Abstract
In the human infundibular (arcuate) nucleus, a subpopulation of neurons coexpress kisspeptin and neurokinin B (NKB), 2 peptides required for normal reproductive function. A homologous group of neurons exists in the arcuate nucleus of rodents, termed KNDy neurons based on the coexpression of kisspeptin, NKB, and dynorphin. To study their function, we recently developed a method to selectively ablate KNDy neurons using NK3-SAP, a neurokinin 3 receptor agonist conjugated to saporin (SAP). Here, we ablated KNDy neurons in female rats to determine whether these neurons are required for estrous cyclicity and the steroid induced LH surge. NK3-SAP or Blank-SAP (control) was microinjected into the arcuate nucleus using stereotaxic surgery. After monitoring vaginal smears for 3–4 weeks, rats were ovariectomized and given 17β-estradiol and progesterone in a regimen that induced an afternoon LH surge. Rats were killed at the time of peak LH levels, and brains were harvested for NKB and dual labeled GnRH/Fos immunohistochemistry. In ovary-intact rats, ablation of KNDy neurons resulted in hypogonadotropic hypogonadism, characterized by low levels of serum LH, constant diestrus, ovarian atrophy with increased follicular atresia, and uterine atrophy. Surprisingly, the 17β-estradiol and progesterone-induced LH surge was 3 times higher in KNDy-ablated rats. Despite the marked increase in the magnitude of the LH surge, the number of GnRH or anterior ventral periventricular nucleus neurons expressing Fos was not significantly different between groups. Our studies show that KNDy neurons are essential for tonic levels of serum LH and estrous cyclicity and may play a role in limiting the magnitude of the LH surge.
In the human infundibular nucleus, there is a subpopulation of neurons that coexpress estrogen receptor-α (1), kisspeptin (2), and neurokinin B (NKB) mRNA (3, 4). These neurons have been termed KNDy neurons based on the coexpression of kisspeptin, NKB, and dynorphin in multiple mammalian species (5). In postmenopausal women, these neurons become hypertrophied and exhibit marked increases in NKB, substance P, and kisspeptin mRNA (2, 3). Neurons expressing dynorphin mRNA also exhibit somatic hypertrophy but the mRNA for dynorphin is reduced (6). The increase in kisspeptin and NKB gene expression in postmenopausal women is secondary to ovarian failure because identical changes are observed in response to ovariectomy of young monkeys (2, 7, 8). Close apposition between kisspeptin/NKB processes and GnRH neurons provides an anatomic framework for KNDy neurons to influence GnRH secretion in the human hypothalamus (4).
Hypogonadotropic hypogonadism (HH) is characterized by insufficient secretion of gonadotropins from the anterior pituitary gland resulting in low levels of circulating sex steroids (9). HH may be acquired or caused by mutations in the genes controlling migration of GnRH neurons, the GnRH receptor or upstream regulators of GnRH neurons, such as kisspeptin or NKB (9–14). Because KNDy neurons coexpress 2 peptides that are critical for GnRH secretion, it seems likely that HH in patients with mutations in the kisspeptin (KISS1) or NKB (TAC3) genes could be due to altered signaling from KNDy neurons. However, kisspeptin and NKB are located in other hypothalamic regions (2, 15, 16), so it is not clear whether neurons in the infundibular nucleus represent the essential element for reproduction.
Research into the function of KNDy neurons has been facilitated by the identification of a homologous group of neurons in the arcuate nucleus of the monkey (7, 8, 17, 18), sheep (19), goat (20), rat (21, 22), and mouse (23). To date, there is evidence that KNDy neurons mediate estrogen negative feedback on LH secretion, relay progesterone (P) inhibition of pulsatile GnRH secretion and modulate the pulsatile release of GnRH (5, 24–26). In the ewe, KNDy neurons have also been shown to be important for the induction of the preovulatory GnRH/LH surge by estrogen (27, 28). In the rodent, however, kisspeptin neurons in the anterior ventral periventricular nucleus (AVPV) trigger the LH surge via activation of GnRH neurons (29, 30) with limited information on a role of KNDy neurons.
To study the function of KNDy neurons, we recently developed a method to selectively ablate them based on their expression of the neurokinin 3 receptor (NK3R) (22, 31). KNDy neurons are targeted using stereotaxic injections of NK3-SAP, a selective NK3R agonist that is conjugated to saporin (SAP), a ribosome-inactivating toxin. Injections of NK3-SAP into the arcuate nucleus produced near-complete ablation of kisspeptin, NKB, and NK3R-immunoreactive neurons with loss of most dynorphin neurons in the arcuate nucleus (31). Specificity was demonstrated by the preservation of neuropeptide Y, proopiomelanocortin, and GnRH neuronal elements in the arcuate nucleus and adjacent median eminence (31). Dopamine neurons are also preserved after injections of NK3-SAP (32), consistent with the lack of NK3R receptor on arcuate neurons that project to the fenestrated capillaries in the median eminence (33).
Using injections of NK3-SAP to ablate KNDy neurons, we previously showed these neurons to be essential for the elevated LH in response to ovariectomy, tonic LH secretion in 17β-estradiol (E2)-treated rats, and the E2 modulation of body weight (31). In the present study, we determined the effects of KNDy neuron ablation in intact rats, by evaluating tonic LH secretion, estrous cyclicity, body weight, and ovarian and uterine histology. A second goal was to evaluate whether KNDy neurons are essential for the LH surge in E2 and P (E2P)-treated ovariectomized (OVX) rats. Portions of this work were previously presented in abstract form (34, 35).
Materials and Methods
Animals
Female Sprague-Dawley rats (∼12 wk old, 200–250 g; Harlan Laboratories) were individually housed in a quiet, temperature-controlled room in the University of Arizona Animal Care Facility (14-hour light, 10-hour dark cycle, lights on at 5 am). Rats had ad libitum access to water and a low-phytoestrogen diet (Harlan Teklad rodent diet 2014; Harlan Laboratories). All protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health guidelines. To determine the number of rats needed for the study, a power analysis was performed using LH data from the OVX E2-treated control and KNDy-ablated rats in our previous study (31). A power of over 0.95 was obtained using n = 7 rats/group. A power of 0.80 was obtained with n = 4.
To ablate NK3R-expressing KNDy neurons, we microinjected NK3-SAP, a conjugate of [MePhe7]-NKB and SAP (Advanced Targeting Systems). [MePhe7]-NKB was selected for conjugation to SAP because it is 1000 times more selective for NK3R than other tachykinin receptors (36, 37). In addition, the binding of [MePhe7]-NKB to SAP did not change its affinity for the NK3R (31). Control rats received injections of Blank-SAP, a scrambled 11-amino acid peptide conjugated to SAP. SAP conjugates were stored at −80°C as a 40-ng/nL stock solution in 0.1M PBS (pH 7.4), and diluted to a working concentration (10 ng/100 nL PBS) immediately before use.
Rats were anesthetized by im injections of a cocktail (1.0 mL/kg) containing ketamine (33.3 mg/mL), xylazine (10.7 mg/mL), and acepromazine (1.3 mg/mL). Stereotaxic surgery was used to make bilateral injections (2 on each side) of either NK3-SAP (n = 9) or Blank-SAP (controls, n = 7) dorsal to the arcuate nucleus. Microinjections were made using a NanoFil 10-μL syringe with a 33-gauge beveled tip needle (World Precision Instruments) inserted into an UltraMicroPump (UMP3) with a Micro 4 controller (World Precision Instruments). The rostral coordinates were: 2.4 mm posterior to bregma, ±0.5 mm lateral and 9.8 mm ventral to the skull surface. Caudal coordinates were 3.5 mm posterior to bregma, ±0.5 mm lateral and 9.7 mm ventral to the skull surface. NK3-SAP or Blank-SAP was infused (10 ng for each injection) over a 5-minute period, and then the infusion needle was left in place for an additional 5 minutes before removal.
Experiment 1. Effects of KNDy neuron ablation on gonadotropin secretion, estrous cyclicity, ovarian and uterine histology, and body weight
After a 1-week recovery from stereotaxic surgery, estrous cycles were monitored by daily vaginal smears (Figure 1). The vaginal smears were interpreted as described by Goldman et al (38). Body weights were measured weekly. From 28 to 32 days after stereotaxic surgery, rats were OVX on diestrous morning using the anesthetic cocktail described above. Blood samples were taken immediately before stereotaxic surgery (via saphenous vein puncture) and at the time of OVX (from the ovarian artery). Blood samples were collected between 7 and 10 am. Saphenous vein punctures were achieved using a 5-mm lancet (Braintree Scientific) and a Microvette capillary tube (Sarstedt). All blood was allowed to clot for 90 minutes and centrifuged. Serum was frozen and stored at −20°C before RIA analysis.
