Abstract
Cystic fibrosis (CF)-related diabetes in humans is intimately related to exocrine pancreatic insufficiency, yet little is known about how these 2 disease processes simultaneously evolve in CF. In this context, we examined CF ferrets during the evolution of exocrine pancreatic disease. At 1 month of age, CF ferrets experienced a glycemic crisis with spontaneous diabetic-level hyperglycemia. This occurred during a spike in pancreatic inflammation that was preceded by pancreatic fibrosis and loss of β-cell mass. Surprisingly, there was spontaneous normalization of glucose levels at 2–3 months, with intermediate hyperglycemia thereafter. Mixed meal tolerance was impaired at all ages, but glucose intolerance was not detected until 4 months. Insulin secretion in response to hyperglycemic clamp and to arginine was impaired. Insulin sensitivity, measured by euglycemic hyperinsulinemic clamp, was normal. Pancreatic inflammation rapidly diminished after 2 months of age during a period where β-cell mass rose and gene expression of islet hormones, peroxisome proliferator-activated receptor-γ, and adiponectin increased. We conclude that active CF exocrine pancreatic inflammation adversely affects β-cells but is followed by islet resurgence. We predict that very young humans with CF may experience a transient glycemic crisis and postulate that pancreatic inflammatory to adipogenic remodeling may facilitate islet adaptation in CF.
Diabetes is a frequent comorbidity of cystic fibrosis (CF), with approximately 80% of individuals carrying severe CF transmembrane conductance regulator (CFTR) mutations developing diabetes by middle age (1). CF-related diabetes (CFRD) occurs primarily due to deficient insulin secretion, especially first phase insulin secretion (2), with a lesser but variable contribution of insulin resistance (3). Historically, deficient insulin secretion in CFRD was assumed to be due to collateral damage to islets secondary to the severe exocrine pancreatic disease that occurs in CF. Those with CF who remain pancreatic sufficient are far less likely to develop diabetes (4). Individuals with CFRD have an approximately 50% loss of β-cells postmortem (5); however, this degree of β-cell loss alone is likely insufficient to cause diabetes (6). Furthermore, nonfirst phase insulin secretion, including late-phase and secretagogue-induced insulin secretion, remains intact in many CFRD patients long after exocrine pancreas decline (7). These points raise the possibility that there is intrinsic β-cell dysfunction in CF islets independent of active exocrine pancreatic disease.
Recent work in CF animal models also suggests that CF induces a degree of β-cell dysfunction independent of major structural pancreatic damage. For example, newborn CF ferrets exhibit loss of first phase insulin secretion, despite a lack of pancreatic inflammation, apoptosis, and structural damage (8). Furthermore, β-cells of CF mice fail to compensate for increased insulin resistance occurring later in life despite minimal pancreatic pathology (9) and CF pigs are born with abnormal glucose tolerance and impaired insulin secretion despite sparing of islet mass (10). Finally, isolated islets from newborn CF ferrets exhibit impaired glucose stimulated insulin secretion (8). Thus, the relative contribution of intrinsic islet dysfunction vs indirect dysfunction due to exocrine pancreatic disease remains unclear in CF.
The relationship between the natural history of glycemia in CF and the progression of exocrine pancreatic disease is presently unknown. In CF ferrets, exocrine pancreas disease begins at birth and by 1 month of age there is extensive pancreatic fibrosis, ductal dilation, and marked loss of pancreatic acini (8). As CF animals age, pancreatic adipogenesis increases and islets remodel within fibrotic areas surrounding large ducts (11). These histologic changes are nearly identical to human CF pancreas pathology (11). In this study, we sought to determine the relation between pancreatic pathology and glycemic status in ferrets from birth to 7 months of age. Our results demonstrate a strong coupling between incipient pancreatic inflammation and glycemic deterioration. Unexpectedly, there was subsequent recovery of β-cell function and glycemic status, coinciding with the expansion of pancreatic adipose tissue.
Materials and Methods
Rearing of CF ferrets
All animal experimentation was approved by the Institutional Animal Care and Use Committee. The previously described CFTR exon-10 disrupted ferret model was used for all studies (12) and both male and female animals were included. CFTR knockout kits were paired with a non-CF littermate at birth and reared as previously described (13) with the following exception. All kits were reared on the antibiotics metronidazole (20 mg/kg, 2 times daily) and piperacillin/tazobactam (4.0 mg/kg, 2 times daily) from birth and enrofloxacin (10 mg/kg, 2 times daily) was initiated at 5 days of age. These antibiotics were maintained throughout life for both CF and non-CF controls.
Oral glucose tolerance tests (OGTTs) and mixed meal tolerance tests (MMTTs)
OGTTs and MMTTs were performed on pairs of age-matched CF and non-CF ferrets fasted for 4 hours. For OGTTs, an oral glucose dose of 1.75-g/kg body weight (glucose tolerance beverage; Thermo Scientific) was administered in 3 minutes. For MMTTs, the meal dose was normalized to a body surface area estimate (8). Mixed meals included Elecare formula (Abbott), administered by gavage until ferrets could drink from a nipple. In weaned animals (∼5 wk), the mixed meal also included canned food (Fancy Feast). Each mixed meal contained a total of 0.026-g carbohydrate/cm2 body surface area estimate (8). Blood glucose was measured on tail pin-prick samples by portable meter (One Touch Lifescan), and plasma was obtained for insulin assays. Owing to the fragile health of young CF ferrets, venous blood draws were not feasible before approximately 2 months of age. Similarly, formal MMTTs and OGTTs were not possible before 1 month of age. Area under the cure (AUC) was calculated using the trapezoid rule.
L-arginine stimulation test (AST)
ASTs were performed on 4-hour fasted CF and non-CF age-matched ferrets. The ferrets were injected iv with L-arginine monohydrochloride (Sigma) 0.25 g/kg in 0.9% saline.
Histology and immunohistochemistry
At necropsy, pancreata were fixed in 10% neutral buffered formalin, processed and embedded in paraffin, and sectioned at 4 μm. Sections were stained with hematoxylin and eosin for light microscopy. Sections were immunohistochemical stained using anti-insulin antibody (MP Biomedicals) (8) and counterstained with hematoxylin. β-Cell mass was quantified as the percentage of parenchymal tissue area positive for insulin staining using ImageJ. Aperio whole-slide scanning technology and a color deconvolution algorithm was used.
RNA quantification in pancreatic samples
RNA expression was measured by the QuantiGene Plex Assay kit (Affymetrix). Bead-based oligonucleotide probe sets specific for ferret were developed by Affymetrix (Supplemental Materials). Probes were generated for 16 target transcripts: TNFα, IL-8, IL-1β, IL-6, chemokine (C-X-C motif) ligand 10 (CXCL10), TGFα, TGFβ1, insulin, glucagon, somatostatin, pancreatic polypeptide (PP), solute carrier family 2 (Glut2), pancreatic and duodenal homeobox 1 (PDX1), peroxisome proliferator-activated receptor gamma (PPARγ), adiponectin, and sex-determining region Y-box 9 (Sox9). Probes were also generated for 4 internal control reference transcripts: hypoxanthine phosphoribosyltransferase 1, peptidylprolyl isomerase B/cyclophilin B (PPIB), ribosomal large protein P0 (RPLP0), and ribosomal protein L32 (RPL32). Pancreatic homogenates were prepared using the QuantiGene Sample Processing kit (Affymetrix). Homogenates were incubated overnight at 54°C with X-MAP beads harboring the oligonucleotide capture extenders, label extenders, and blocking probes. Samples were then incubated with the preamplifier, amplifier, and labeled probes at 50°C for 1 hour followed by binding to streptavidin-conjugated R-phycoerythrin at room temperature for 30 minutes. Streptavidin-conjugated R-phycoerythrin fluorescence was measured on a Luminex instrument (Bio-Plex). The RNA levels for target genes were normalized to levels of PPIB, which was chosen from the 4 references genes as the best internal control based on transcript abundance and consistency across age and genotype. RPLP0 and RPL32 were also valid internal controls but often saturated the assay unless diluted. Hypoxanthine phosphoribosyltransferase 1 transcript levels varied with age. For all assays the same lot of probes was used for data generated in this manuscript. The QuantiGene Plex Assay kit (Affymetrix) cites an intraassay and interassay % coefficient of variation (CV) of less than 15% and less than 20%, respectively. We also empirically calculated the intraassay and interassay %CV for 11 RNAs evaluated using triplicate measurements (Table 1). For all 11 RNAs, the average intraassay CV was 9.9% and the average interassay CV was 14.9%.
