Abstract
Context:
In postmenopausal osteoporosis, combining denosumab and teriparatide increases hip and spine bone mineral density more than either monotherapy.
Objective:
The objective of the study was to determine the effects of 2 years of combination therapy on bone microarchitecture and estimated strength.
Design:
This was an open-label, randomized controlled trial.
Participants and Methods:
We performed high-resolution peripheral quantitative computed tomography at the distal tibia and radius in 94 postmenopausal osteoporotic women randomized to 2 years of teriparatide 20 μg sc daily, denosumab 60 mg sc every 6 months, or both.
Results:
Total volumetric bone mineral density (vBMD) at the radius and tibia, trabecular vBMD at the radius, and cortical vBMD at the tibia all increased more in the combination group than both monotherapy groups (P < .002 for all comparisons with combination). Cortical thickness at the tibia also increased more in the combination group (8.1% ± 4.3%) than both other groups (P < .001). Cortical porosity at both the radius and tibia increased progressively over the 24-month treatment period in the teriparatide group but was stable in both other groups (P < .001 teriparatide vs both other groups). Trabecular vBMD at the tibia increased similarly in all groups, whereas radius trabecular vBMD increased more in the combination group than the other groups (P < .01 for both comparisons). Finite element analysis-estimated strength improved or was maintained by all treatments at both the radius and tibia.
Conclusions:
Two years of combined teriparatide and denosumab improves bone microarchitecture and estimated strength more than the individual treatments, particularly in cortical bone. These findings suggest that this regimen may be beneficial in postmenopausal osteoporosis.
As assessed by HR-pQCT, two years of combined teriparatide and denosumab improves bone microarchitecture and estimated strength more than the individual treatments, particularly in cortical bone.
Treatment with single-drug therapy remains the standard of care for postmenopausal osteoporosis despite the fact that no single medication is able to completely restore skeletal integrity in patients with advanced disease. Prior studies of combination bisphosphonate and PTH therapy have not demonstrated sustained additive effects on areal bone mineral density (BMD) at either the spine or hip (1–4). In contrast, in the Denosumab and Teriparatide Administration (DATA) study, we reported that 24 months of combined denosumab and teriparatide increases BMD at the femoral neck, total hip, and lumbar spine more than either drug alone (5). The greater effect of combined denosumab (DMAB) and teriparatide (TPTD) on BMD may be due to the ability of DMAB to fully inhibit TPTD-induced bone resorption but only partially inhibit TPTD-induced bone formation, as evidenced by the changes in serum markers of bone turnover (5).
Whereas both anabolic and antiresorptive drug-induced increases in BMD are associated with antifracture efficacy (6–8), BMD, as measured by dual-energy x-ray absorptiometry (DXA), is unable to differentiate the microarchitectural changes in cortical and trabecular bone that underlie these increases. Moreover, it has been reported that changes in microarchitectural parameters are associated with fracture incidence in postmenopausal women independent of BMD (9–11). To understand the underlying microarchitectural changes causing the increases in BMD as measured by DXA in the DATA study, we performed high-resolution quantitative computed tomography (HR-pQCT) and previously reported that 12 months of combined DMAB and TPTD therapy improves cortical bone density, cortical microarchitecture, and estimated bone strength as measured by microfinite element analysis (μFEA) at the distal radius and tibia more than either drug alone (12). In this prospectively designed extension of the DATA-HRpQCT study, we hypothesized that 2 years of combined DMAB and TPTD therapy would sustain these larger improvements in microarchitecture and estimated strength as compared to monotherapy.
Materials and Methods
Subjects
The characteristics of the DATA subjects have been described in detail (5). In brief, 94 postmenopausal women at least 45 years of age with a high risk of fracture were recruited at a single clinical site (Massachusetts General Hospital, Boston, Massachusetts). High risk of fracture was defined as a T-score of −2.5 or lower at the spine or hip or a T-score of −2.0 or lower with at least one BMD-independent risk factor (fracture after age 50 y, parental hip fracture after age 50 y, previous hyperthyroidism, inability to get up from a chair with arms raised, or current smoking) (13) or a T-score of −1.0 or less with a history of a fragility fracture. Women were excluded for use of glucocorticoids or oral bisphosphonates within 6 months of enrollment; use of estrogen, selective estrogen receptor modulators, or calcitonin within 3 months of enrollment; or any prior use of iv bisphosphonates, PTH, or strontium ranelate.