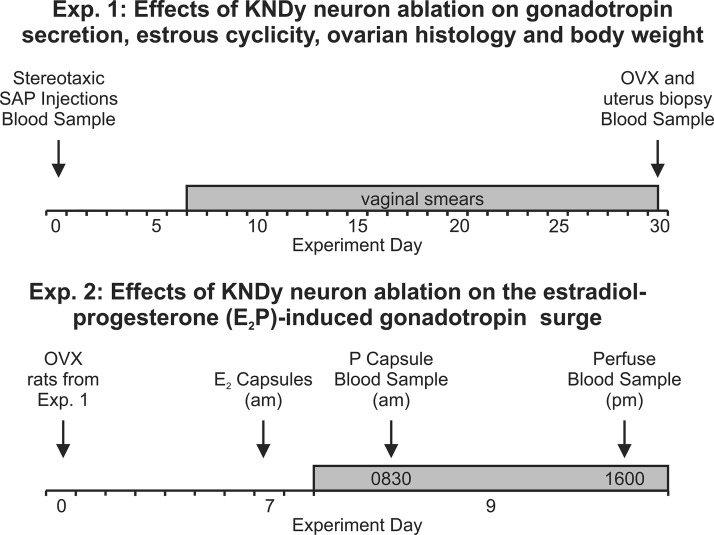
Figure 1.
Schematic diagrams of experimental protocols. In experiment 1, rats were injected with Blank-SAP or NK3-SAP (d 0), and daily vaginal smears were started on experimental day 7 and conducted until ovariectomy (shown at d 30 in this example). In experiment 2, rats were implanted with an E2 capsule 7 days after ovariectomy, and on the morning of day 9, they were implanted with a P capsule to induce an afternoon LH surge. Rats were killed at the expected time of peak LH levels (shown here at 4 pm), and the brains were harvested for immunohistochemistry.
The ovaries and samples of the dorsal horns of the uterus were fixed in 10% formalin. The ovaries were weighed after removal of fat. The tissues were processed overnight and paraffin embedded. The ovaries were oriented to section the largest cross-sectional area, and the uterus was oriented for transverse sections. After facing the block, a total of 150 serial sections were collected using a rotary microtome (4 μm thick). Sections were stained with hematoxylin and eosin. For morphological analysis, a representative section of the largest cross-sectional area of 1 ovary and 1 uterine horn was blindly selected for each rat (39). Quantitative analysis was conducted using an image-combining computer microscope (Nikon) equipped with a motorized stage (Ludl Electronics Products), a Lucivid miniature cathode ray tube, and NeuroLucida software (MicroBrightField).
Ovary sections were systematically scanned (×20 Plan apochromatic objective, NA 0.50), and the follicles and corpora lutea were manually marked and counted. Follicles that were not undergoing atresia were classified into 3 main categories: small (primordial or primary), medium (secondary), or large (antral) (40–42). Apoptotic cells were identified by shrinkage of the granulosa cells with condensed nuclear chromatin or fragmentation of the nuclei. Follicular atresia was evaluated by the method described by Braw and Tsafriri (41): in stage I atresia, 5%–10% of the granulosa cells were apoptotic; in stage II atresia, 10%–50% of the granulosa cells were apoptotic; and in stage III atresia there was collapse of the follicle, fragmentation of the basal lamina, and degeneration of the oocyte (41). The follicle type that preceds type III atresia cannot be determined accurately (41). Uterine sections were also systematically scanned, and the endometrial glands were marked and counted. The outer (adjacent to the muscular wall) and inner (epithelial lining) boundaries of the endometrium were outlined using the computer microscope and these values were used to calculate endometrial area. Ovarian and endometrial parameters were compared between groups using 2 tailed Student's t tests.
Experiment 2. Effects of KNDy neuron ablation on the E2P-induced gonadotropin surge
A well-characterized E2P-replacement regimen was used to elicit an LH surge (Figure 1) (43, 44). Seven days after OVX (at ∼8:30 am, under isoflurane anesthesia), the rats were implanted with 2 sc SILASTIC capsules (outer diameter, 3.18 mm; inner diameter, 1.57 mm; effective length, 20 mm; Dow Corning Corp) filled with E2 (150 μg/mL in sesame oil). Forty-eight hours later, an additional sc capsule containing P (50 μg/mL in sesame oil) was implanted, and blood was collected via saphenous vein puncture. Rats were administered an overdose of sodium pentobarbital (200 mg/kg ip) between 3:30 and 4:30 pm on the afternoon of P capsule implantation, at the time of the peak LH surge (44). A blood sample was collected via cardiac puncture. Serum was processed and stored as described above.
Experiment 3. Effects of KNDy neuron ablation on Fos-activation in GnRH neurons and the AVPV during the E2P-induced LH surge
After the overdose of sodium pentobarbital, rats were perfused with 200-mL heparinized PBS followed by 400-mL 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Brains were harvested and postfixed in 4% paraformaldehyde for approximately 1 hour followed by cryoprotection in ascending sucrose solutions (10%, 20%, and 30% in PBS, each overnight). Brains were blocked using a rat brain matrix (Braintree Scientific) and frozen sectioned on a sliding microtome (40 μm, coronal plane). Sections were stored in cryoprotectant solution for later processing (45).
The GnRH antibody (clone HU4H; Henryk Urbanski) was raised in mouse against the GnRH decapeptide (Table 1). This antibody has been extensively characterized including preadsorption experiments (33, 46). The Fos antibody (Table 1) was raised in rabbit against amino acids 4–17 of human c-fos (PC38 AB-5, lot D0001099; EMD Biosciences, Inc). The specificity of this antiserum has been verified by preadsorption experiments (47) and negative staining in Fos knockout mice (48). Controls included subjecting sections to the dual-label procedure with the omission of one or both of the primary antibodies.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Source | Species Raised | Dilution |
|---|---|---|---|---|---|
| Fos | Amino acids 4–17 of human c-Fos | AB-5 PC38 | EMD Biosciences | Rabbit polyclonal | 1:5000 |
| GnRH | GnRH decapeptide | Clone HU4H | Dr Henryk Urbanski | Mouse monoclonal | 1:5000 |
| NKB | Amino acids 50–79 of mouse pro-NKB | NB300-201 | Novus Biologicals | Rabbit polyclonal | 1:15 000 |
Every 10th section was Nissl stained to facilitate the identification of hypothalamic landmarks. Dual-labeled fluorescent immunohistochemistry of Fos and GnRH was performed on sections matched to plates 29, 32, 33/34, and 36 of a rat brain atlas (49). These brain levels have been previously used to characterize the extent of GnRH activation during the LH surge (50). Unless indicated, multiple PBS rinses were performed between steps. For antigen retrieval, sections were treated with 15mM sodium citrate (pH 8.8) for 30 minutes at 80°C followed by peroxidase inhibition in 0.3% H2O2 in PBS for 30 minutes. Sections were blocked for 1 hour in 3% normal goat serum (Vector Laboratories) and 0.4% Triton X-100 in PBS and directly incubated with rabbit anti-Fos (1:5000 in blocking solution) for 48 hours. The Fos antibody was visualized with tyramide signal amplification that consisted of the following steps: incubation with biotinylated goat antirabbit (1:5000 in blocking solution; Vector Laboratories) for 2 hours, avidin-biotin complex (in PBS with 0.4% Triton X-100; Vector Laboratories) for 30 minutes, biotinyl tryamide (1:200 in PBS with 0.005% H2O2; PerkinElmer) for 20 minutes and SA Alexa Fluor 568 (1:200 in PBS with 0.4% Triton X-100; Life Technologies) at 37°C for 3 hours. Sections were blocked and directly incubated with mouse anti-GnRH (1:5000 in blocking solution) for 48 hours. The GnRH antibody was visualized with highly cross-adsorbed goat antimouse conjugated to Alexa Fluor 488 (1:500 in blocking solution; Life Technologies) for 2 hours. The sections were counterstained with 3μM 4′,6-diamidino-2-phenylindole (Life Technologies), mounted on gelatinized slides, and coverslipped with ProLong Antifade medium (Life Technologies).