Table 1.
Intraassay and Interassay %CV for RNA Detection
| Gene | GCG | SST | INS | PP | TNFα | IL-8 | IL-1β | CXCL10 | IL-6 | TGFα | TGFβ1 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intraassay %CV | 9.5 | 9.0 | 10.2 | 9.7 | 10.0 | 13.6 | 8.8 | 10.0 | 7.0 | 10.7 | 10.1 | 9.9 |
| Interassay %CV | 11.4 | 18.4 | 13.2 | 17.2 | 17.7 | 14.8 | 15.3 | 12.1 | 17.1 | 15.7 | 11.4 | 14.9 |
Abbreviations: CV, coefficient of variation; GCG, glucagon; SST, somatostatin; INS, insulin; PP, pancreatic polypeptide; TNFα, tumor necrosis factor α; IL-8, interleukin-8; IL-1β, interleukin 1 β; CXCL10, chemokine (C-X-C Motif) ligand 10; IL-6, interleukin 6; TGFα, transforming growth factor α; TGFβ1, transforming growth factor β1.
Measurements of plasma insulin, IL-6, IL-8, TNFα, TGFβ1, and cortisol
Plasma insulin was measured using human anti-insulin antibody and a Bio-Plex Assay (Millipore) (for antibodies, please see Table 2). This assay only detects mature insulin. Plasma IL-6, IL-8, and TNFα were measured using a Canine Bio-Plex Assay (Millipore). Plasma TGFβ1 was measured using a mouse/rat/porcine/canine TGFβ1 ELISA (R&D Systems). Plasma cortisol was measured by ELISA (Calbiotech). The manufacturer reported %CV (intraassay/interassay) for these analytes are as follows: insulin (<10%/<15%), IL-6 (<5%/<15%), IL-8 (<5%/<15%), TNFα (<5%/<15%), TGFβ1 (<3.5%/<8.3%), and cortisol (<7.3%/<11.3%).
Table 2.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Insulin | Unknown | Human insulin magnetic bead | Millipore, HMHEMAG-34K | Capture monoclonal, detection monoclonal | Kit |
| IL-6 | Unknown | Canine IL-8 magnetic bead panel | Millipore, CCYTOMAG-90K | Vendor will not disclose | Kit |
| IL-8 | Unknown | Canine TNFα magnetic bead panel | Millipore, CCYTOMAG-90K | Vendor will not disclose | Kit |
| TNFα | Unknown | Canine IL-6 magnetic bead panel | Millipore, CCYTOMAG-90K | Vendor will not disclose | Kit |
| TGFβ1 | Unknown | Mouse/rat/porcine/canine TGFβ1 immunoassay | R&D Systems, MB100B | Capture monoclonal, detection polyclonal | Kit |
| Cortisol | Unknown | Cotisol ELISA | Calbiotech, CO103S | Anticortisol monoclonal antibody | Kit |
| Insulin | Unknown | Insulin polyclonal antibody | MP Biomedical, 651041 | Guinea pig | 1:2000 |
In situ hybridization for pancreatic insulin and glucagon RNA
Pancreata were fixed in 10% neutral-buffered formalin, processed and embedded in paraffin, and sectioned at 10 μm. Sections were baked for 1 hour at 60°C. After baking, slides were rinsed in xylene 2 times for 10 minutes per rinse, incubated in 100% ethanol 2 times for 10 minutes per rinse, and then air dried. Slides were then processed using the QuantiGene ViewRNA (QVT0012; Affymetrix) kit. Slides were incubated in 1x pretreatment (QVT0500; Affymetrix) buffer at 95°C for 10 minutes, followed by 15 minutes protease digestion at 40°C and postfixation with 10% neutral buffered formalin for 5 minutes. The type 1 and type 6 RNA targeting probes were diluted into probe set diluent (QVT0511; Affymetrix) and then simultaneously incubated on tissue sections for 3 hours at 40°C in a humidified chamber. After hybridization, slides were washed at room temperature with Affymetrix proprietary washing buffer. Slides were then incubated with preamplifier oligonucleotide and amplifier oligonucleotide at 40°C for 20 minutes in a humidified chamber. To develop the chromogenic signal for each probe, alkaline phosphatase-conjugated type 6 detection probe was hybridized to slides at 40°C for 20 minutes followed by reaction with Fast Blue substrate (QVT0506; Affymetrix) in 0.1M Tris maleate buffer. The reaction was then terminated with Stop QT buffer (QVT0517; Affymetrix) to deactivate remaining alkaline phosphatase and the process was repeated with alkaline phosphatase-conjugated type 1 detection probe and Fast-Red substrate (QVT0505; Affymetrix) in naphthol buffer. Lastly, slides were rinsed in PBS 3 times for 5 minutes each and then immersed in Vectashield Mounting Medium (Vector Labs H-1500) before coverslips were applied. Both type 1 and type 6 RNA targeting probes were purchased from Affymetrix. Ferret accessions XM_004759670(INSULIN) and XM_004743954(GLUCAGON) were submitted to Affymetrix for design and synthesis of RNA targeting probes. In this case, type 6 RNA targeting probe was designed to detect ferret insulin mRNA and type 1 RNA targeting probe was designed to detect ferret glucagon mRNA.
Hyperinsulinemic euglycemic and hyperglycemic clamps
Hyperinsulinemic euglycemic clamps were performed in 4-hour fasted conscious ferrets (14). Percutaneous catheters were nonsurgically placed in the tail artery and cephalic veins during brief isoflurane anesthesia. Catheter patency was maintained using heparin (10 IU mL−1) in 0.9% saline flushed at 1-hour intervals. Ferrets were infused with insulin (Humulin R; Eli Lilly) at 4 mU/kg·min for 120 minutes. Variable rates of 50% dextrose were infused to maintain euglycemia (110 mg/dL) while measuring blood glucose every 10 minutes. Infusions were delivered via the cephalic vein catheter, and the tail artery catheter was used for blood sampling. Hyperglycemic clamps (14) were performed in 4-hour fasted conscious ferrets. Percutaneous catheters were nonsurgically placed in the cephalic and jugular veins during brief isoflurane anesthesia and maintained as above. At time 0, dextrose was infused at 50 mg/kg·min. The rate of dextrose administration was varied thereafter based on blood glucose measurements at 2.5, 5, and 10 minutes and every 10 minutes thereafter with a glycemic goal of 240 mg/dL. Blood samples (200 μL) were obtained at 0, 2.5, 5, 10, 20, 40, and 60 minutes for quantification of plasma insulin levels. Infusions were delivered via the cephalic vein catheter and the jugular vein was used for blood sampling.
