young adults living in three different environments to examine whether FGF23 levels differ across populations with varying levels of economic transition and industrialization. In this ecological analysis, we found significantly higher levels of phosphate intake, fractional excretion of phosphate, and FGF23 levels in the United States compared with Ghana. Even after adjusting for demographics, FGF23 levels remained significantly higher in the United States compared with Seychelles or Ghana. Whereas no significant association was noted between FGF23 and plasma phosphate levels, FGF23 was significantly and positively correlated with phosphate intake and the fractional excretion of phosphate in the pooled sample. We also found that dietary phosphate intake was significantly and positively associated with the fractional excretion of phosphate.
The results of this study are supported by our previous study demonstrating higher urinary phosphate excretion and FGF23 levels in a small sample of adults living in the urban United States compared with adults living in a rural village in Nigeria (18), suggesting a gradient of FGF23 levels across the economic transition. This previous pilot study did not have information on dietary factors and included only two sites with a small number of individuals in each site. The present study includes study populations from three sites across a gradient of industrialization and demonstrates that dietary phosphate intake differs significantly by the level of industrialization. FGF23 levels in the present study were no longer significant after adjusting for site, but this was likely due to the low variability in both phosphate intake and urinary phosphate excretion within each site. The pooling of three sites with marked differences in FGF23 levels and net gastrointestinal phosphate absorption likely facilitated discernment of their association. The correlation between FGF23 levels and dietary phosphate intake was fairly weak, consistent with previous studies,(8, 12, 26), and this weak association may be due to difficulties in accurately assessing dietary phosphate intake (16). We also noted higher FGF23 levels in women compared with men across all sites, whereas plasma phosphate levels, within each site, were similar between men and women. Higher FGF23 values in young women vs young men may be due to the effects of estradiol on FGF23 production. Both FGF23 mRNA and FGF23 serum levels increase in ovariectomized rats after treatment with 17β-estradiol (27). In addition, menopause, a state of low estradiol levels, is associated with lower urinary phosphate excretion (28), with estradiol treatment for menopause increasing urinary phosphate excretion (13). Higher levels of FGF23 among women may have also been a function of lower iron stores, especially because the majority of these women were likely premenopausal (21, 22).
Populations living in highly industrialized societies may have higher population mean levels of FGF23 compared with populations living in less industrialized societies due to differences in diet. Diets characterized by a high intake of meats and processed foods preserved with phosphate additives, common in industrialized societies, will have higher phosphate content compared with diets high in vegetable protein and low in processed foods. Diets consistently high in phosphate, especially inorganic phosphate, will lead to compensatory increases in FGF23 levels to maintain serum phosphate levels within a tight range Elevated circulating levels of both phosphate and FGF23 levels have been associated with an increased risk for adverse cardiovascular outcomes including cardiac hypertrophy, atrial fibrillation, stroke, and mortality (29–33). For example, in a community health study of adults older than age 40 years living in Manhattan, New York, a plasma FGF23 value greater than 90 RU/mL was associated with a 50% higher risk for a stroke over a 12-year follow-up period after adjustment for GFR, demographic factors, and vascular risk factors (33). Although it remains premature to suggest a safe upper level of phosphate intake, increasing transparency regarding the phosphate content of food will provide consumers more knowledge regarding their individual phosphate intake. Currently the US Food and Drug Administration regulations do not require food producers to provide phosphate content on nutrition labels (34).
The strengths of this study include the use of three large populations of young adults living in environments with different diets and access to processed foods. The collection of demographic, anthropometric, and laboratory data were completed using standardized survey instruments and collection procedures. The limitations of the study include the use of spot urine samples to calculate the fractional excretion of phosphate because timed urine collections may provide a more accurate and comprehensive description of phosphate absorption and excretion. The misclassification of urinary phosphate excretion was likely nondifferential and would have biased our findings toward the null. Because food manufacturers are currently not required to report the amount of phosphate added to foods (34), total phosphate intake was likely underestimated, especially within the US site. All participants were instructed to fast for 10 hours prior to the clinic visit when blood samples were collected, and standard protocols were used for blood and urine collection across all study sites. However, it is possible that some variance in plasma phosphate levels was due to differences in the time of sample collection, given the known circadian changes in circulating phosphate levels (35). This study also used an assay that measures the C-terminal fragment of FGF23, which may be influenced by iron deficiency, and to address this, we excluded individuals with FGF23 values greater than 200 RU/mL. Most study participants with FGF23 levels greater than 200 RU/mL were US women. We did not have information on serum iron or ferritin levels and cannot confirm that participants with FGF23 values greater than 200 RU/mL had low serum iron levels. Whereas the results with the C-terminal fragment and intact FGF23 assays have yielded qualitatively similar associations in most settings (36), the findings of this study should be confirmed using an assay that measures intact FGF23 levels.
Conclusion
This study suggests that the distribution of FGF23 levels in a given population may be influenced by the level of industrialization, likely due to differences in access to foods preserved with phosphate additives. Because higher FGF23 levels are associated with a heightened risk for cardiovascular disease, future studies should further explore the association between chronic dietary phosphate intake, FGF-23 levels, and their association with health outcomes.