Digital images of brain sections were captured using a Nikon E1000 microscope equipped with a epifluorescent attachment, a motorized stage (Ludl Electronic Products), a Uniblitz model VMM-D1 shutter driver (Vincent Associates), a Photometrics Coolsnap FX camera (Roper Scientific), and the appropriate filter sets. Images were taken in a systematic step-wise fashion using MetaMorph imaging software (Molecular Devices) and a ×20 Nikon Plan Fluor objective (NA 0.50) and assembled into montages. The sections were systematically scanned through the oculars and the location of single-label GnRH and dual-label GnRH/Fos neurons were marked on the captured image montages. Counts were summed across the 4 sections for each animal and the numbers for each animal were used to compute group means. Fos labeling intensity in photomontages of the AVPV (plates 33–34; Paxinos and Watson) was adjusted to highlight labeled nuclei. The AVPV was outlined with the aid of the 4′,6-diamidino-2-phenylindole counterstain and the number of Fos neurons were mapped and counted. The average number of Fos neurons per hemisection of AVPV (±SEM) was calculated for each rat, and these data were used to compute group means. These data were compared between groups using 2-tailed Student's t tests.
Immunohistochemical verification of KNDy neuron ablation
To evaluate the loss of KNDy neurons in NK3-SAP rats, sections corresponding to plates 56 and 64 of a rat brain atlas (49) were processed using a pro-NKB antibody (Table 1). This antibody (NB300-201 lot A2; Novus Biologicals) was raised in rabbit against residues 50–79 of mouse pro-NKB. This antibody has been previously characterized (22, 31, 33). Preadsorption with the synthetic peptide used for immunization (Novus Biologicals) prevented specific labeling.
Briefly, sections were rinsed in PBS to remove cryoprotectant, subjected to a 0.3% H2O2 incubation for removal of endogenous peroxidases, rinsed, and blocked in 3% normal goat serum with 0.4% Triton X-100 in PBS. The pro-NKB antibody (1:15 000) was diluted in blocking solution and sections were incubated for 48 hours at 4°C. After rinses, sections were incubated in secondary antibody (biotinlyated goat antirabbit, 1:600 dilution) in blocking solution for 2 hours followed by rinses and incubation with avidin-biotin complex (Vector Laboratories) in PBS with 0.4% Triton X-100. Sections were rinsed in PBS followed by rinses in 0.175M sodium acetate. Tissue was then incubated in filtered, nickel-intensified 3,3′diaminobenzidine solution (1.25-g nickel [II] sulfate, 10-mg 3,3′diaminobenzidine in 50-mL 0.175M sodium acetate with 41.5 μL of 30% H2O2). Finally, sections were rinsed and mounted onto subbed slides, dehydrated, and coverslipped.
Arcuate brain regions were outlined (×4 Plan objective) and the number of NKB-immunoreactive neurons was counted (×20 Plan apochromatic objective, NA 0.50) using an image-combining computer microscope with NeuroLucida software (described above). Counts per animal were averaged for middle and posterior arcuate hemisections. Group averages were compared using 2-tailed Student's t tests.
Hormone assays
Serum samples were assayed by the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research in Reproduction (Charlottesville, VA). Serum LH and FSH (10-μL sample, in singlet) were assayed using a multiplex panel assay (Milliplex MAP for Luminex xMAP Technology, RPT-86K-02). Because undiluted serum from KNDy-ablated rats at the time of the E2P surge was above the reportable range (30 ng/mL), these samples were reassayed with a 1:10 dilution. The assay had an intraassay variability of 7.3% and an interassay variability of 10.3%. Values falling below the sensitivity of the assays (which only occurred in NK3-SAP rats on d 30) were assigned the minimal detectable values of 0.24 ng/mL for LH and 2.4 ng/mL for FSH. An LH surge was defined as 2 SDs above the mean LH value in the morning (51). Data were analyzed using two-way ANOVA with repeated measures (SAP treatment vs time) followed by Tukey's post hoc test.
Results
Morphological verification of KNDy neuron ablation
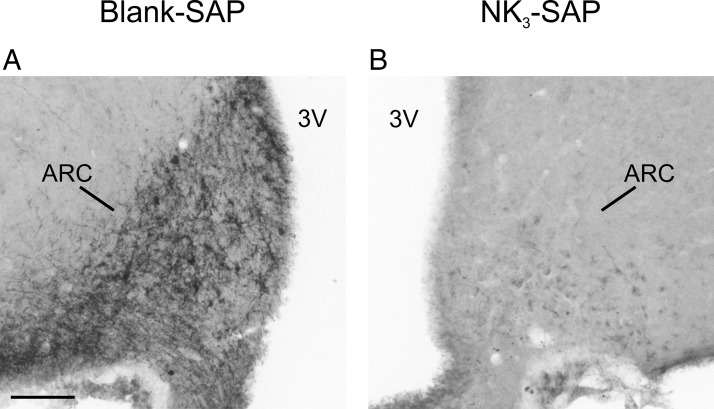
Consistent with previous descriptions, the morphological appearance of Nissl-stained sections through the arcuate nucleus was identical in Blank-SAP and NK3-SAP injected rats (31). Because loss of NKB neurons in the arcuate nucleus is a sensitive marker for KNDy neuron ablation (31), NKB immunohistochemistry was used to characterize the effects of Blank-SAP and NK3-SAP injections. In Blank-SAP rats, the arcuate nucleus is characterized by intensely stained NKB cell bodies accompanied by a dense plexus of NKB fibers that extends to the median eminence (Figure 2A). Rats with accurate targeting of NK3-SAP injections exhibited near-complete loss of NKB neurons within the arcuate nucleus (Figure 2B and Table 2). In sections where NKB neurons were absent, there was a complete loss of the NKB axonal plexus within the arcuate neuropil (Figure 2B). These data agree with studies showing that the NKB axons within the arcuate nucleus originate from KNDy neurons (22, 52, 53).
Figure 2.
Photomicrographs of pro-NKB immunohistochemistry from a representative Blank-SAP and NK3-SAP rat. A, The Blank-SAP control shows intense staining of NKB neuronal elements in the arcuate nucleus. There is jet-black staining of scattered cell bodies with a dense plexus of NKB axons. B, In contrast, rats with accurate targeting of NK3-SAP injections did not show the intense jet-black staining of neurons but only a faint cellular blush consistent with background staining. In addition, the dense plexus of NKB axons within the arcuate neuropil was absent. 3V, third ventricle; ARC, arcuate nucleus. Scale bar, 100 μm (applies to A and B).
Table 2.
Numbers of Immunoreactive Neurons From Control (Blank-SAP) or KNDy-Ablated (NK3-SAP) Rats
| NKB Neurons Midarcuate | NKB Neurons Posterior Arcuate | GnRH Neurons | Dual-Labeled GnRH/Fos Neurons | AVPV Neurons Expressing Fos | |
|---|---|---|---|---|---|
| Blank-SAP | 12.3 ± 0.5 (n = 6) | 47.8 ± 4.2 (n = 4) | 84.9 ± 4.6 (n = 7) | 14.6 ± 4.6 (n = 7) | 28.1 ± 3.2 (n = 6) |
| NK3-SAP | 1.0 ± 0.4a (n = 4) | 2.3 ± 0.9a (n = 3) | 85.8 ± 9.8 (n = 4) | 18.8 ± 1.4 (n = 4) | 38.9 ± 9.3 (n = 4) |
Data are excluded from NK3-SAP-injected rats with less than 90% ablation of KNDy neurons. Values represent the mean ± SEM.
Significantly different, Blank-SAP vs NK3-SAP, P < .001.
Depending on the accuracy of stereotaxic targeting, variable loss of NKB neurons was observed in rats injected with NK3-SAP. In 5 rats, there was preservation of 30%–50% of NKB neurons in the arcuate nucleus after NK3-SAP injections, indicating incomplete KNDy neuron ablation. These incompletely ablated rats were considered missed injections and were not included in the statistical analyses or morphological studies of ovary, endometrium, GnRH/Fos, and AVPV Fos. In all data and figures, only rats in which there was greater than 90% (93.9 ± 0.9%, n = 4) degeneration of arcuate NKB neurons are termed NK3-SAP or KNDy-ablated rats.