Results
Glycemic abnormalities in CF ferrets occur in age-related phases
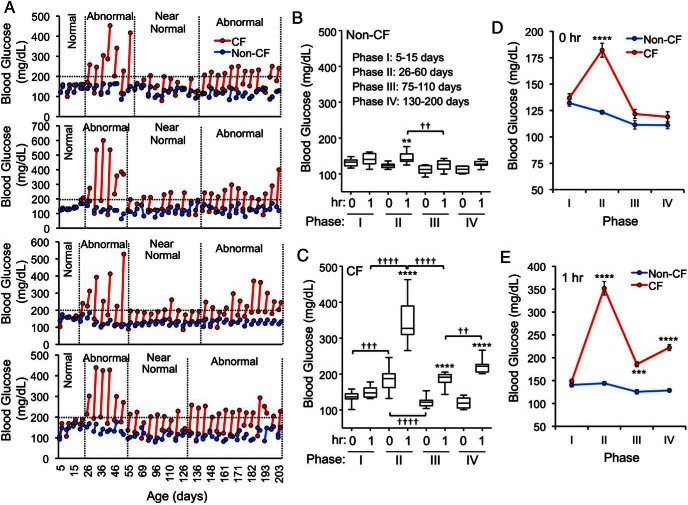
The progression of CFRD from birth onward has not been well characterized. To this end, we performed routine pre- and postmeal glucose monitoring in CF and non-CF ferrets. This revealed that CF animals consistently had elevated premeal and/or 1-hour postprandial blood glucose starting at approximately 26 days of age (Figure 1A). Unexpectedly, glucose levels spontaneously recovered to near normal in CF animals starting at about 60 days; however, this improvement was not sustained. Abnormal glycemic control returned again at approximately 4 months of age, when CF ferrets frequently had 1-hour postprandial glucose values above 200 mg/dL. Although the exact timing varied modestly from animal-to-animal, 4 consensus phases consistently emerged that demonstrated substantial differences. To summarize, in the CF animals, glycemia during the phases was: I, normal; II, very elevated; III, near normal; and IV, elevated (Figure 1, B and C). By contrast, non-CF animals experienced stable normoglycemia throughout all the phases. Compared with non-CF animals, CF premeal glucose levels were significantly higher during phase II, whereas 1-hour postprandial glucose levels were higher during phases II–IV (Figure 1, D and E). Furthermore, there were significant changes to the degree of premeal and postprandial glucoses disturbances between the various phases in CF animals, with a marked recovery between phases II–III and a worsening between phases I–II and III–IV (Figure 1C).
Figure 1.
Phase-dependent glucose abnormalities in CFTR knockout ferrets after feeding. A, Blood glucose values before and 1 hour after feeding for 4 paired CF and non-CF ferrets. Each line connects the 2 glucose values at a given age. B and C, Average blood glucose values before and 1 hour after feeding for (B) non-CF and (C) CF ferrets at each of the indicated age ranges (“phases”). Blood glucose was measured every 5 days and the average of multiple measures for each animal within each age range were used to calculate means and error in each group. D, Baseline blood glucose (0 h) before feeding for the dataset in B and C. E, Blood glucose at 1 hour after feeding for the dataset in B and C. Between 7 and 13 independent animals were evaluated for each genotype and phase. The mean ± SEM are presented in D and E. Statistical analysis was performed by one-way ANOVA and a Newman-Keuls multiple comparison; B and C, Asterisks mark comparisons between 0 and 1 hour and daggers mark bracketed comparisons between phases with P values as †† or **, P < .01; ††† or ***, P < .001; †††† or ****, P < .0001. D and E, Asterisks mark genotypic comparisons within a single phases with ***, P < .001; ****, P < .0001.
Correspondence of age-related glycemic phases to evolving CF pancreatic histopathology
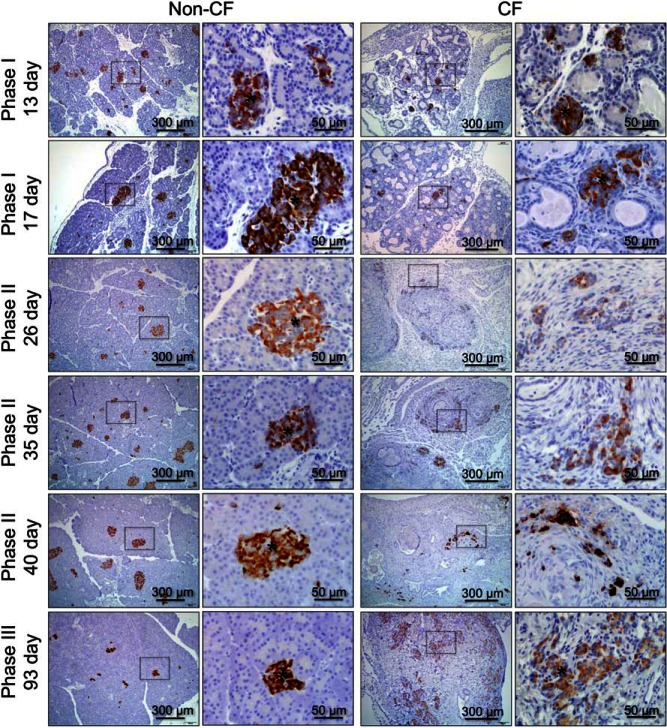
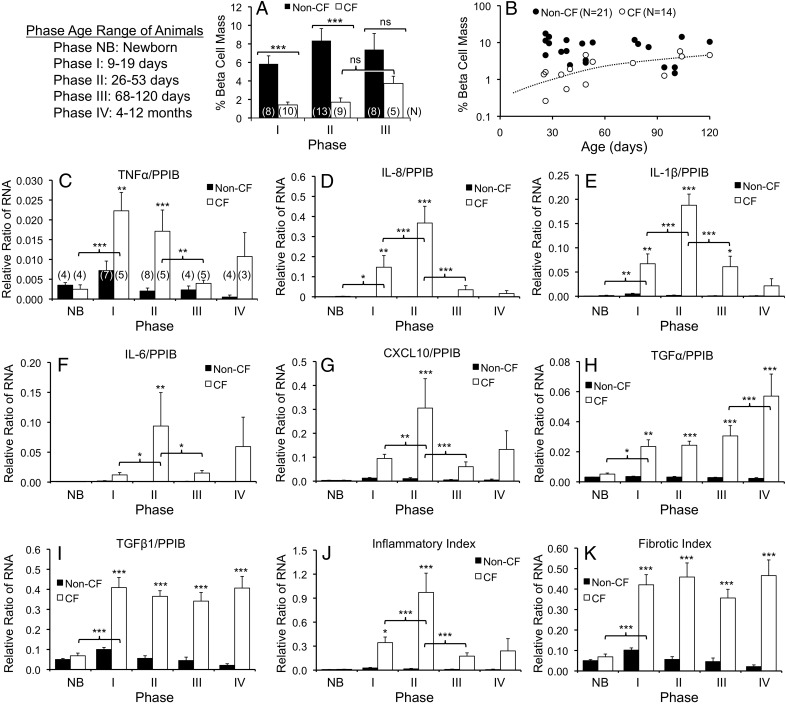
We previously found that pancreatic β-cell mass does not differ between newborn CF and non-CF ferrets (non-CF 7.6 ± 0.7% vs CF 6.3 ± 0.8% parenchymal area) (8), whereas CF ferrets older than 3 months of age have large aggregated islets that cluster in fibrotic regions of the pancreas (11). We hypothesized that changes in islet structure and β-cell abundance would correspond temporally with the glycemic transitions from phases I through III. In CF animals, there was a marked restructuring of insulin-positive islets that initiated during phase II (d 26–60), insulin-expressing β-cells were most often seen as scattered individual cells or small clusters, as opposed to being associated with defined islets as observed in age-matched non-CF controls or younger phase I (d 9–19) CF animals (Figure 2 and Supplemental Figure 1). During phase III, when transient glycemic recovery is observed, CF animals experienced a reemergence of larger insulin-positive structures aggregated within fibrotic regions of the pancreas (Figure 2 and Supplemental Figure 1). As previously described, pancreatic β-cell mass does not significantly differ between non-CF (7.6 ± 0.7%) and CF (6.3 ± 0.8%) newborn animals (8). However, β-Cell mass was significantly reduced in CF vs non-CF animals during both phase I (4.1-fold, P < .001) and phase II (4.9-fold, P < .001) (Figure 3A). The most significant change in β-cell mass of CF animals occurred between newborn animals (8) and phase I (4.4-fold decline, P < .001), whereas β-cell mass in CF animals did not significantly differ during phases I and II. These findings demonstrate that a loss in β-cell mass precedes the severe hyperglycemia observed in phase II of CF animals. By contrast, β-cell mass during phase III (68–120 d old) was not significantly different between genotypes; however, the 2.2-fold rise in β-cell mass in CF animals between phase II and III also did not reach significance. β-Cell mass increased with age after age 26 days in CF (P = .0053, r = 0.7003, Pearson correlation), but not in non-CF animals (Figure 3B). These findings demonstrate that a reduction in β-cell mass precedes the severe hyperglycemia of phase II and that partial recovery of β-cell mass during the phase II–III transition appears to be associated with islet restructuring and an improvement in glycemia. Whether alterations in β-cell function occur during these phases and impact the extent of glycemic abnormalities remains to be determined. To partly address this question, we assessed markers associated with β-cell function as detailed below.