Protocol
Subjects were stratified by age (younger than 65 y vs 65 y or older) and by previous bisphosphonate use. Subjects were then randomized to 2 years of open-label treatment of TPTD 20 μg sc once daily, DMAB 60 mg sc every 6 months, or both (combination [COMBO]) medications. Calcium and vitamin D were prescribed to achieve total daily intakes of 1200 mg of calcium and 25-hydroxyvitamin D concentrations greater than 20 ng/mL. The study was approved by the Partners Healthcare Institutional Review Board and registered on ClinicalTrials.gov (number NCT00926380).
High-resolution quantitative computed tomography
Volumetric density and microarchitecture of the distal radius and distal tibia were assessed at 0, 3, 6, 12, 18, and 24 months using HR-pQCT (XtremeCT; Scanco Medical AG). Details of the HR-pQCT image acquisition and assessments are described in detail elsewhere and are summarized below (12). Specifically, the volume of interest (VOI) shared by a participant's 0-, 3-, 6-, 12-, 18-, and 24-month scans was identified using the manufacturer's two-dimensional region-matching software, which relies on the periosteal contours. In this shared VOI, we used semiautomated software to segment cortical and trabecular regions that uses a threshold-based algorithm. Total, trabecular, and cortical volumetric bone density (vBMD; milligrams hydroxyapatite per cubic centimeter) were determined in addition to microarchitecture parameters of cortical thickness (millimeters), trabecular thickness (millimeters), trabecular number (millimeters−1), and trabecular separation (millimeters).
The cortical bone compartment was then further segmented via an autocontouring process that generated periosteal and endosteal contours (14). All automatically generated contours were inspected, and if the contour deviated from the visually apparent periosteal or endosteal margin, semimanual adjustment was made. From this extended cortical analysis, cortical tissue mineral density (TMD; milligrams hydroxyapatite per cubic centimeter) and cortical porosity (percentage) were assessed at months 0, 12, and 24 using the shared VOI at these visits. We used the μFEA to estimate stiffness (kilo Newtons per millimeter) and failure load (kilo Newtons) at months 0 and 24 using the shared VOI at months 0, 12, and 24. Failure load was estimated as per previously published methods by scaling the resultant load from a 1% compressive strain until 2% of all elements reached an effective strain of greater than 7000 microstrain (15).
Two operators performed the endosteal contouring (the same operator was assigned for all scans for each subject), and a third operator reviewed all periosteal and endosteal contouring for quality assurance. Image quality was graded on a 1–3 scale, and subjects with significant motion artifact (grade 3) were excluded from analysis. Scans with a fracture in the region of interest were also excluded from the analysis.
Statistical analysis
We performed a modified intention-to-treat analysis, which includes all data from women completing at least one postbaseline study visit. To compare the between-group changes in each standard parameter, we used a repeated-measures ANOVA. Within-group changes between months 0–24 and 12–24 were assessed by a paired t test. Statistical analyses were performed with SAS for Windows (version 9.3; SAS institute, Inc). The data are presented as mean (SD) unless otherwise specified. Two-sided values of P ≤ .05 are considered significant.
Results
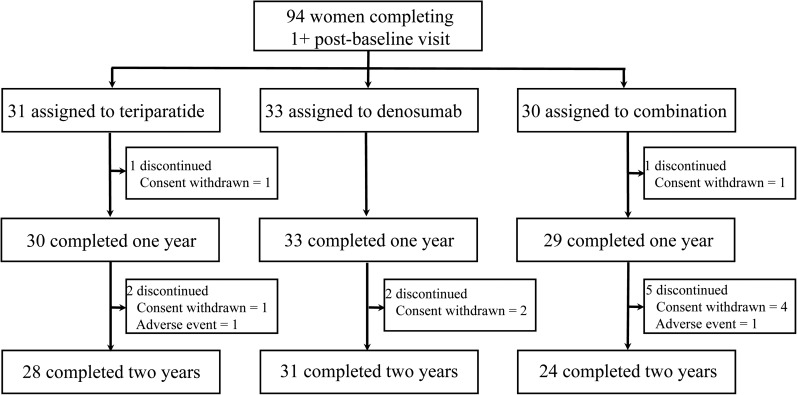
Of the 100 women enrolled in DATA, 94 women completed at least one postbaseline visit and are included in the modified intention-to-treat analysis (Figure 1). When comparing all six visits, the shared VOI averaged 89% at the tibia and 82% at the radius. As reported previously, baseline HR-pQCT density and microstructural characteristics were similar among the three groups with the exception of cortical thickness, at the tibia, which was greater in the combination therapy group than in the denosumab group (12).
Figure 1.
Subject disposition.