KNDy neuron ablation resulted in decreased serum LH, constant diestrus, increased type III follicular atresia, and endometrial atrophy
Serum LH in rats before the stereotaxic surgery was similar between groups (Blank-SAP, 1.4 ± 0.2 ng/mL, n = 7 vs NK3-SAP, 1.4 ± 0.3 ng/mL, n = 3) (Figure 3A). Approximately 30 days after injections, serum LH was significantly reduced in KNDy-ablated rats (0.3 ± 0.06 ng/mL, n = 4) compared with control rats (1.6 ± 0.3 ng/mL, n = 7) (Figure 3A).
Figure 3.
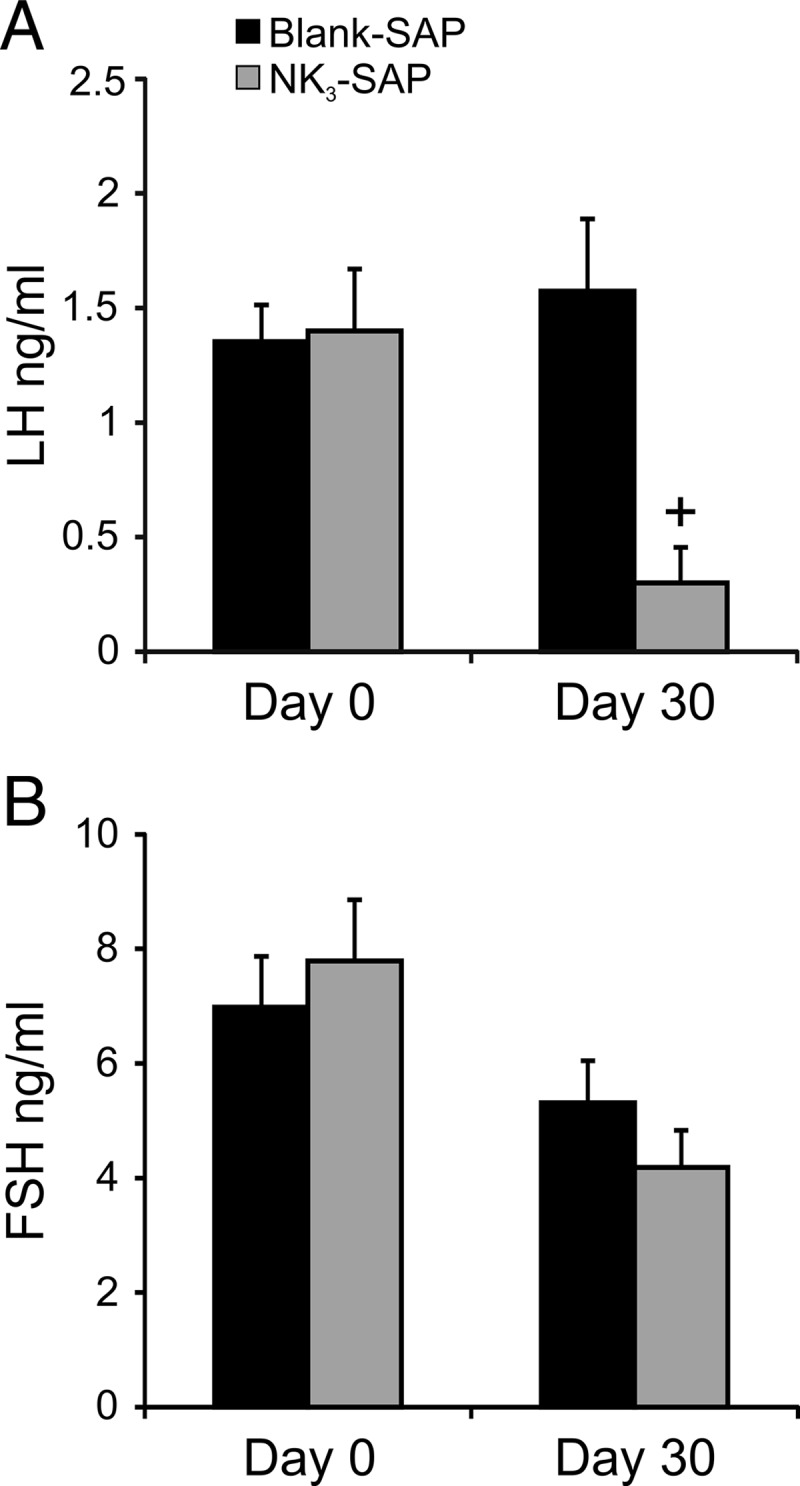
Serum LH (A) and FSH (B) immediately before (d 0) and approximately 30 days after stereotaxic injections of Blank-SAP or NK3-SAP (mean ± SEM). At day 30, serum LH was significantly reduced in KNDy-ablated rats compared with Blank-SAP controls. Serum FSH was not significantly different between Blank-SAP and NK3-SAP rats. n = 7 Blank-SAP rats, n = 3 (d 0) or 4 (d 30) NK3-SAP rats. +, significantly different, Blank-SAP vs NK3-SAP, P = .003.
There was no significant difference in serum FSH between groups before the stereotaxic surgery (Blank-SAP, 7.0 ± 0.9 ng/mL, n = 7 vs NK3-SAP, 7.8 ± 1.1 ng/mL, n = 3) (Figure 3B). Serum FSH was also not different between control and NK3-SAP rats, 30 days after injections (Blank-SAP, 5.3 ± 0.7 ng/mL, n = 7 vs NK3-SAP, 4.2 ± 0.6 ng/mL, n = 4) (Figure 3B).
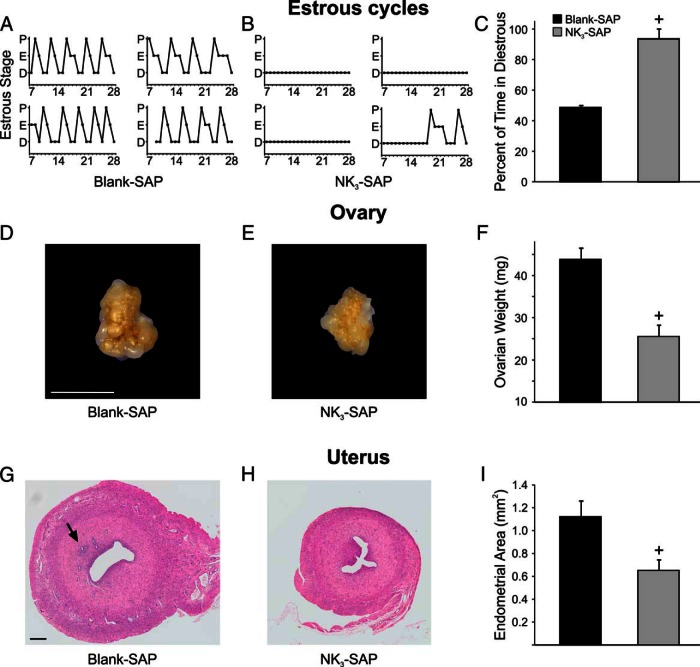
Vaginal smears of control rats (n = 7) showed typical 4- to 5-day estrous cycles (Figure 4A). Vaginal smears from most of the KNDy-ablated rats (n = 3) showed constant diestrus (predominantly leukocytes) for the entire time (Figure 4B). One KNDy-ablated rat exhibited 14 days of constant diestrus followed by a 7-day cycle and the beginning of the next cycle (Figure 4B). Diestrous vaginal smears were detected in control animals 48.6 ± 1.4% of the time, compared with 93.5 ± 6.5% in KNDy-ablated rats (Figure 4C). Estrous smears (cornified epithelial cells) were observed 32.5% of the time in Blank-SAP rats, compared with 4.3% in NK3-SAP rats. Finally, proestrous smears cells (round, nucleated cells) were present 18.9% of the time in in Blank-SAP rats, compared with 2.2% in NK3-SAP rats.
Figure 4.