Figure 2.
Age-dependent changes in islet structure and insulin protein expression in the pancreas. Pancreatic paraffin sections of non-CF (left) and CF (right) animals were immunostained for insulin and counterstained with hematoxylin. Ages of the animals evaluated are given on the left. The right column of photomicrographs for each genotype is an enlargement of the boxes region in the left set of photomicrographs. Supplemental Figure 1 shows H&E-stained sections for the same age groups. Asterisks mark discernable islets.
Figure 3.
Age-dependent changes in β-cell mass and pancreatic proinflammatory cytokine and chemokine gene expression. A, Morphometric analysis of β-cell mass expressed as the % pancreatic parenchymal immunostaining positive for insulin. There were significant differences by Mann-Whitney U test (***, P < .001; ns, not significant). Statistical comparisons for β-cell mass between newborn non-CF (7.6 ± 0.7%, N = 22) and CF (6.3 ± 0.8%, N=12) animals and phase I animals utilized previously published data and the same method for morphometry (8). In this comparison, β-cell mass of newborn CF animals and phase I CF animals was significantly different (P < .001). The results present the mean ± SEM, and the number of independent animals in each group as marked in brackets on the bar graph. B, There was a significant positive correlation between β-cell mass and age in CF animals by Pearson's correlation test (P = .0053, r = 0.7003) and a lack of correlation in non-CF. C–K, Lysates of CF and non-CF pancreata were used for detection of proinflammatory cytokine and chemokine RNA transcripts. Each sample was evaluated simultaneously for the RNA expression of the target genes and the housekeeping gene PPIB. C–I, The ratios of RNA transcript abundance for (C) TNFα to PPIB, (D) IL-8 to PPIB, (E) IL-1β to PPIB, (F) IL-6 to PPIB, (G) CXCL10 to PPIB, (H) TGFα to PPIB, and (I) TGFβ1 to PPIB for the various age ranges shown. J, The sum of proinflammatory RNA to PPIB RNA ratios for TNFα, IL-8, IL-1, IL-6, and CXCL10 was used to create an inflammatory index. K, The sum of TGFα to PPIB and TGFβ1 to PPIB RNA ratios were used to create a fibrotic index. Results in C–K show the mean ± SEM with significant differences by one-way ANOVA and Bonferroni post hoc test for the marked genotypic comparisons or within a genotype when marked by brackets: *, P < .05; **, P < .01; ***, P < .001. The number of independent animals in each group of (C–K) is indicated in brackets on the bar graph in C.
Pancreatic proinflammatory cytokines are associated with glycemic disturbances in CF ferrets
We previously demonstrated that histologic pancreatic inflammation and fibrosis in CF ferrets becomes severe near the end of the first month of age (8). Here, we examined RNA expression patterns of proinflammatory factors across the glycemic phases. TNFα, IL-8, IL-1β, IL-6, and CXCL10 RNA levels were significantly higher in CF than non-CF ferrets during phase II. Each of these cytokines/chemokines, except TNFα, peaked during phase II with a significant increase during the phase I–II transition and significant decrease during the phase II–III transition (Figure 3, C–G, and Supplemental Figure 2). TNFα peaked during phase I and progressively declined in successive phases. A composite inflammatory index, which sums these inflammatory RNA levels, peaked in phase II, and diminished thereafter (Figure 3J). In CF animals, there was a significant age-dependent rise in the inflammatory index from birth to 53 days of age (P < .0001), as well as a significant age-dependent decline in this index from 26 to 120 days of age (P = .0306) (Supplemental Figure 2). TGFα and TGFβ1 RNA levels rose significantly in phase I and remained elevated thereafter (Figure 3, H and I), as did a composite fibrotic index (Figure 3K). These findings support the notion that heightened pancreatic inflammation may underlie phase II hyperglycemia in CF animals and that the reduction in pancreatic inflammation may contribute to the subsequent glycemic recovery.
Contrasting pancreatic levels, circulating plasma levels of IL-6, IL-8, TNFα, and TGFβ1 demonstrated no significant differences between genotypes during phase II (Supplemental Figure 3). Rather, only newborn CF animals demonstrated significant elevations in circulating IL-6, IL-8, and TNFα (Supplemental Figure 3). Interestingly, plasma TGFβ1 was significantly reduced in CF animals at 12–27 days of age (Supplemental Figure 3D). These findings stress the importance of local rather than systemic proinflammatory changes.
Mixed meal glycemic intolerance precedes oral glucose intolerance in CF ferrets
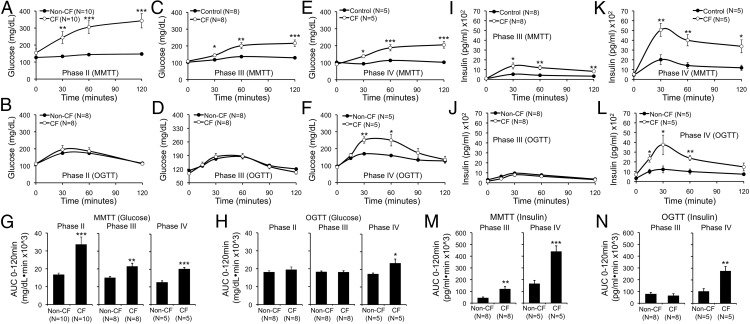
To more formally evaluate glucose metabolism, we performed MMTTs and OGTTs (Figure 4). CF animals exhibited the most severe glucose intolerance during MMTT within the phase II age bracket, with less severe impairment thereafter (Figure 4, A, C, and E). By contrast, during OGTT, CF animals exhibited normal glucose tolerance until phase IV (Figure 4, B, D, and F). These phases of impaired glucose tolerance were also reflected by glucose AUC analysis (Figure 4, G and H).
Figure 4.
Differential detection of abnormal glucose tolerance in CF ferrets following MMTT vs OGTT. A–F, Blood glucose during (A, C, and E) MMTTs or (B, D, and F) OGTTs on fasted paired CF and non-CF ferrets at the indicated phases (phase II, 1–2 mo; phase III, 2–4 mo; phase IV, 4–12 mo). G and H, Area under the curve (0–120 min) for glucose during MMTT and OGTT for data in A–F. I–L, Plasma insulin during (I and K) MTTT and (J and L) OGTT for the tests shown in C–F. M and N, Area under the curve analysis (0–120 min) for insulin during MMTT and OGTT for data in I–L. Results show the mean ± SEM with significant differences for marked comparisons between genotypes by 2-tailed Student's t test: *, P < .05; **, P < .01; ***, P < .001.
Insulin secretion and insulin sensitivity in CF ferrets
Due to the fragile health of CF animals before 2 months of age, it was not possible to perform venous blood draws during phases I and II, thus insulin measurements were limited to phases III–IV. Fasting insulin levels did not differ between CF and non-CF ferrets (Figure 4, I–L). In tests done in CF animals with normal glucose tolerance (ie, phase III OGTT) (Figure 4, J and N), insulin levels were normal. By contrast, insulin levels were elevated in CF ferrets during tests demonstrating hyperglycemia (Figure 4, I and K–N). Although these findings show that substantial insulin secretion can occur in CF animals during sustained hyperglycemia, they do not distinguish whether hyperglycemia occurs due to insulin resistance and/or insufficient insulin secretion. Likewise, although the elevated insulin levels observed 30–120 minutes during hyperglycemic portions of the OGTT and MMTT are by definition insufficiently elevated to control glycemia, they also could indicate insulin resistance. To address these questions, we performed euglycemic hyperinsulinemic and hyperglycemic clamps to assess insulin sensitivity and secretion, respectively.