Volumetric bone mineral density
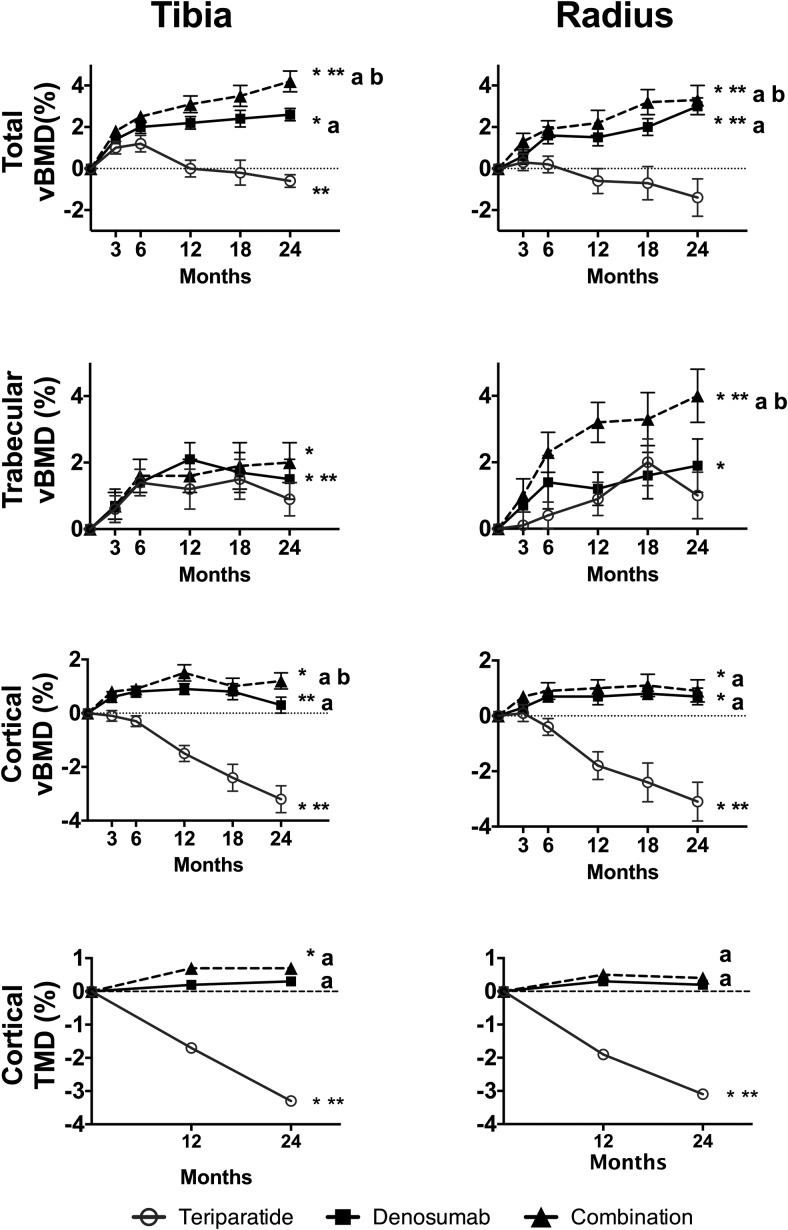
Changes in total and compartmental vBMD at the tibia and radius are shown in Figure 2.
Figure 2.
Mean percentage change (SEM) from baseline at the distal tibia and radius in vBMD and TMD over 24 months. *, P < .05 vs baseline; **, P < .05 vs 12 months for 12- to 24-month change; a, P < .05 vs TPTD alone; b, P < .05 vs DMAB alone.
Tibia
Over the 24-month treatment period, total vBMD at the tibia increased by 4.2% ± 2.1% in the COMBO group (P = .001 within group) and by 2.6% ± 1.7% in the DMAB group (P = .001 within group) but did not change in the TPTD group (P = .311 within group). The changes in the COMBO group were greater than those in the DMAB and TPTD groups (P = .001 for both comparisons). Between months 12 and 24, total vBMD at the tibia continued to increase in the COMBO group (0.7% ± 0.9%, P = .001 vs 12 mo), did not change with DMAB, but decreased with TPTD (−0.6% ± 1.5%, P = .046 vs 12 mo).