Effects of KNDy neuron ablation on estrous cycles (A–C), ovary (D–F), and uterus (G–I). Representative graphs show 4- to 5-day estrous cycles in 4 Blank-SAP rats (A) and constant diestrus in 3 out of 4 NK3-SAP rats (B). One NK3-SAP rat showed a long period of constant diestrous, followed by a 7-day cycle and the beginning of the next cycle. C, Vaginal smears from NK3-SAP rats showed a marked increase in the percentage of time spent in diestrous compared with control rats. D and E, Representative photographs of the ovaries from a Blank-SAP and NK3-SAP rat. F, The ovaries weighed significantly less in NK3-SAP rats. G and H, Representative photomicrographs of paraffin-embedded, hematoxylin and eosin-stained uterine sections from a Blank-SAP and NK3-SAP rat. The arrow in G marks endometrial glands, which were not present in 3 out of 4 NK3-SAP rats. I, The endometrial area was significantly reduced in KNDy-ablated rats. Scale bars, 5 mm (in D; applies to D and E) and 200 μm (in G; applies to G and H). n = 7 Blank-SAP and n = 4 NK3-SAP rats. All data represents mean ± SEM. +, significantly different, Blank-SAP vs NK3-SAP (P < .001 for C and F; P < .05 for I).
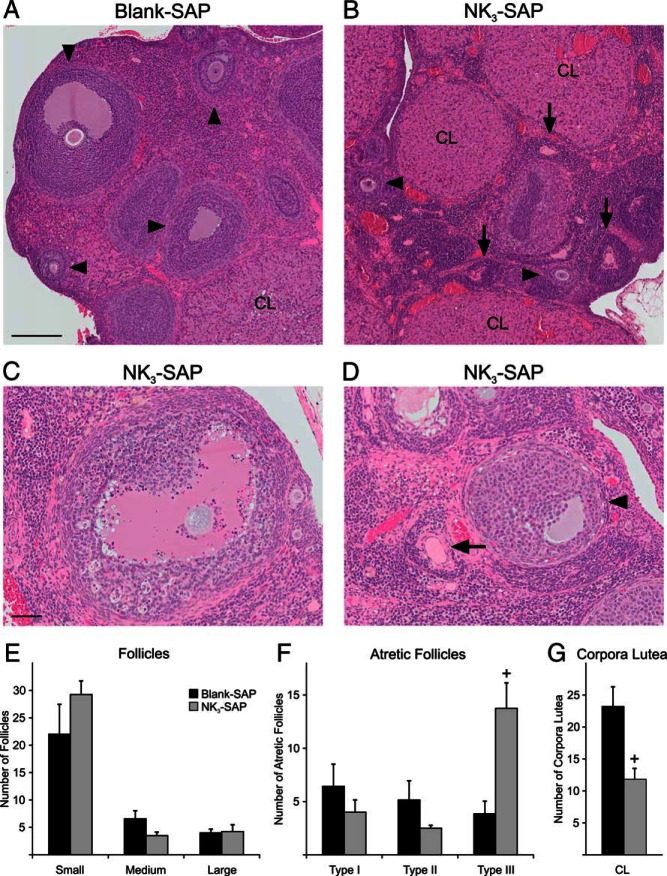
The ovaries were smaller in appearance and weighed significantly less in KNDy-ablated animals (Blank-SAP, 43.8 ± 2.6 mg, n = 7 vs NK3-SAP 25.5 ± 2.6 mg, n = 4) (Figure 4, D–F). Histological sections of the ovaries from both groups showed follicles in various stages of development, follicles undergoing atresia and corpora lutea (Figure 5). There were no significant differences in the number of small (primordial and primary), medium (secondary), and large (antral) follicles between control and KNDy-ablated rats (Figure 5E). In addition, no significant differences were detected in the numbers of follicles undergoing type I and type II atresia. However, there was a 3-fold increase in follicles exhibiting type III atresia in the KNDy-ablated rats compared with control rats (Blank SAP, 3.9 ± 1.2 type III atretic follicles/section, n = 7 vs NK3-SAP, 13.8 ± 2.4 type III atretic follicles/section, n = 4) (Figure 5F).
Figure 5.
A–D, Photomicrographs of hematoxylin and eosin-stained ovarian sections from Blank-SAP (A) and NK3-SAP rats (B–D). The arrowheads (A, B, and D) show developing (secondary or antral) follicles and the arrows show type III follicular atresia. C, Photomicrograph of a large antral follicle with numerous apoptotic cells indicative of type II atresia. D, Type III follicular atresia (arrow) is characterized by collapse of the follicle and fragmentation of the basal lamina. An adjacent viable antral follicle is also seen (arrowhead). E–G, Quantitative morphology showing the number of viable follicles (E) atretic follicles (F) and corpora lutea per section from Blank-SAP and NK3-SAP rats. In KNDy-ablated rats, the number of follicles exhibiting type III atresia is increased, with decreased numbers of corpora lutea. CL, corpora lutea. Scale bars, 200 μm (in A; applies to A and B) and 50 μm (in C; applies to C and D). n = 7 Blank-SAP and n = 4 NK3-SAP rats. +, significantly different, Blank-SAP vs NK3-SAP (P < .01 for type III atresia; P < .05 for corpora lutea).
Although 3 out of the 4 KNDy-ablated rats exhibited constant diestrus for the full 3 weeks, corpora lutea were still observed (Figure 5). The morphological life of corpora lutea has been calculated to be from 13 to 17 days and is markedly prolonged in hypophysectomized rats due to loss of prolactin surges (54). Therefore, the corpora lutea observed in these NK3-SAP rats were likely to have been formed before KNDy neuron ablation. Nevertheless, the number of corpora lutea was significantly reduced in the KNDy-ablated animals (Blank SAP, 23.1 ± 3.2 corpora lutea/section, n = 7 vs NK3-SAP, 11.8 ± 1.8 corpora lutea/section, n = 4).
The endometrium of the KNDy-ablated rats appeared atrophic (Figure 4, G and H). Quantitative analysis showed that the area of the endometrium was significantly reduced from 1.12 ± 0.1 mm2 in the control rats (n = 7) to 0.65 ± 0.09 mm2 (n = 4) in the NK3-SAP rats (Figure 4I). Inspection of the uterine sections showed endometrial glands in every control animal (12.3 ± 2.8 glands/uterine cross-section, n = 7). In contrast, endometrial glands were absent in 3 out of the 4 KNDy-ablated rats. The remaining rat exhibited 19 glands, despite having endometrial area that was similar to the other ablated rats (0.7 mm2) and vaginal smears indicating constant diestrus.
Blank-SAP and NK3-SAP rats gained similar amounts of weight (∼15 g) during the 4-week period after SAP injections. The body weight of Blank-SAP rats was 251.2 ± 5.2 g before surgery and increased to 266.8 ± 4.0 g (mean ± SEM, n = 7). The NK3-SAP rats weighed 246.6 ± 3.9 g before surgery and increased to 261.85 ± 2.6 g (n = 4). Thus, despite the evidence that estradiol is reduced in KNDy-ablated rats (diestrous vaginal smears and endometrial atrophy), body weights were not significantly different. These findings are consistent with our previous studies that show KNDy neurons are required for the estradiol modulation of body weight (31).
The E2P-induced gonadotropin surge was markedly increased in KNDy-ablated rats
An LH surge (defined as 2 SDs above the mean from the morning) was observed in all rats except 1 Blank-SAP animal. Serum LH was lower in NK3-SAP animals on the morning of P capsule implantation (Blank-SAP, 6.1 ± 1.2 ng/mL, n = 7 vs NK3-SAP 0.5 ± 0.1 ng/mL, n = 4) (Figure 6A). Unexpectedly, the peak levels of LH during the surge were more than 3 times greater in KNDy-ablated rats compared with that of Blank-SAP rats (Blank-SAP, 16.5 ± 2.1 ng/mL, n = 7 vs NK3-SAP, 53.5 ± 16.5 ng/mL, n = 4) (Figure 6A). Serum LH in KNDy-ablated rats increased by more than 100-fold from the morning to the afternoon (Figure 6A).
Figure 6.