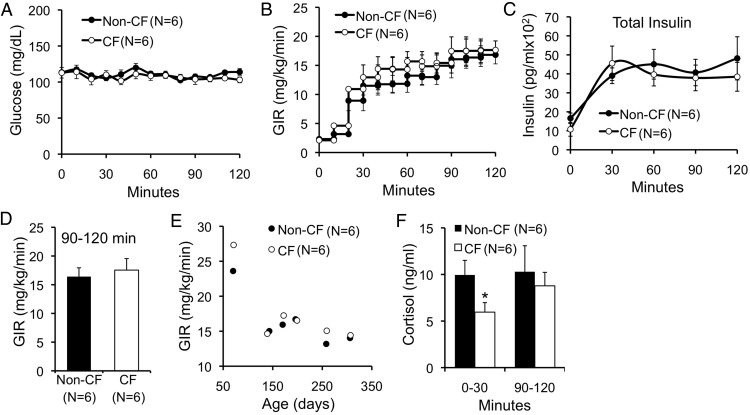
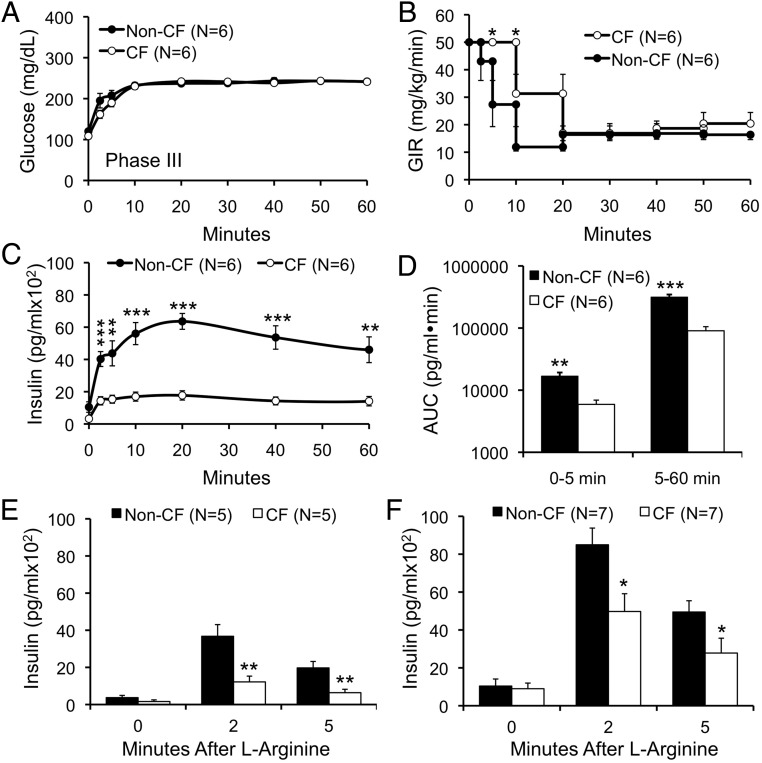
Insulin sensitivity was normal as demonstrated by hyperinsulinemic euglycemic clamps performed in conscious ferrets ranging 70–307 days of age (Figure 5, A–E). Plasma cortisol, an index of counterregulation and stress, was significantly lower in CF animals during the first 30 minutes of the clamp (Figure 5F). Given normal whole-body insulin sensitivity, we hypothesized that the primary reason for hyperglycemia during MMTT and OGTT in CF ferrets was insufficient insulin secretion. To directly assess insulin secretion, we performed hyperglycemic clamps at a target blood glucose of 240 mg/dL in conscious ferrets. These clamps showed that first-phase insulin response (FPIR) was significantly reduced in phase III CF animals (Figure 6). Later phase insulin secretion was also significantly reduced in CF as compared with non-CF animals (Figure 6). AUC insulin levels for FPIR (AUC0–5 min) and later phase insulin (AUC5–60 min) were 2.9- and 3.5-fold reduced in CF compared with non-CF animals, respectively (Figure 6D). Importantly, these clamps show that phase III healthy ferrets should rapidly achieve serum insulin levels of over 5000 pg/mL when challenged with blood glucose of 240 mg/dL. By contrast, phase III CF ferrets during MMTT achieved insulin levels under 1500 pg/mL (Figure 4I), despite blood glucose levels sustained above 200 mg/dL (Figure 4C). As an independent measure of FPIR, we also employed ASTs. There was a significant reduction in FPIR among CF compared with non-CF ferrets during phases III and IV by AST (Figure 6, E and F). Absolute FPIR insulin levels increased in CF animals from phase III–IV, and the reduction in FPIR relative to non-CF ferrets was less dramatic in phase IV (∼2-fold) as compared with phase III (∼3-fold) animals, suggesting a partial recovery of FPIR. Taken together, these data demonstrate a marked impairment in insulin secretion in CF ferrets. Although insulin levels are higher in CF vs non-CF during abnormal MMTT and OGTT, these insulin levels appear insufficiently elevated for the degree of hyperglycemia.
Figure 5.
Whole-animal insulin sensitivity in CF and non-CF ferrets. A–E, Hyperinsulinemic euglycemic clamps in conscious CF and non-CF ferrets ranging from 70–307 days of age, depicting (A) blood glucose, (B) glucose infusion rate (GIR), (C) total insulin, and (D) GIR during the last 30 minutes of the clamp. E, Relationship between GIR and age. F, Mean cortisol levels at the indicated time range in clamped animals shown in A–E.
Figure 6.
Hyperglycemic clamps and L-arginine stimulations tests detect a reduction in first phase and later phase insulin response in CF ferrets. A–C, Hyperglycemic clamps in conscious CF and non-CF ferrets ranging from 63–125 days of age, depicting (A) blood glucose, (B) glucose infusion rate (GIR), and (C) plasma insulin levels. D, Area under the curve for insulin responses between 0–5 and 5–60 minutes for data shown in C. E and F, First phase insulin secretion was measured in age matched CF and non-CF ferrets at (E) 2–4 months (phase III) and (F) 4–12 months (phase IV) by iv ASTs. Results show the mean ± SEM with significant differences by 2-tailed Student's t test for marked genotypic comparisons: *, P < .05; **, P < .01; ***, P < .001.
Resurgence of islet function at the transcriptional level occurs during phase III of CF pancreatic remodeling
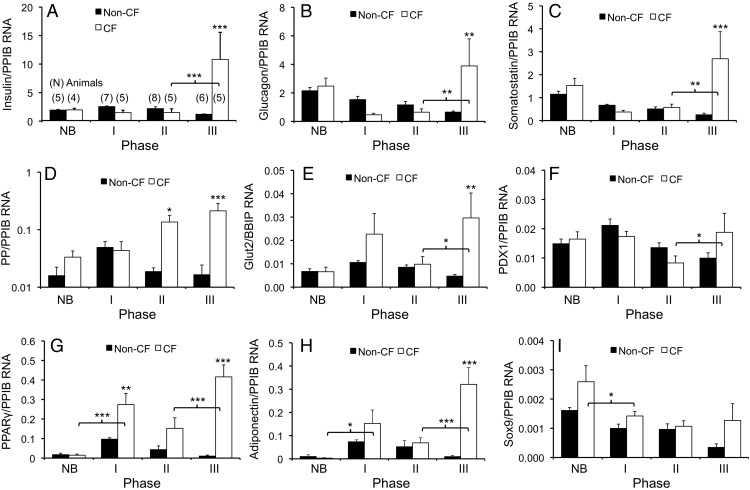
The above data indicate a recovery in β-cell mass and function during phase III. Because other islet cell types were also likely affected by the pancreatic inflammation and fibrosis, we surveyed islet hormone transcripts in whole pancreas. Unexpectedly, there were marked increases of multiple hormonal RNA levels during phase III. With the exception of PP, which rose during phase II, the islet hormone RNAs significantly rose during the transition from phase II to III in CF animals (Figure 7, A–D). In fact, phase III expression of islet hormones in CF pancreata was significantly higher than even non-CF pancreata for insulin (9.0-fold, P < .001), glucagon (6.0-fold, P < .01), somatostatin (10.7-fold, P < .001), and PP (12.9-fold, P < .001) (Figure 7, A–D). These findings were independent of the housekeeping gene used to normalize expression, as reference to PPIB, RPLP0, or RPL32 yielded statistically similar patterns (Supplemental Figure 4). Hence, these data indicate that the improved capacity of CF animals to maintain glycemia during phase III likely reflects a broad improvement in islet environment and function. A significant rise in Glut2 RNA expression in CF pancreata during the phase II–III transition also supports this concept (P < .01) (Figure 7E).