Cortical vBMD at the tibia increased by 1.2% ± 1.6% in the COMBO group (P = .003 within group), did not change in the DMAB group, and decreased in the TPTD group by 3.2% ± 2.7% (P < .001 within group) over the 24-month treatment period. The increase in the COMBO group was greater than those in the DMAB and TPTD groups (P = .002 vs DMAB, P < .001 vs TPTD). Between months 12 and 24, cortical vBMD was maintained in the COMBO group but decreased by −0.7% ± 1.3% in the DMAB group (P = .007 vs 12 mo) and by −1.7% ± 1.5% in the TPTD group (P < .003 vs 12 mo).
Cortical TMD at the tibia increased by 0.7% ± 1.2% in the COMBO group (P = .013 within group), did not change in the DMAB group, and decreased by −3.3% ± 2.7% in the TPTD group (P < .001 within group, P < .001 for comparisons with both other groups). Between months 12 and 24, cortical TMD did not change in the COMBO or DMAB group but decreased in the TPTD group by −1.7% ± 1.3% (P < .001 vs 12 mo).
Over 24 months, trabecular vBMD at the tibia increased by 2.0% ± 2.8% in the COMBO group (P = .003 within group), by 1.5% ± 3.1% in the DMAB group (P = .015 within group), but did not change in the TPTD group (P = .100 within group). There were no significant between-group differences in trabecular BMD change.
Radius
Over the 24-month treatment period, total vBMD at the radius increased by 3.3 ± 3.2 in the COMBO group (P < .001 within group), by 3.0 ± 2.0 in the DMAB group (P < .001 within group), and did not change in the TPTD group (P = .122 within group). The increases in the COMBO group were larger than those in the DMAB and TPTD groups (P = .005 vs DMAB, P < .001 vs TPTD). Between months 12 and 24, total vBMD continued to increase in the COMBO group (2.0% ± 2.5%, P = .003 vs 12 months) and in the DMAB group (1.0% ± 2.0%, P = .016 vs 12 mo) but not the TPTD group (P = .275 vs 12 mo).
Cortical vBMD at the radius increased by 0.9% ± 1.6% at 24 months in the COMBO group (P = .024 within group), by 0.7% ± 1.5% in the DMAB group (P = .014 within group), but decreased by −3.1% ± 3.8% in the TPTD group (P < .001 within group). The changes in the COMBO group were greater than those in the TPTD group (P < .001). Between months 12 and 24, radial cortical vBMD in the TPTD further decreased by −1.3% ± 2.0% (P < .003 vs 12 mo) but did not change in the other groups.
Cortical TMD at the radius did not change in the COMBO or DMAB groups over the 24 months of treatment but decreased by −3.1% ± 3.7% in the TPTD group (P < .001 within group, P < .001 for both comparisons vs TPTD). Between months 12 and 24, cortical TMD did not change in the COMBO or DMAB group but decreased in the TPTD group (−1.3% ± 1.8%, P < .001 vs 12 mo).
At the radius, trabecular vBMD increased by 4.0% ± 3.4% in the COMBO group (P < .001 within group), by 1.9% ± 4.1% in the DMAB group (P < .001 within group), and did not change in the TPTD group (P = .178 within group). The changes in the COMBO group were larger than those in the DMAB and TPTD groups (P < .001 vs DMAB, P = .002 vs TPTD). Between months 12 and 24, trabecular vBMD increased by 1.4% ± 2.2% in the COMBO group (P = .015 vs 12 mo) but did not change in the monotherapy groups.
Cortical microarchitecture
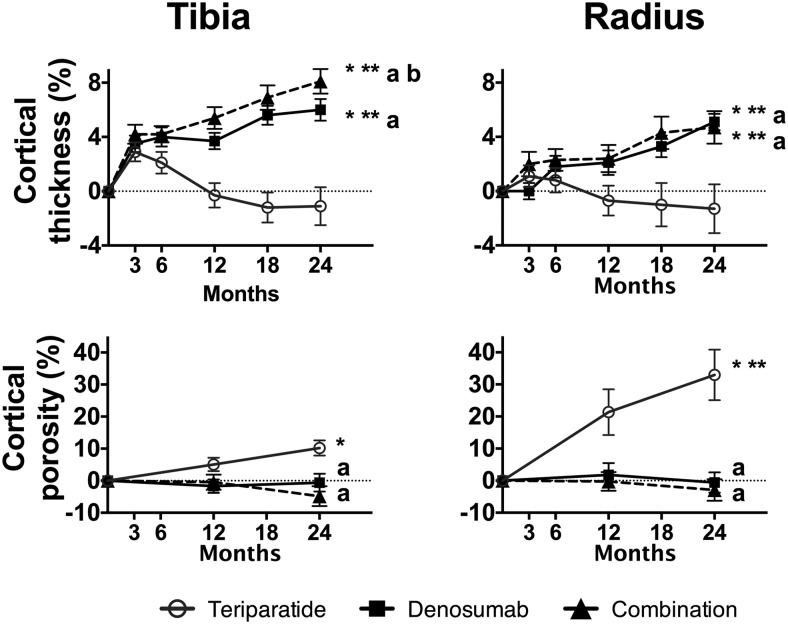
Changes in cortical thickness and porosity at the tibia and radius are shown in Figure 3.