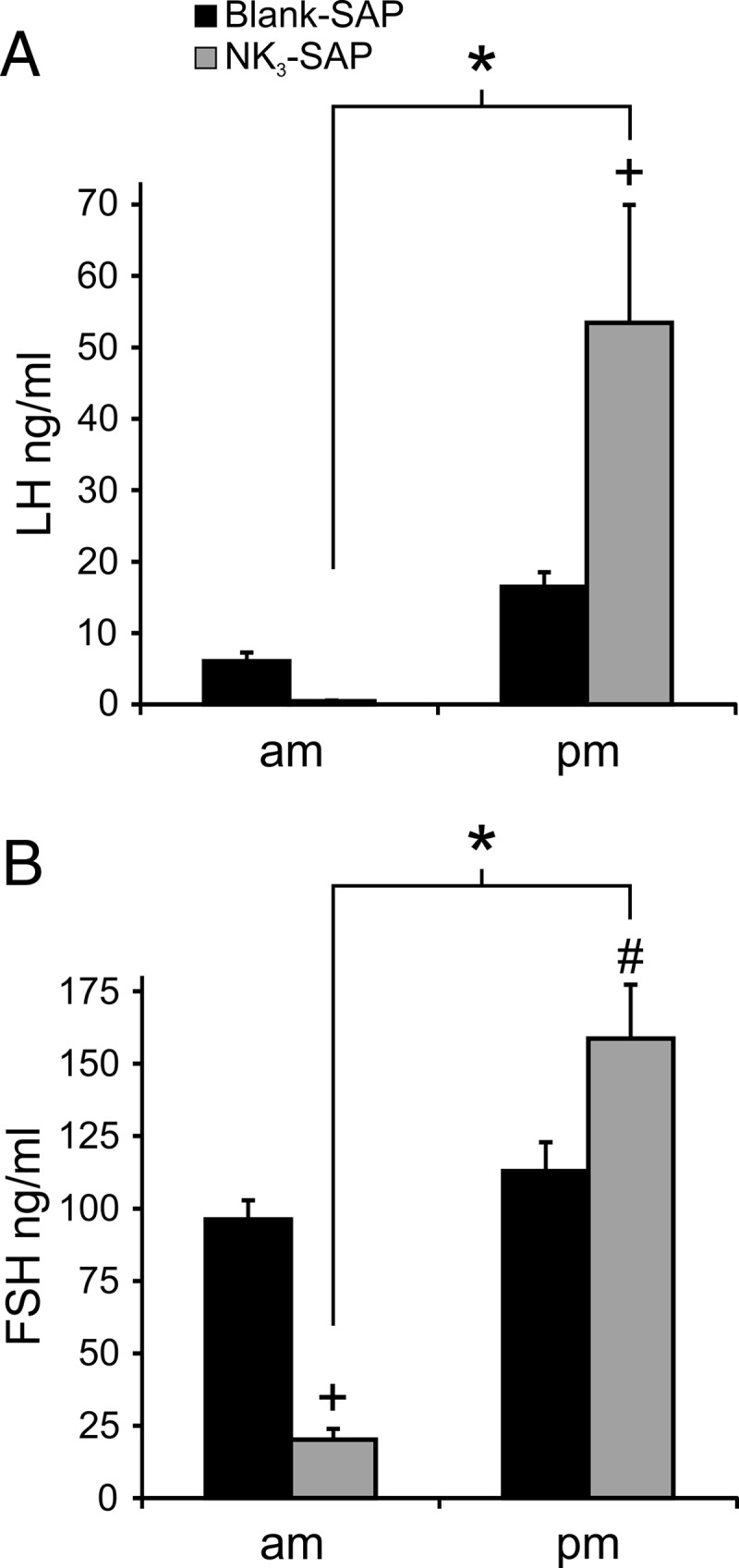
Serum LH (A) and FSH (B) in Blank-SAP and NK3-SAP rats receiving an E2P regimen to induce an LH surge in the afternoon (mean ± SEM). The level of LH during the surge was 3-fold higher in KNDy-ablated rats relative to controls. The FSH surge was also higher in KNDy-ablated rats. The am blood sample was taken at 8:30 am, and the pm blood sample was taken between 3:30 and 4:30 pm. n = 7 Blank-SAP and n = 4 NK3-SAP rats. +, significantly different, Blank-SAP vs NK3-SAP, P < .001; #, significantly different, Blank-SAP vs NK3-SAP, P < .01; *, significantly different than am, within NK3-SAP, P < .001.
The surge in serum FSH was also increased by KNDy neuron ablation. Serum FSH was significantly lower in NK3-SAP rats on the morning of the surge (Blank-SAP 96.2 ± 6.6 ng/mL, n = 7 vs NK3-SAP 20.3 ± 2.7 ng/mL, n = 4) (Figure 6B). At the time of the LH surge, serum FSH was significantly greater in NK3-SAP rats than Blank-SAP controls (Blank-SAP, 113.0 ± 9.9 ng/mL, n = 7 vs NK3-SAP, 158.7 ± 18.6 ng/mL, n = 4). The change in serum FSH from the morning to the afternoon within KNDy-ablated rats was more than 7-fold (Figure 6B).
KNDy neuron ablation did not alter Fos expression in GnRH or AVPV neurons at the time of the LH surge
The number of GnRH neurons counted in 4 sections was nearly identical in control and KNDy-ablated rats (Figure 7 and Table 2). In addition, there was no significant difference in the percentage of GnRH neurons expressing Fos (Blank-SAP, 16.6 ± 4.9% GnRH/Fos neurons, n = 7 vs KNDy ablated, 22.4 ± 1.8% GnRH/Fos, n = 4). Representative maps of Fos-positive nuclei in the AVPV of a Blank-SAP and NK3-SAP rat are shown in Figure 8. The number of Fos-expressing cells counted in the AVPV was not significantly different between groups (Table 2).
Figure 7.
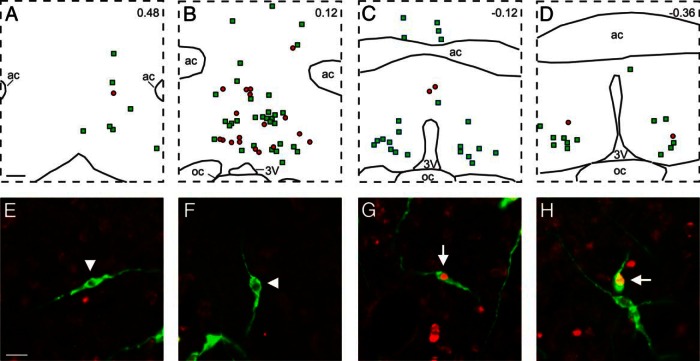
A–D, Computer-assisted maps of GnRH (green squares) and GnRH/Fos (red circles) neurons in a Blank-SAP rat. The distance from bregma is indicated in upper right corners. E–H, Fluorescent photomicrographs of GnRH (green) and Fos (red) immunoreactivity. E and F show single-labeled GnRH neurons (arrowheads), and G and H show green GnRH neurons with red nuclear staining for Fos neurons (arrows). Although the LH surge was markedly amplified in KNDy-ablated rats, there was no change in number of neurons expressing GnRH or GnRH/Fos between Blank-SAP and NK3-SAP rats (Table 2). 3V, third ventricle; ac, anterior commissure; oc, optic chiasm. Scale bars, 250 μm (in A; applies to A–D) and 20 μm (in E; applies to E–H).
Figure 8.
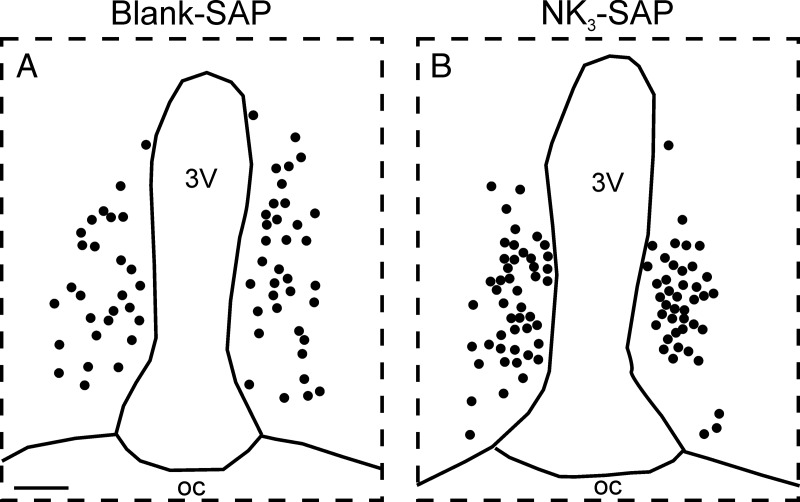
Computer-assisted maps of Fos-positive nuclei in the AVPV of a Blank-SAP (A) and NK3-SAP rat (B) killed at the peak of the LH surge. There was no significant difference in the number of AVPV Fos cells between groups. 3V, third ventricle; oc, optic chiasm. Scale bar, 100 μm (in A; applies to A and B).