Figure 7.
Age-dependent changes in pancreatic RNA markers of endocrine function. A–I, Lysates of CF and non-CF pancreata were used for simultaneous detection of target RNA transcripts normalized to the housekeeping gene PPIB. The ratios of RNA transcript abundance for the indicated phases (ie, age groups) are shown for (A) insulin to PPIB, (B) glucagon to PPIB, (C) somatostatin to PPIB, (D) PP to PPIB, (E) Glut2 to PPIB, (F) PDX1 to PPIB, (G) PPARγ to PPIB, (H) adiponectin to PPIB, and (I) Sox9 to PPIB. Results in A–I show the mean ± SEM with significant differences by one-way ANOVA and Bonferroni post hoc test for the marked genotypic comparisons or within a genotype when marked by brackets: *, P < .05; **, P < .01; ***, P < .001. The number of independent animals in various groups for all graphs is marked in brackets above the bar graph in A. Phase NB, newborn; phase I, 9–19 days; phase II, 26–53 days; phase III, 68–120 days; phase IV, 4–12 months.
To better appreciate the dynamic changes in islet structure occurring during the various phases of CF pancreatic injury and remodeling, we performed in situ hybridization for both insulin and glucagon RNA (Figure 8). There was a decrease in the number of insulin RNA-positive β-cells and glucagon RNA-positive α-cells during phase II in CF animals as compared with non-CF controls. This decrease was also accompanied by disorganization of islet structure. During phase III, CF islet-like structures emerged and both insulin and glucagon RNA expression appeared more intense than that in non-CF controls (Figure 8). Such findings support the quantitative assessment of insulin and glucagon RNA in whole pancreatic samples (Figure 7).
Figure 8.
In situ hybridization for pancreatic insulin and glucagon RNA. Pancreatic paraffin sections of non-CF (left) and CF (right) animals were used for RNA in situ hybridization for insulin (blue) and glucagon (red). Ages of the animals evaluated are given on the left. The right column of photomicrographs for each genotype is an enlargement of the boxed region in the left set of photomicrographs.
Potential factors promoting β-cell recovery in CF animals
To better understand the pancreatic environment responsible for facilitating islet recovery, we evaluated factors known to enhance β-cell function and/or regeneration (PDX1, PPARγ, adiponectin) (15–17) and a marker for acinar to duct cell reprogramming (Sox9) (18). PDX1 and Sox9 RNA levels were not significantly different between genotypes for the various ages (Figure 7, F and I). However, PDX1 was significantly induced between phase II and III in CF pancreata (P < .05), consistent with enhanced insulin RNA expression during that time frame. Most interestingly, PPARγ and adiponectin RNA expression during phase III was 40- and 34-fold increased over non-CF controls, respectively (P < .001) (Figure 7, G and H). Both transcripts also increased 3- to 5-fold (P < .001) during glycemic recovery (phase II–III).
Discussion
Exocrine pancreatic disease is a major risk factor for CFRD, yet in humans, CFRD does not typically develop until 1–2 decades after the onset of exocrine pancreatic insufficiency. Exocrine pancreatic disease is rapidly progressive in humans with CF; by 7 weeks of age, 60% of infants have pancreatic insufficiency and by 1 year of age, 90% (19). However, pancreatic sufficiency, as indexed by fecal elastase, can dynamically change in the first few years of life with 46% of CF patients moving between insufficient and sufficient status (20, 21). Severe neonatal exocrine pancreatic disease increases subsequent CFRD risk (22). These findings served as impetus to study the earliest interactions between exocrine and endocrine pancreatic disease in CF. Early CFRD pathogenesis is difficult to address in young children due to the limited ability to perform semiinvasive clinical studies and inaccessibility of pancreatic tissue. Using CF ferrets, we herein demonstrate that the CF endocrine pancreas responds dynamically to evolving exocrine disease.
We found that CF ferrets experience transient diabetic-level hyperglycemia at approximately 1 month of age. This glycemic crisis occurs during peak pancreatic inflammation and is accompanied significant loss of insulin-positive β-cells. However, the decline in β-cells mass precedes the glycemic crisis by approximately 2 weeks, suggesting that functional impairment of β-cells occurs as inflammation increases. Although we predict that insulin responses would be reduced during this phase of glycemic crisis, we were unable to directly evaluate this in the younger CF animals owing their fragile health that precluded venous blood draws. Several elevated inflammatory markers (IL-8, IL-1β, IL-6, and CXCL10) peak during the glycemic crisis and are known to be involved in acute pancreatitis (23, 24). CXCL10 is induced in acinar cells during acute pancreatitis and is associated with apoptosis of this cellular compartment (25, 26). Importantly, IL-1β and IL-6 inhibit glucose stimulated insulin secretion in isolated islets (27), and thus may contribute to impaired postprandial glucose tolerance during peak inflammation in CF ferrets. Two other inflammatory cytokines associated with chronic pancreatitis and activation of pancreatic stellate cell, TNFα and TGFβ1, experienced peak expression before the glycemic crisis, suggesting that their expression marks an earlier phase CF exocrine pancreatic disease associated with pancreatic stellate cell-mediated fibrosis (23, 24, 28, 29). Transgenic mice overexpressing TGFβ1 in the β-cell have striking similarities to the CF pancreas during glycemic crisis, with replacement of exocrine acinar cells by fibrotic and adipose tissues, and disorganized islets without loss of β-cell mass (30). However, when both TNFα and TGFβ1 are overexpressed in the mouse β-cell, there is loss of insulin-positive cells and diabetes ensues (30). TGFβ1 induces insulin gene expression while repressing β-cell mitogenesis (31, 32). Given that the β-cell mass at 1–2 months was reduced approximately 5-fold in CF pancreata, yet insulin mRNA expression was only reduced 1.5-fold, the remaining β-cells likely had higher insulin content, supporting the notion that TGFβ1 may induce insulin gene expression as previously observed (31, 32).
Ultimately, there was significant recovery of normoglycemia after the glycemic crisis, and this was accompanied by a reorganization of islet-like structures in CF ferrets. Although this recovery was associated with a nonsignificant doubling of β-cell mass, there was a large and significant enhancement in pancreatic insulin, glucagon, and somatostatin gene expression. Thus, partial recovery of postprandial glycemic control in CF ferrets during phase III appears to be influenced by a gain in islet function. Despite this transient recovery in glycemia, hyperglycemic clamp data demonstrated that CF insulin secretory capacity by the β-cell during phase III remained lower than that of non-CF control animals. By contrast to other islet endocrine hormones, PP RNA increases significantly during the glycemic crisis before recovery. The biologic significance of this finding is currently unknown, but is of interest given that PP gene expression marks a population of progenitor cells that give rise to both β-cells and δ-cells, as determined by lineage tracing and cellular ablation experiments in mice (33, 34). PP cell hyperplasia has also been observed in human type 1 diabetes (35). Others have reported that elevated blood glucose can drive reversible changes in pancreatic islet structure and function by promoting transdifferentiation of β-cells to α-cells (36). However, our RNA in situ hybridization studies that demonstrated relatively few α-cells during the glycemic crisis suggests this mechanisms is likely not at play. Regardless of the origins of endocrine cells of the remodeled CF islet, our data suggests that after exocrine destruction and associated inflammation, the endocrine cells of the CF islet have a previously unknown capacity to reform functional islets capable of temporarily controlling blood glucose.