Figure 3.
Mean percentage change (SEM) from baseline at distal tibia and radius in cortical thickness and cortical porosity over 24 months. *, P < .05 vs baseline; **, P < .05 vs 12-months for 12- to 24-month change; a, P < .05 vs TPTD alone; b, P < .05 vs DMAB alone.
Tibia
Cortical thickness at the tibia increased by 8.1% ± 4.3% after 24 months of treatment in the COMBO group (P < .001 within group), by 6.0% ± 4.5% in the DMAB group (P < .001 within group), but did not change in the TPTD group (P = .425). The changes in the COMBO group were larger than those in the monotherapy groups (P < .001 for both comparisons vs COMBO). Between months 12 and 24, cortical thickness continued to increase in the COMBO (1.8% ± 1.9%, P < .001 vs 12 mo) and DMAB groups (1.5% ± 2.9%, P = .011 vs 12 mo) but not the TPTD group.
Cortical porosity at the tibia was unchanged in the COMBO and DMAB groups after 24 months of treatment but increased in the TPTD group by 10.2% ± 12.1% (P < .001 within group). The changes in the TPTD group were larger than those in the COMBO group (P < .001 vs COMBO). Cortical porosity did not change in any group between months 12 and 24.
Radius
Over the 24-month treatment period, cortical thickness at the radius increased by 4.7% ± 5.3% in the COMBO group (P < .001 within group), by 5.1% ± 3.1% in the DMAB group (P < .001 within group), and did not change in the TPTD group (P = .459 within group). The changes in the COMBO group were larger than those in the TPTD group (P < .001 vs TPTD). Between months 12 and 24, cortical thickness increased in the COMBO (3.8% ± 4.6%, P = .006 vs 12 mo) and DMAB (2.6% ± 4.3%, P = .006 vs 12 mo) groups but did not change in the TPTD group.
Cortical porosity at the radius increased by 33.0% ± 40.1% in the TPTD group (P < .001 within group) but did not change in the DMAB or COMBO group. The changes in cortical porosity in the TPTD group were larger than those in the COMBO group (P < .001). Between months 12 and 24, cortical porosity continued to increase in the TPTD group (13.0% ± 21.2%, P = .04 vs 12 mo) but not the other groups.
Trabecular microarchitecture
As shown in Table 1, changes in trabecular microarchitecture were small in all groups and generally did not differ between treatment groups.
Table 1.
Mean Percentage Change (SD) From Baseline in Trabecular Microarchitecture at 24 Months
| Change in Tibia (SD), % |
Change in Radius: (SD), % |
|||||
|---|---|---|---|---|---|---|
| Teriparatide | Denosumab | Combination | Teriparatide | Denosumab | Combination | |
| Tb.Th | 6.0 (10.2)a | 4.7 (11.1)a | 2.5 (7.8) | 3.3 (10.1) | 2.5 (7.4) | 3.2 (7.9) |
| Tb.Sp | 5.1 (11.5)a | 3.1 (12.0) | 0.6 (8.8) | 2.1 (10.3) | 0.8 (8.0) | −1.4 (7.5) |
| Tb.N | −3.9 (10.0) | −1.9 (10.4) | −0.1 (8.8) | −1.3 (9.6) | −0.3 (7.8) | 1.4 (7.8) |
Abbreviations: Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
P < .05 vs baseline.