The reproductive axis of rats with incomplete KNDy neuron ablation was similar to controls
Analysis of estrous cycles appeared to be a good predictor of the extent of KNDy neuron ablation. With one exception, rats with incomplete KNDy neuron ablation exhibited regular 4- to 5-day estrous cycles. Similar to Blank-SAP rats, diestrous (58.9%), estrous (25.8%), or proestrous (15.3%) smears were detected in the incompletely ablated rats (n = 5). In experiment 1, the serum LH of incompletely ablated rats was 1.2 ± 0.08 ng/mL on day 0 and 1.0 ± 0.2 ng/mL on day 30 (n = 5). Serum FSH in experiment 1 was 6.2 ± 0.3 ng/mL on day 0 and 5.7 ± 0.5 ng/mL on day 30 (n = 5). Ovarian weights were also similar to controls (41.5 ± 3.8 mg, n = 5). The E2P-induced gonadotropin surge was not amplified in incompletely ablated rats (LH, 18.2 ± 6.4 ng/mL, n = 5; FSH, 90.6 ± 7.8 ng/mL, n = 5). Finally, similar to the other groups of rats, incompletely ablated rats gained approximately 15 g over the course of experiment 1. They weighed 253.3 ± 4.4 g before the time of the SAP injection and 268.0 ± 5.9 g 4 weeks later.
Discussion
The present study underscores the importance of KNDy neurons in reproductive function. When over 90% of KNDy neurons were ablated, estrous cycles were characterized predominantly by a state of constant diestrus. Tonic blood levels of serum LH were reduced as well as the size of the ovaries. Hypogonadism was evident by the constant diestrous vaginal smears and endometrial atrophy, both signs of reduced levels of circulating estrogens. Microscopically, the ovaries of KNDy-ablated rats showed follicular development to the antral stage, reflecting continued secretion of FSH (55). However, there was increased type III follicular atresia with decreased corpora lutea, suggesting that the acyclicity resulted in follicles that underwent atresia rather than ovulating and progressing to corpora lutea. The low serum LH was not secondary to a defect in the anterior pituitary gland or GnRH neurons because an E2P stimulus was still able to generate an afternoon GnRH/LH surge.
The occurrence of insufficient serum LH for maintenance of gonadal function in KNDy-ablated rats indicates that HH can be caused by alterations in the function of KNDy neurons. Low levels of serum LH, absent menstrual cycles, small ovaries, and uterine atrophy are characteristic of women with HH due to mutations in the genes encoding kisspeptin, NKB, or their respective receptors (11–14, 56, 57). Serum FSH is more variable, with reports of normal or nearly normal FSH in patients with TAC3 (NKB) and TACR3 (NK3R) mutations (13, 56). Pulsatile GnRH administration restores serum LH and gonadal function, indicating that the HH is secondary to insufficient GnRH secretion (11, 56). It is not known whether these individuals will respond with an LH surge if given a regimen of steroid replacement designed to stimulate the surge (58). Because we did not ablate KNDy neurons before puberty, the full phenotype of congenital HH, in which there is abnormal pubertal progression, was not addressed in the present study.
In the present study, HH only occurred in rats in which KNDy neurons were reduced to less than 10% of the control population. Rats with ablation of 50%–70% of KNDy neurons were similar to controls in gonadotropin secretion, estrous cyclicity, and ovarian weights. These findings underscore the importance of histologically verifying the extent of cell loss when using NK3-SAP to ablate KNDy neurons. A previous study showed that at least 70%–90% of GnRH neurons need to be removed or dysfunctional before an adverse reproductive phenotype is detected (59). Thus, like GnRH neurons, there is considerable redundancy in KNDy neuron circuitry.
Surprisingly, the E2P-induced LH surge was markedly increased in KNDy-ablated rats. The occurrence of a robust gonadotropin surge after KNDy-neuron ablation indicates that the neuronal network for triggering the LH surge, including GnRH neurons, is intact. Studies of transgenic knockout mice show that kisspeptin signaling is required for the LH surge (60–62), but the present study shows that KNDy neurons are not the critical subgroup of kisspeptin neurons. These findings agree with classic studies showing that the neural network that stimulates the LH surge in the rodent is generated outside of the arcuate nucleus (63). Kisspeptin neurons in the AVPV and adjacent periventricular region are particularly important for the LH surge (64). AVPV neurons are modulated by estrogen positive feedback (65), receive circadian signals (66), project to GnRH neurons (67), and are activated during the surge (29). Because the diffusion of NK3-SAP does not extend to the rostral hypothalamus, AVPV kisspeptin neurons are not lesioned in KNDy-ablated rats (31). The unaltered Fos expression in the AVPV during the LH surge is additional evidence that the AVPV is preserved in KNDy-ablated rats.
The mechanism for the amplified E2P-induced LH surge in KNDy-ablated rats is not known. It could be secondary to changes at the level of the anterior pituitary gland, such as increased sensitivity to GnRH or an increase in the releasable pool of LH. Alternatively, the higher levels of LH could be due to increased secretion of GnRH into the portal capillary system. To address whether there was an increase in the number of activated GnRH neurons, we performed dual-label GnRH/Fos immunohistochemistry, an established method for investigating subpopulations of GnRH neurons participating in the surge (29, 50, 68, 69). This procedure showed no change in the number of GnRH neurons or the percentage of GnRH neurons expressing Fos. The number of neurons expressing Fos in the AVPV was also not significantly different between control and KNDy-ablated rats. Thus, the amplified LH surge does not appear to be secondary to recruitment of additional GnRH or AVPV neurons. However, these findings do not address whether there is increased firing of the Fos-expressing GnRH neurons or increased secretion of GnRH into the portal system.
We hypothesize that KNDy neuron ablation removes an inhibitory influence that otherwise limits the magnitude of the LH surge. A likely candidate is dynorphin, because it has been shown to consistently inhibit LH secretion. Administration of prodynorphin-derived opioid peptides blocks the LH surge in the rat (70). In addition, the inhibitory effects of NK3R agonists on LH secretion are via κ-opioid receptors, the primary receptor for dynorphin (71). In the ewe, dynorphin neurons in the arcuate nucleus mediate the inhibition of GnRH pulses by P (72, 73). Interestingly, a recent study showed that injection of a κ-opioid receptor agonist into the preoptic area increases the E2-induced LH surge, similar to their amplification of the LH surge after NK3-SAP injections into the arcuate nucleus (32). Finally, central infusion of a κ-opioid receptor agonist on proestrous afternoon markedly amplifies the LH surge, suggesting that endogenous κ-opioid tone limits the size of the preovulatory LH surge in intact rats (74). The striking amplification of the LH surge shown here provides evidence that KNDy neurons are the source of the endogenous κ-opioid tone that limits the LH surge on proestrous afternoon.
It has been previously shown that a modest knockdown of kisspeptin expression in the arcuate nucleus of adult rats will inhibit pulsatile LH secretion and lengthen estrous cycles but have no effect on the LH surge (75). Consistent with our studies, injections of diphtheria toxin to ablate kisspeptin cells in genetically engineered adult mice results in acyclicity and infertility (76). However, these results do not address the specific function of KNDy neurons, because the systemic injections of diphtheria toxin targeted all kisspeptin cells, including those in the AVPV, arcuate, and anterior pituitary gland. Finally, transgenic Tac2 (NKB) knockout mice exhibit ovarian atrophy, impaired estrous cycles, and infertility but recover their reproductive function over time (77). Further studies will be needed to determine whether recovery of estrous cyclicity also occurs in KNDy-ablated rats.
In laboratory rats and mice, ovariectomy causes obesity, and conversely, E2 treatment reduces body weight in OVX rodents (78–80). In the present study, body weights were equal between the NK3-SAP and Blank-SAP rats, despite the clear evidence (diestrous vaginal smears and endometrial atrophy) that estrogen secretion was reduced by KNDy neuron ablation. These findings agree with our previous demonstration that KNDy neurons are required for the increase in body weight that occurs secondary to E2 withdrawal (31).