A recovery of insulin secretion capacity is not without precedent. It is well known that shortly after initiation of therapy for type 1 diabetes, there often is a temporary resurgence of insulin secretion capacity known as the “honeymoon” phase (37). An analogous process can also occur after therapy initiation for type 2 diabetes (38). However, in contrast to other forms of diabetes, the recovery is spontaneous in the CF ferrets. Although insulin secretion could not be directly assessed during phase II of CF animals, due to their fragile health, by analogy to pancreatic insulin RNA (whole pancreas and in situ hybridization) there appears to be a resurgence of insulin production capacity by the pancreas in CF ferrets during phase III.
Humans with CF develop significant exocrine pancreatic disease very early in life, and thus may have similar relationship between the failure of the exocrine pancreas and the function of the endocrine pancreas as seen in CF ferrets. CFRD in very young children and infants has been described (39, 40) but considered to be rare. The incidence of CFRD is 2% in children, 15%–19% in adolescents, and 40%–50% in adult CF patients (41, 42). Furthermore, glycemic abnormalities in CF children 6–9 years of age predicts more rapid onset of CFRD (41). Our results in ferrets suggest that very young children with CF may experience a similar transient glycemic crisis of short duration. We know of only 2 previous studies of glucose tolerance in very young children with CF. Both studies are cross-sectional but do support the possibility of worsened glycemia in very young CF children. In the most recent such study, there was a higher prevalence of abnormal glucose tolerance in children under age 6 as compared with ages 6–9.9 (43). Likewise, a 1969 study found the highest rates of abnormal glucose tolerance in children ages 0–5 years, with lower rates throughout the remainder of childhood (44). Although it is difficult to draw firm relative age comparisons between humans (∼80-y life span) and ferrets (∼8-y life span), if one directly extrapolates relative age based on life span, a 2-month-old ferret would be approximately equivalent to a 2-year-old toddler. However, using the dog as a guide (which is the closest domestic relative to ferrets), the first 2 years of ferret life would be equivalent to 11.7 years of human life. In this calculation, a 2-month-old ferret would be close to a 14-month-old toddler. Thus, 1- to 2-year-old CF toddlers, which is the time frame of exocrine pancreas instability in human CF (20, 21), may represent the equivalent age window to which CF ferrets (1–2 mo) demonstrate the most significant exocrine disease inflammation and postprandial glucose intolerance.
Interestingly, during the glycemic crisis (phase II) in CF ferrets, glucose intolerance was detected only by mixed meal and not by oral glucose testing. The reasons underlying this difference in sensitivity to detect glycemic abnormalities are not clear, but could include differing involvement of incretins in response to the 2 stimuli or a role for amino acid driven gluconeogenesis in CF animals. Although whole-body insulin sensitivity was normal in CF ferrets, metabolic tracer and imaging studies would be needed to separate potential hepatic insulin resistance from changes in peripheral glucose disposal as has been demonstrated in humans with CF (45, 46). One limitation of our study was the inability to collect serum analytes (such as insulin) during phase II (glycemic crisis), owing to the fragile health of CF animals at this age, which precluded measurements of first phase insulin release and glycemic clamp. However, because hyperglycemic clamps during phase III demonstrated a reduction in both first phase and later phase insulin secretion in CF animals, it is likely that insulin secretion is further reduced during phase II given the reduction in pancreatic insulin and β-cell mass. The reason(s) why this reduced insulin secretion did not manifest as glucose intolerance during phase III OGTTs remains unclear, but may relate to observations of enhanced peripheral glucose utilization observed in exocrine-insufficient CF patients without diabetes (46). This mechanism of metabolic adaptation has been suggested to be a response to increased peripheral energy needs. Nonetheless, the differential sensitivity to glucose intolerance between OGTT and MMTT may have implications for the clinical screening of young CF patients.
Glycemic recovery in CF ferrets was associated with induction of the β-cell promoting gene PDX1 and with enhanced expression of β-cell functional gene Glut2. Additional factors appear to also enhance islet adaptation to the markedly altered pancreatic environment. Interestingly, islet resurgence in CF ferrets is associated with appearance of factors that promote adipogenesis, suggesting that fat accumulation in the CF pancreas may play a protective role in maintaining islet function. The expression of the adipose-centric genes PPARγ and adiponectin was induced in CF pancreata concordant with a decline in inflammation and recovery of glycemic status. Adiponectin expression is induced by PPARγ activation (47–49), and thus suggests activation of the PPARγ transcriptional program in CF pancreas. The antidiabetic effects of adiponectin are classically mediated by insulin sensitization in muscle and liver (50, 51). More recently, adiponectin has been associated with direct protective effects on the β-cell (17), where adiponectin accumulates, induces β-cell insulin expression, and protects the β-cell from glucotoxicity (17, 52, 53). The concordant rise in adiponectin and insulin gene expression within CF pancreata during glycemic recovery supports the notion of a causative link. Of additional interest, adipose-tissue mesenchymal stem cells may be β-cell protective by promoting antiinflammatory immunomodulation (54). We thus postulate that pancreatic adipogenesis and PPARγ activity help promote islet resurgence in CF.
In summary, our study of early glucose abnormalities in CF ferrets has led to several important findings potentially relevant to the diagnosis and treatment of CFRD. First, there is a transient glycemic crisis in 1-month-old ferrets associated with pancreatic inflammation and preceded by a loss of insulin-positive cells. Because early life human CF pancreas pathology is similar, one might predict a similar glycemic crisis occurs in young humans with CF. Second, CF ferrets experience glycemic recovery that is associated with resurgence of the endocrine pancreas. Third, the strong concordance between active exocrine pancreatic disease and hyperglycemia suggests that exocrine pancreatic disease is the primary driver of CFRD. Fourth, factors were identified that may support endocrine resurgence, including decreased inflammation and pancreatic expression of PPARγ and adiponectin. Because humans with CF do not develop CFRD until many years after exocrine pancreas destruction, the adaptive islet-protective mechanisms discovered in CF ferrets are also likely relevant to CF children. In summary, these findings suggest that the CF pancreas has a unique capacity to adapt the endocrine pancreas during and after inflammatory destruction of the exocrine pancreas.
Acknowledgments
We thank Dr Deborah J. Nelson for helpful discussion of this work.
Author contributions: Y.Y. and X.S. did the experimental design, researched data and organization, and wrote/reviewed the manuscript; K.G.-C. did the experimental design and researched data and organization; W.X., B.L., N.H., and S.R.T. researched data; A.U., L.H.P., M.H., and K.L.O. did the experimental design, discussed and interpreted data, and reviewed/edited the manuscript; K.W. conducted the statistical interpretation of data and reviewed the manuscript; A.W.N. and J.F.E. did the experimental design, reviewed and interpreted data, and wrote the manuscript.
This work was supported by National Institutes of Health Grants R24 DK096518 (to J.F.E., A.W.N.), R24 HL123482 (to J.F.E.), and R01 DK097820 (to A.U. and A.W.N.), a Fraternal Order of Eagles Diabetes Research Center scholar award (A.W.N.), the University of Iowa Center for Gene Therapy Grant DK54759, and the Carver Chair in Molecular Medicine (J.F.E.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AST
- L-arginine stimulation test
- AUC
- area under the cure
- CF
- cystic fibrosis
- CFRD
- CF-related diabetes
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CV
- coefficient of variation
- CXCL10
- chemokine (C-X-C motif) ligand 10
- FPIR
- first-phase insulin response
- Glut2
- solute carrier family 2
- MMTT
- mixed meal tolerance test
- OGTT
- oral glucose tolerance test
- PDX1
- pancreatic and duodenal homeobox 1
- PP
- pancreatic polypeptide
- PPARγ
- peroxisome proliferator-activated receptor gamma
- PPIB
- peptidylprolyl isomerase B/cyclophilin B
- RPL32
- ribosomal protein L32
- RPLP0
- ribosomal large protein P0
- Sox9
- sex-determining region Y-box 9.