Microfinite element analysis
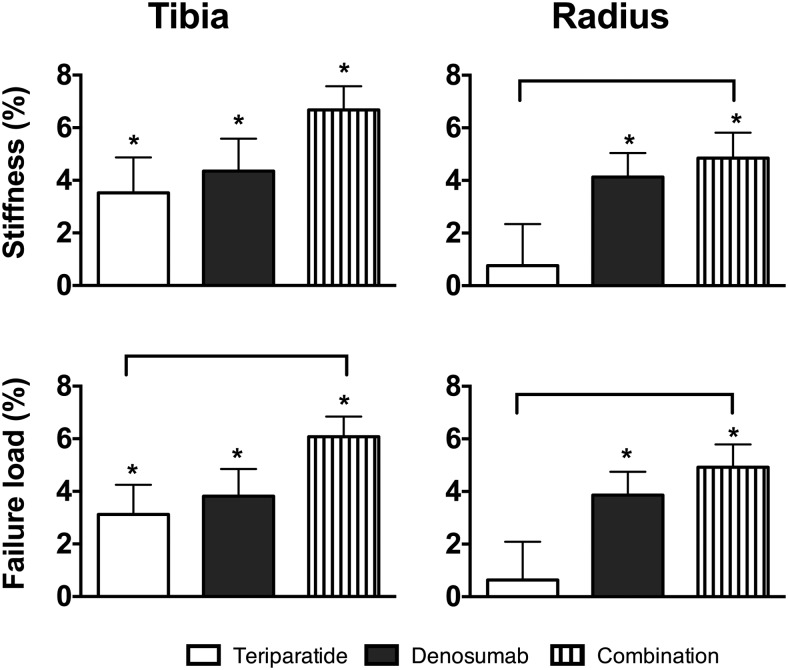
Estimated stiffness and failure load increased similarly in all groups at the tibia (Figure 4). The increases in failure load at the tibia were larger in the COMBO group than the TPTD group (P = .045).
Figure 4.
Mean percentage change (SEM) from baseline at distal tibia and radius in stiffness and failure load at 24 months. *, P < .05 vs baseline. Brackets denote a value of P < .05 between group comparisons.
At the radius, stiffness increased by 4.8% ± 4.2% in the COMBO group (P < .001 within group), by 4.0 ± 4.7% in the DMAB group (P < .001 within group), but did not change in the TPTD group. The increases in stiffness in the COMBO group were larger than those in the TPTD group (P = .050 vs TPTD). Similarly, failure load at the radius increased by 4.9% ± 3.8% in the COMBO group (P < .001 within group), by 3.8% ± 4.5% in the DMAB group (P < .001 within group), and did not change in the TPTD group. The increases in the COMBO group were larger than those in the TPTD group (P = .026 vs TPTD).
Discussion
In this study, we demonstrate that 2 years of treatment with combined TPTD and DMAB increases cortical thickness and cortical vBMD more than either treatment alone at the tibia and more than teriparatide monotherapy at the radius. Changes in trabecular microarchitecture were similar among the three groups. Together the treatment-induced changes in cortical and trabecular microarchitecture maintained or increased bone strength in all groups. At the radius, the combination of both drugs increased stiffness and failure load more than TPTD monotherapy.
The effects of DMAB monotherapy and TPTD monotherapy observed in the present study are similar to those reported in prior HR-pQCT studies (16–18). For example, in a study of 11 postmenopausal women treated with TPTD for 18 months (10 of whom received bisphosphonate therapy prior to TPTD), cortical vBMD decreased at the distal radius and tibia, although FEA estimates of bone strength were unchanged (18). In a separate study of postmenopausal women treated with TPTD or PTH (1–84) for 18 months, cortical vBMD decreased and cortical porosity increased at the tibia and radius. FEA-estimated strength was preserved in women treated with TPTD but decreased in women treated with PTH (1–84) (16). Also similar to our findings, 12 months of DMAB increased cortical thickness and cortical vBMD at both the distal tibia and radius in postmenopausal osteoporotic women (17). Of note, however, the decrease in tibial trabecular and cortical vBMD during year 2 of DMAB therapy was unexpected, although, to our knowledge, there are no comparable long-term studies of DMAB's effects on HR-pQCT parameters. These findings contrast with improvement in both trabecular and cortical density at the hip as measured by quantitative computed tomography with 3 years of DMAB therapy vs placebo (19). Also, using a cortical bone-mapping technique at the hip with computed tomography, 3 years of DMAB increased average cortical thickness and average cortical density more than placebo (20).
The observed progressive decreases in cortical vBMD over 2 years at the radius with TPTD monotherapy contrast with prior studies using DXA reporting that radius diaphyseal BMD decreases only during the first year of treatment (5). It is possible that the TPTD-induced decreases in cortical vBMD and cortical TMD reflect a decrease in bone matrix age and mineralization and/or increased cortical porosity. However, prior studies have reported both increases and decreases in mineral to matrix ratio in response to TPTD therapy (21–23). Also of note, in seven men treated with TPTD for 18 months and in six women treated with TPTD for 18–36 months, paired iliac crest biopsies showed that treatment increased the heterogeneity of mineralization due to a higher percentage of less mineralized matrix (23). The clinical relevance of the increase in cortical porosity observed in the TPTD group is unclear, and it should be noted that the large percentage decreases in cortical porosity we report correspond to only small absolute decreases (average of 3.8%–3.1% at the radius). Although the absolute changes in cortical porosity are small, the overall degree of cortical porosity may be of clinical significance based on work by Burghardt et al (14) evaluating change in biomechanical indices using models with cortical porosity masked and unmasked. The degree of intracortical porosity that contributed to a calculated deficit in stiffness between the two models ranged from 0.3% to 6.0% at the tibia and from 0.1% to 2.0% at the radius.