Acknowledgments
We thank Tom Mattix for his assistance with graphics.
This work was supported in part by National Institutes of Health Grants 1R01DK90360-1A1 (to R.D.-A.) and 5R01DK080763-04 (to A.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- FGF23
- fibroblast growth factor 23
- GFR
- glomerular filtration rate
- METS
- Modeling the Epidemiological Transition Study
- VIDA
- Vitamin D Ancillary Study.
References
- 1. Houston J, Isakova T, Wolf M. Phosphate metabolism and fibroblast growth factor 23 in chronic kidney disease. In: Kopple JD, Massry SG, Kalantar-Zadeh K, eds. Nutritional Management of Renal Disease. 3rd ed London: Academic Press; 2013:285–308. [Google Scholar]
- 2. Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+ dependent phosphate co-transport activity and 1α, 25-dihydroxyvitamin D3 production. J Biol Chem. 2004;278:2206–2211. [DOI] [PubMed] [Google Scholar]
- 3. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2003;19:429. [DOI] [PubMed] [Google Scholar]
- 4. Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143(8):3179–3182. [DOI] [PubMed] [Google Scholar]
- 5. Abramowitz M, Muntner P, Coco M, et al. Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol. 2010;5(6):1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhingra R, Gona P, Benjamin EJ, et al. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail. 2010;12(8):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16(3):186–188. [DOI] [PubMed] [Google Scholar]
- 8. Gutierrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the Health Professionals Follow-up Study. Clin J Am Soc Nephrol. 2011;6(12):2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Houston J, Smith K, Isakova T, Sowden N, Wolf M, Gutierrez OM. Associations of dietary phosphorus intake, urinary phosphate excretion, and fibroblast growth factor 23 with vascular stiffness in chronic kidney disease. J Renal Nutr. 2013;23(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–1196. [DOI] [PubMed] [Google Scholar]
- 11. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8):3144–3149. [DOI] [PubMed] [Google Scholar]
- 12. Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90(3):1519–1524. [DOI] [PubMed] [Google Scholar]
- 13. Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA. Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: the Heart and Soul Study. Am J Kidney Dis. 2011;58(5):737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takeda E, Yamamoto H, Yamanaka-Okumura H, Taketani Y. Increasing dietary phosphorus intake from food additives: potential for negative impact on bone health. Adv Nutr. 2014;5(1):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107(1):42–50. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Renal Nutr. 2007;17(5):350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutierrez OM. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Adv Chronic Kidney Dis. 2013;20(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckberg K, Kramer H, Wolf M, et al. Impact of Westernization on fibroblast growth factor 23 levels among individuals of African ancestry. Nephrol Dial Transplant. 2015;30(4):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The World Bank. http://beta.data.worldbank.org Washington, DC. [Google Scholar]
- 20. Luke A, Bovet P, Forrester TE, et al. Protocol for the modeling the epidemiologic transition study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health. 2011;11:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinkenbeard EL, Farrow EG, Summers LJ, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29(2):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braithwaite V, Jarjou LM, Goldberg GR, Prentice A. Iron status and fibroblast growth factor-23 in Gambian children. Bone. 2012;50(6):1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oehlert GW. A note on the delta method. Am Stat. 1992;46(1):27–29. [Google Scholar]
- 25. Durazo-Arvizu RA, Camacho P, Bovet P, et al. 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr. 2014;100(3):908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. di Giuseppe R, Kuhn T, Hirche F, et al. Potential predictors of plasma fibroblast growth factor 23 concentrations: Cross-sectional analysis in the EPIC-Germany study. PLoS One. 2015;10(7):e0133580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carrillo-Lopez N, Roman-Garcia P, Rodriguez-Rebollar A, Fernandez-Martin JL, Naves-Diaz M, Cannata-Andia JB. Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol. 2009;20(9):2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uemura H, Irahara M, Yoneda N, et al. Close correlation between estrogen treatment and renal phosphate reabsorption capacity. J Clin Endocrinol Metab. 2000;85(3):1215–1219. [DOI] [PubMed] [Google Scholar]
- 29. Miyamura M, Fujita S, Morita H, et al. Circulating fibroblast growth factor 23 has a U-shaped association with atrial fibrillation prevalence. Circ J. 2015;79(8):1742–1748. [DOI] [PubMed] [Google Scholar]
- 30. Hsu HJ, Wu MS. Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337(2):116–122. [DOI] [PubMed] [Google Scholar]
- 31. Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–390. [DOI] [PubMed] [Google Scholar]
- 32. Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–551. [DOI] [PubMed] [Google Scholar]
- 33. Wright CB, Dong C, Stark M, et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology. 2014;82(19):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hariharan D, Vellanki K, Kramer H. The Western diet and chronic kidney disease. Curr Hypertens Rep. 2015;17(3):16. [DOI] [PubMed] [Google Scholar]
- 35. Carruthers BM, Copp DH, Mcintosh HW. Diurnal variation in urinary excretion of calcium and phosphate and its relation to blood levels. J Lab Clin Med. 1964;63:959–968. [PubMed] [Google Scholar]
- 36. Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21(9):1427–1435. [DOI] [PubMed] [Google Scholar]