References
- 1. Lewis C, Blackman SM, Nelson A, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Street ME, Spaggiari C, Ziveri MA, et al. Insulin production and resistance in cystic fibrosis: effect of age, disease activity, and genotype. J Endocrinol Invest. 2012;35:246–253. [DOI] [PubMed] [Google Scholar]
- 3. Hardin DS, Ahn C, Rice J, Rice M, Rosenblatt R. Elevated gluconeogenesis and lack of suppression by insulin contribute to cystic fibrosis-related diabetes. J Investig Med. 2008;56:567–573. [DOI] [PubMed] [Google Scholar]
- 4. Blackman SM, Hsu S, Vanscoy LL, et al. Genetic modifiers play a substantial role in diabetes complicating cystic fibrosis. J Clin Endocrinol Metab. 2009;94:1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iannucci A, Mukai K, Johnson D, Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15:278–284. [DOI] [PubMed] [Google Scholar]
- 6. O'Riordan SM, Dattani MT, Hindmarsh PC. Cystic fibrosis-related diabetes in childhood. Horm Res Paediatr. 2010;73:15–24. [DOI] [PubMed] [Google Scholar]
- 7. Handwerger S, Roth J, Gorden P, et al. Glucose intolerance in cystic fibrosis. N Engl J Med. 1969;281:451–461. [DOI] [PubMed] [Google Scholar]
- 8. Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontes G, Ghislain J, Benterki I, et al. The ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator is associated with progressive insulin resistance and decreased functional β-cell mass in mice. Diabetes. 2015;64:4112–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uc A, Olivier AK, Griffin MA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond). 2015;128:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol. 2014;184:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun X, Yan Z, Yi Y, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult CFTR-knockout ferrets. Am J Respir Cell Mol Biol. 2014;50:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sui H, Yi Y, Yao J, et al. Quantifying insulin sensitivity and entero-insular responsiveness to hyper- and hypoglycemia in ferrets. PLoS One. 2014;9:e90519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic β cell. Mol Cell Endocrinol. 2008;294:1–9. [DOI] [PubMed] [Google Scholar]
- 16. Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60:1664–1672. [DOI] [PubMed] [Google Scholar]
- 18. Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bronstein MN, Sokol RJ, Abman SH, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533–540. [DOI] [PubMed] [Google Scholar]
- 20. Benahmed NA, Manene D, Barbot L, Kapel N. Fecal pancreatic elastase in infants under 2 years of age. Ann Biol Clin (Paris). 2008;66:549–552. [DOI] [PubMed] [Google Scholar]
- 21. O'Sullivan BP, Baker D, Leung KG, Reed G, Baker SS, Borowitz D. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013;162:808–812.e801. [DOI] [PubMed] [Google Scholar]
- 22. Soave D, Miller MR, Keenan K, et al. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes. 2014;63:2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24(suppl 1):45–51. [DOI] [PubMed] [Google Scholar]
- 24. Frossard JL, Hadengue A, Pastor CM. New serum markers for the detection of severe acute pancreatitis in humans. Am J Respir Crit Care Med. 2001;164:162–170. [DOI] [PubMed] [Google Scholar]
- 25. Singh L, Arora SK, Bakshi DK, Majumdar S, Wig JD. Potential role of CXCL10 in the induction of cell injury and mitochondrial dysfunction. Int J Exp Pathol. 2010;91:210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh L, Bakshi DK, Majumdar S, Vasishta RK, Arora SK, Wig JD. Expression of interferon-γ- inducible protein-10 and its receptor CXCR3 in chronic pancreatitis. Pancreatology. 2007;7:479–490. [DOI] [PubMed] [Google Scholar]
- 27. Southern C, Schulster D, Green IC. Inhibition of insulin secretion from rat islets of Langerhans by interleukin-6. An effect distinct from that of interleukin-1. Biochem J. 1990;272:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimizu K. Mechanisms of pancreatic fibrosis and applications to the treatment of chronic pancreatitis. J Gastroenterol. 2008;43:823–832. [DOI] [PubMed] [Google Scholar]
- 30. Sanvito F, Nichols A, Herrera PL, et al. TGF-β 1 overexpression in murine pancreas induces chronic pancreatitis and, together with TNF-α, triggers insulin-dependent diabetes. Biochem Biophys Res Commun. 1995;217:1279–1286. [DOI] [PubMed] [Google Scholar]
- 31. Sayo Y, Hosokawa H, Imachi H, et al. Transforming growth factor β induction of insulin gene expression is mediated by pancreatic and duodenal homeobox gene-1 in rat insulinoma cells. Eur J Biochem. 2000;267:971–978. [DOI] [PubMed] [Google Scholar]
- 32. Sjöholm A, Hellerström C. TGF-β stimulates insulin secretion and blocks mitogenic response of pancreatic β-cells to glucose. Am J Physiol. 1991;260:C1046–C1051. [DOI] [PubMed] [Google Scholar]
- 33. Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. [DOI] [PubMed] [Google Scholar]
- 34. Herrera PL, Huarte J, Zufferey R, et al. Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc Natl Acad Sci USA. 1994;91:12999–13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gepts W, De Mey J, Marichal-Pipeleers M. Hyperplasia of “pancreatic polypeptide”-cells in the pancreas of juvenile diabetics. Diabetologia. 1977;13:27–34. [DOI] [PubMed] [Google Scholar]
- 36. Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun. 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. [DOI] [PubMed] [Google Scholar]
- 38. Retnakaran R. Novel strategies for inducing glycemic remission during the honeymoon phase of type 2 diabetes. Can J Diabetes. 2015;39:S142–S147. [DOI] [PubMed] [Google Scholar]
- 39. Casas L, Berry DR, Logan K, Copeland KC, Royall JA. Cystic fibrosis related diabetes in an extremely young patient. J Cyst Fibros. 2007;6:247–249. [DOI] [PubMed] [Google Scholar]
- 40. Lombardi F, Raia V, Spagnuolo MI, et al. Diabetes in an infant with cystic fibrosis. Pediatr Diabetes. 2004;5:199–201. [DOI] [PubMed] [Google Scholar]
- 41. Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013;1:52–58. [DOI] [PubMed] [Google Scholar]
- 42. Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mozzillo E, Raia V, Fattorusso V, et al. Glucose derangements in very young children with cystic fibrosis and pancreatic insufficiency. Diabetes Care. 2012;35:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Milner AD. Blood glucose and serum insulin levels in children with cystic fibrosis. Arch Dis Child. 1969;44:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hardin DS, LeBlanc A, Para L, Seilheimer DK. Hepatic insulin resistance and defects in substrate utilization in cystic fibrosis. Diabetes. 1999;48:1082–1087. [DOI] [PubMed] [Google Scholar]
- 46. Moran A, Pyzdrowski KL, Weinreb J, et al. Insulin sensitivity in cystic fibrosis. Diabetes. 1994;43:1020–1026. [DOI] [PubMed] [Google Scholar]
- 47. Maeda N, Takahashi M, Funahashi T, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. [DOI] [PubMed] [Google Scholar]
- 48. Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. [DOI] [PubMed] [Google Scholar]
- 49. Iwaki M, Matsuda M, Maeda N, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. [DOI] [PubMed] [Google Scholar]
- 50. Padmalayam I, Suto M. Role of adiponectin in the metabolic syndrome: current perspectives on its modulation as a treatment strategy. Curr Pharm Des. 2013;19:5755–5763. [DOI] [PubMed] [Google Scholar]
- 51. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rao JR, Keating DJ, Chen C, Parkington HC. Adiponectin increases insulin content and cell proliferation in MIN6 cells via PPARγ-dependent and PPARγ-independent mechanisms. Diabetes Obes Metab. 2012;14:983–989. [DOI] [PubMed] [Google Scholar]
- 53. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and β cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015;2015:878535. [DOI] [PMC free article] [PubMed] [Google Scholar]