The cortical microarchitectural changes observed with combined TPTD and DMAB therapy continue to most closely resemble DMAB monotherapy and not a mixture of TPTD and DMAB effects. This is likely due to the capacity of DMAB to fully block TPTD's stimulation of osteoclast activity throughout the 24-month treatment period, as demonstrated by the complete suppression of the bone resorption marker, serum c-telopeptide, in women treated with combination therapy (5).
The increase in cortical thickness at the tibia with combination therapy was greater than either drug alone and continued to increase during the second year of treatment. This continued increase may be related to sustained modeling-based formation in women treated with both drugs as we have hypothesized previously (5). It is also notable that in women treated with either combination therapy or DMAB monotherapy, cortical thickness increased more at the tibia than at the radius. This finding is consistent with those reported in with other osteoporosis therapies including alendronate, ibandronate, risedronate, odanacatib, and strontium (24–28). The mechanisms underlying this differential response between the radius and tibia are unclear but may be related to differential effects of weight-bearing and mechanical stress at the tibia and radius. Because mechanical loading is known to reduce sclerostin production by osteocytes (29), it is possible that osteoblast function can be better maintained at this site during antiresorptive therapy.
There are several limitations to the current study that deserve mention. First, the lack of a placebo group limits our ability to make firm conclusions about the effect of each of the three therapies compared with observation alone. That said, because the effects on most measured parameters are robust, it unlikely that they are unrelated to treatment. Additionally, whereas we measured cortical porosity using well-validated methods (30), because the actual spatial resolution of HR-pQCT is approximately 130 μm, we may be underestimating cortical porosity (31–33). Furthermore, the μFEA calculations assume fixed material properties, yet cortical TMD increased in the combination group at the tibia, and therefore, the estimated bone strength values may be underestimated in the combination group. Conversely, cortical TMD decreased in the TPTD group, and thus, estimated bone strength values may be overestimated.
One final limitation is the method used to measure cortical thickness. Because cortical thickness is calculated as a ratio of cortical area to the cortical periosteal diameter, any factor that increases cortical area would contribute to the increase in cortical thickness observed in the DMAB and combination therapy groups. Increases in cortical area may be due to the expansion of cortical bone at the periosteal surface or at the endosteal surface or even due to infilling of cortical pores. Because the serial HR-pQCT image registration assumed constant periosteal contours, any therapy-induced effects on the periosteal circumference would not be detected by the methods that we used. Also, because there was no significant change in cortical porosity observed with DMAB or combination treatment, we may presume that the increase in cortical thickness seen with these treatments are driven by an increase in cortical bone at the endosteal cortical border. This is consistent with the increase in cortical area and concurrent decrease in trabecular area over 24 months observed in the DMAB and combination groups (data not shown). Finally, although change in mineralization influencing detection of cortical edges is a consideration and therefore could affect the measurement of cortical area, cortical TMD did not increase in the denosumab or combination treatment groups during months 12–24, so changes in mineralization contributing to edge detection of the cortical area would not likely explain the change in cortical area.
In summary, 2 years of combined TPTD and DMAB therapy resulted in the most favorable changes in bone quality at peripheral skeletal sites, particularly in cortical bone. Moreover, consistent with the DXA-derived BMD results and central sites, the differential beneficial microarchitectural changes observed with combination therapy at year 1 are maintained at year 2. Together with the previously reported DXA-derived increased in axial BMD, these findings continue to support the consideration of combined TPTD and DMAB therapy in patients at a high risk of fracture and should help guide further optimization of combination treatment regimens.
Acknowledgments
We thank the study volunteers for their participation.
Eli Lilly and Amgen did not have any role in the study design, data analysis, or data interpretation.
This study was registered (clinicaltrials.gov) with the number NCT00926380.
This work was supported by the shared National Institutes of Health/National Center for Research Resources Equipment Grant 1 S10 RR023405, National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1F32AR065359–01 (to J.N.T.), Eli Lilly, and Amgen.