In summary, ablation of over 90% of KNDy neurons reduced tonic levels of serum LH and resulted in constant diestrus, ovarian atrophy with increased type III follicular atresia and endometrial atrophy. In contrast, levels of serum LH in the E2P-induced gonadotropin surge were markedly elevated in KNDy-ablated rats. Because the number of GnRH or AVPV neurons expressing Fos were not significantly different between control and KNDy-ablated rats, the increased magnitude of the LH surge does not appear to be secondary to recruitment of additional GnRH or AVPV neurons. Our studies show that KNDy neurons are essential for tonic levels of serum LH, ovarian function and reproductive cyclicity, and suggest a role for these neurons in limiting the magnitude of the LH surge. These data support the hypothesis that dysfunction in KNDy neuron signaling could be the mechanism of HH in patients with mutations in the genes encoding kisspeptin, NKB, or their receptors.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute on Aging Grants R01 AG032315 and R01 AG047887. The hormone assays were performed at the Ligand Assay and Analysis Core, University of Virginia Center for Research in Reproduction supported by the National Institute of Child Health and Human Development Grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVPV
- anterior ventral periventricular nucleus
- E2
- 17β-estradiol
- E2P
- E2 and P
- HH
- hypogonadotropic hypogonadism
- KNDy
- arcuate neurons coexpressing kisspeptin, NKB, and dynorphin
- NKB
- neurokinin B
- NK3-SAP
- NK3R agonist conjugated to SAP
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomized
- P
- progesterone
- SAP
- saporin.
References
- 1. Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., 3rd Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. [DOI] [PubMed] [Google Scholar]
- 2. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. [DOI] [PubMed] [Google Scholar]
- 3. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 4. Borsay BÁ, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance P immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100:141–152. [DOI] [PubMed] [Google Scholar]
- 5. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arucate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. [DOI] [PubMed] [Google Scholar]
- 8. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153. [DOI] [PubMed] [Google Scholar]
- 9. Silveira LF, Latronico AC. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98:1781–1788. [DOI] [PubMed] [Google Scholar]
- 10. Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539. [DOI] [PubMed] [Google Scholar]
- 11. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 12. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635. [DOI] [PubMed] [Google Scholar]
- 15. Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384:429–442. [DOI] [PubMed] [Google Scholar]
- 16. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. [DOI] [PubMed] [Google Scholar]
- 17. Alçin E, Sahu A, Ramaswamy S, et al. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2013;25:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 20. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. [DOI] [PubMed] [Google Scholar]
- 22. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. [DOI] [PubMed] [Google Scholar]
- 23. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2015;112:13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538. [DOI] [PubMed] [Google Scholar]
- 28. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21:305–311. [DOI] [PubMed] [Google Scholar]
- 31. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helena CV, Toporikova N, Kalil B, et al. KNDy neurons modulate the magnitude of the steroid-induced luteinizing hormone surges in ovariectomized rats. Endocrinology. 2015;156:4200–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. [DOI] [PubMed] [Google Scholar]
- 34. Krajewski-Hall SJ, Mittelman-Smith MA, Williams H, LaFrance KJ, McMullen NT, Rance NE. A role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the regulation of estrous cycles and the estrogen modulation of body temperature. Program of the 42th Annual Meeting of the Society for Neuroscience, New Orleans, LA, 2012 (Abstract 585.502). [Google Scholar]
- 35. Krajewski-Hall SJ, Mittelman-Smith MA, McMullen NT, Rance NE. Ablation of arcuate KNDy neurons amplifies the LH surge in steroid-primed, ovariectomized rats. Program of the 43th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2013 (Abstract 274.201). [Google Scholar]
- 36. Corboz MR, Rivelli MA, Eckel SP. Bronchoconstrictor effect of the tachykinin NK3-receptor agonists [MePhe7]-neurokinin B and senktide in the isolated guinea pig lung. Exp Lung Res. 2010;36:509–521. [DOI] [PubMed] [Google Scholar]
- 37. Drapeau G, d'Orléans-Juste P, Dion S, Rhaleb NE, Regoli D. Specific agonists for neurokinin B receptors. Eur J Pharmacol. 1987;136:401–403. [DOI] [PubMed] [Google Scholar]
- 38. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. [DOI] [PubMed] [Google Scholar]
- 39. Yoshida M, Sanbuissyo A, Hisada S, Takahashi M, Ohno Y, Nishikawa A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J Toxicol Sci. 2009;34(suppl 1):SP189–SP197. [DOI] [PubMed] [Google Scholar]
- 40. Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. [DOI] [PubMed] [Google Scholar]
- 41. Braw RH, Tsafriri A. Effect of PMSG on follicular atresia in the immature rat ovary. J Reprod Fertil. 1980;59:267–272. [DOI] [PubMed] [Google Scholar]
- 42. Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. [DOI] [PubMed] [Google Scholar]
- 43. Wise PM, Rance N, Barraclough CA. Effects of estradiol and progesterone on catecholamine turnover rates in discrete hypothalamic regions in ovariectomized rats. Endocrinology. 1981;108:2186–2193. [DOI] [PubMed] [Google Scholar]
- 44. Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136:3604–3610. [DOI] [PubMed] [Google Scholar]
- 45. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. [DOI] [PubMed] [Google Scholar]
- 46. Urbanski HF. Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod. 1991;44:681–686. [DOI] [PubMed] [Google Scholar]
- 47. Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res. 1998;798:127–139. [DOI] [PubMed] [Google Scholar]
- 48. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier, Inc; 2007. [Google Scholar]
- 50. Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA. 1990;87:5163–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cholanian M, Krajewski-Hall SJ, McMullen NT, Rance NE. Chronic oestradiol reduces the dendritic spine density of KNDy (kisspeptin/neurokinin B/dynorphin) neurones in the arcuate nucleus of ovariectomised Tac2-enhanced green fluorescent protein transgenic mice. J Neuroendocrinol. 2015;27:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Malven PV, Sawyer CH. A luteolytic action of prolactin in hypophysectomized rats. Endocrinology. 1966;79:268–274. [DOI] [PubMed] [Google Scholar]
- 55. Hunzicker-Dunn M, Mayo K. Gonadotropin signaling in the ovary. In: Neill JD, Plant TM, Pfaff DW, et al., eds. Knobil and Neill's Physiology of Reproduction. 3rd ed San Diego, CA: Elsevier; 2006:547–592. [Google Scholar]
- 56. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295. [DOI] [PubMed] [Google Scholar]
- 57. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shaw ND, Srouji SS, Histed SN, Hall JE. Differential effects of aging on estrogen negative and positive feedback. Am J Physiol Endocrinol Metab. 2011;301:E351–E355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uenoyama Y, Nakamura S, Hayakawa Y, et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol. 2015;27:187–197. [DOI] [PubMed] [Google Scholar]
- 63. Barraclough CA. Sex steroid regulation of reproductive neuroendocrine processes. In: Greep RO, Astwood EB, eds. Handbook of Physiology, Endocrinology II, Part 1. Baltimore, MD: Waverly Press; 1973:29–56. [Google Scholar]
- 64. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 66. Piet R, Fraissenon A, Boehm U, Herbison AE. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J Neurosci. 2015;35:6881–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156:2582–2594. [DOI] [PubMed] [Google Scholar]
- 68. Lee WS, Smith MS, Hoffman GE. cFos activity identifies recruitment of luteinizing hormone-releasing hormone neurons during the ascending phase of the proestrous luteinizing hormone surge. J Neuroendocrinol. 1992;4:161–166. [DOI] [PubMed] [Google Scholar]
- 69. Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. [DOI] [PubMed] [Google Scholar]
- 70. Zhang Q, Gallo RV. Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine. 2002;18:27–32. [DOI] [PubMed] [Google Scholar]
- 71. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904. [DOI] [PubMed] [Google Scholar]
- 72. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. [DOI] [PubMed] [Google Scholar]
- 73. Goodman RL, Holaskova I, Nestor CC, et al. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152:3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Q, Gallo RV. Presence of κ-opioid tone at the onset of the ovulatory luteinizing hormone surge in the proestrous rat. Brain Res. 2003;980:135–139. [DOI] [PubMed] [Google Scholar]
- 75. Beale KE, Kinsey-Jones JS, Gardiner JV, et al. The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology. 2014;155:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. [DOI] [PubMed] [Google Scholar]
- 77. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156:1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol (Copenh). 1973;72:551–568. [DOI] [PubMed] [Google Scholar]
- 79. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. [DOI] [PubMed] [Google Scholar]
- 80. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]