Disclosure Summary: R.M.N. is a consultant for Eli Lilly, M.L.B. has received research funding from Amgen and Merck and serves on an advisory board for Eli Lilly and Merck, and B.Z.L. is a consultant for Eli Lilly, Amgen Inc, and Merck. The other authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- COMBO
- combination therapy
- DATA
- Denosumab and Teriparatide Administration
- DMAB
- denosumab
- DXA
- dual-energy x-ray absorptiometry
- μFEA
- microfinite element analysis
- HR-pQCT
- high-resolution quantitative computed tomography
- TMD
- tissue mineral density
- TPTD
- teriparatide
- vBMD
- volumetric BMD
- VOI
- volume of interest.
References
- 1. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. [DOI] [PubMed] [Google Scholar]
- 2. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–511. [DOI] [PubMed] [Google Scholar]
- 3. Finkelstein JS, Wyland JJ, Leder BZ, et al. Effects of teriparatide retreatment in osteoporotic men and women. J Clin Endocrinol Metab. 2009;94:2495–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol M. 2010;95:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA Extension Study): a randomized controlled trial. J Clin Endocrinol M. 2014;99:1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Austin M, Yang YC, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:1785–1790. [DOI] [PubMed] [Google Scholar]
- 8. Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004;34:599–604. [DOI] [PubMed] [Google Scholar]
- 9. Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res 2014;29:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. [DOI] [PubMed] [Google Scholar]
- 11. Vico L, Zouch M, Amirouche A, et al. High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res. 2008;23:1741–1750. [DOI] [PubMed] [Google Scholar]
- 12. Tsai JN, Uihlein AV, Burnett-Bowie SA, et al. Comparative effects of teriparatide, denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture, and estimated strength: the DATA-HRpQCT study. J Bone Miner Res. 2015;30:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Black DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12:519–528. [DOI] [PubMed] [Google Scholar]
- 14. Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30:842–848. [DOI] [PubMed] [Google Scholar]
- 16. Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. [DOI] [PubMed] [Google Scholar]
- 17. Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22:357–362. [DOI] [PubMed] [Google Scholar]
- 19. Genant HK, Libanati C, Engelke K, et al. Improvements in hip trabecular, subcortical, and cortical density and mass in postmenopausal women with osteoporosis treated with denosumab. Bone. 2013;56:482–488. [DOI] [PubMed] [Google Scholar]
- 20. Poole KE, Treece GM, Gee AH, et al. Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res. 2015;30:46–54. [DOI] [PubMed] [Google Scholar]
- 21. Gamsjaeger S, Buchinger B, Zoehrer R, Phipps R, Klaushofer K, Paschalis EP. Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone. 2011;49:1160–1165. [DOI] [PubMed] [Google Scholar]
- 22. Paschalis EP, Glass EV, Donley DW, Eriksen EF. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrinol Metab. 2005;90:4644–4649. [DOI] [PubMed] [Google Scholar]
- 23. Misof BM, Roschger P, Cosman F, et al. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003;88:1150–1156. [DOI] [PubMed] [Google Scholar]
- 24. Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25:2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapurlat RD, Laroche M, Thomas T, Rouanet S, Delmas PD, de Vernejoul MC. Effect of oral monthly ibandronate on bone microarchitecture in women with osteopenia-a randomized placebo-controlled trial. Osteoporos Int. 2013;24:311–320. [DOI] [PubMed] [Google Scholar]
- 26. Bala Y, Chapurlat R, Cheung AM, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Miner Res. 2014;29:380–388. [DOI] [PubMed] [Google Scholar]
- 27. Rizzoli R, Chapurlat RD, Laroche JM, et al. Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Results of a 2-year study. Osteoporos Int. 2012;23:305–315. [DOI] [PubMed] [Google Scholar]
- 28. Cheung AM, Majumdar S, Brixen K, et al. Effects of odanacatib on the radius and tibia of postmenopausal women: improvements in bone geometry, microarchitecture, and estimated bone strength. J Bone Miner Res. 2014;29:1786–1794. [DOI] [PubMed] [Google Scholar]
- 29. Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–890. [DOI] [PubMed] [Google Scholar]
- 31. Burghardt AJ, Pialat JB, Kazakia GJ, et al. Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2013;28:524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tjong W, Kazakia GJ, Burghardt AJ, Majumdar S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med Physics. 2012;39:1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jorgenson BL, Buie HR, McErlain DD, Sandino C, Boyd SK. A comparison of methods for in vivo assessment of cortical porosity in the human appendicular skeleton. Bone. 2015;73:167–175. [DOI] [PubMed] [Google Scholar]