Abstract
Calbindin-D(28K) (Calb1), a high-affinity calcium buffer/sensor, shows abundant expression in neurons and has been associated with a number of neurobehavioral diseases, many of which are sexually dimorphic in incidence. Behavioral and physiological end points are affected by experimental manipulations of calbindin levels, including disruption of spatial learning, hippocampal long-term potentiation, and circadian rhythms. In this study, we investigated novel aspects of calbindin function on social behavior, anxiety-like behavior, and fear conditioning in adult mice of both sexes by comparing wild-type to littermate Calb1 KO mice. Because Calb1 mRNA and protein are sexually dimorphic in some areas of the brain, we hypothesized that sex differences in behavioral responses of these behaviors would be eliminated or revealed in Calb1 KO mice. We also examined gene expression in the amygdala and prefrontal cortex, two areas of the brain intimately connected with limbic system control of the behaviors tested, in response to sex and genotype. Our results demonstrate that fear memory and social behavior are altered in male knockout mice, and Calb1 KO mice of both sexes show less anxiety. Moreover, gene expression studies of the amygdala and prefrontal cortex revealed several significant genotype and sex effects in genes related to brain-derived neurotrophic factor signaling, hormone receptors, histone deacetylases, and γ-aminobutyric acid signaling. Our findings are the first to directly link calbindin with affective and social behaviors in rodents; moreover, the results suggest that sex differences in calbindin protein influence behavior.
Calbindin-D(28K) (Calb1) is exclusively expressed in brain in which it functions as a high-affinity calcium buffer/sensor in neurons (1–4). Calb1 is expressed in both pyramidal and nonpyramidal neurons and in γ-aminobutyric acid secreting (GABAergic) inhibitory neurons including the Purkinje cells of the cerebellum and interneurons in the prefrontal cortex, amygdala, and hippocampus (5–8). Moreover, Calb1 is sexually dimorphic (females have more than males) in the cerebellum and prefrontal cortex of juvenile mice, two brain regions associated with cognitive and affective behaviors impacted by sex-specific neurobehavioral disorders (9). Calbindin is also a biomarker for the sexually dimorphic nucleus of the preoptic area: males have significantly greater numbers of calbindin-positive cells than females (10, 11). This population of Calb1 cells is regulated by both estrogen receptor-α and androgen receptors during development (12, 13). Differences in expression are effected only by developmental actions of steroids, and calbindin immunoreactivity in the sexually dimorphic nucleus of the preoptic area is not changed by exposure to steroid hormones in adults (13).
Reduced calbindin has been associated with a number of neurobehavioral diseases, many of which are sexually dimorphic. There are significantly fewer calbindin-immunopositive neurons in the cerebral cortex of individuals with schizophrenia, but not bipolar disorder, compared with control subjects (14, 15). During aging, calbindin-containing neurons in the basal forebrain gradually die, and this process is accelerated in individuals with Alzheimer's disease (16). Decreased calbindin immunoreactivity in the hippocampus is also associated with temporal lobe epilepsy (17, 18). Postmortem studies report fewer and smaller Purkinje cells in individuals with autism (19, 20). Transgenic mouse models of fragile X syndrome have lower-than-normal numbers of calbindin-immunoreactive neurons (21, 22) and significantly decreased Calb1 expression (23). In other transgenic mouse models of neurological disease, altering Calb1 levels affects the phenotype. For example, suppressing Calb1 expression worsens the disease phenotype in an Alzheimer's disease model (24), whereas Calb1 overexpression in dopamine-producing cells has a neuroprotective effect in a Parkinson's model (25).
Behavioral and physiological end points are also affected by experimental manipulations of calbindin levels. In rats, overexpression of calbindin in the dentate gyrus produces deficits in water maze learning and T-maze reversal learning (26). Male transgenic Calb1 knockdown mice have deficits in spatial learning in the Morris water maze and the eight-arm radial maze as compared with controls (27). Complete Calb1 knockout (KO) mice have disrupted circadian rhythms and impaired hippocampal long-term potentiation maintenance as compared with wild types (28, 29).
Here we investigated novel aspects of calbindin function on social behavior, anxiety-like behavior, and fear conditioning in adult mice of both sexes by comparing wild-type (WT) with littermate Calb1 KO mice. We hypothesized that a lack of calbindin would alter inhibitory neurotransmission by disrupting downstream genes related to synaptic plasticity. Because neocortical GABAergic interneurons temper excessive stimulation from excitatory neurons, and molecular and cellular dysfunction of these neurons leads to cognitive, affective, and behavioral impairments (6, 30), we hypothesized that Calb1 KOs would differ in the behaviors tested compared with WT littermates. Moreover, we predicted sex differences in the behavioral responses of wild-type mice would be eliminated in Calb1 KO mice. Next, we examined gene expression in the amygdala and prefrontal cortex (PFC), two areas of the brain intimately connected with limbic system control of the behaviors tested, in response to sex and genotype.
Materials and Methods
Animals
All procedures were approved by, and conducted in accordance with, the University of Virginia Animal Care and Use Committee guidelines. Breeding dams and sires, both of which were heterozygous for the null Calb1 allele, produced the mice used for all experiments (31). To set up the breeding colony, mice were ordered from Jackson Labs (stock number 003079), the background strain was C57BL/6J. Offspring were genotyped before weaning (postnatal day 21), and all homozygotes (WT and complete knockouts) were group housed by sex and age and maintained on a 12-hour light, 12-hour dark cycle (lights on at 1:00 pm). At all times the animals had access to water and food (number 7912; Harlan Teklad) ad libitum.
Between 50 and 70 days of age, all mice were gonadectomized, and at that time each received an estradiol-filled SILASTIC brand implant (Dow Corning, Corp; tubing 1.98 mm inner diameter × 3.17 mm outer diameter). Estradiol was prepared in sesame oil (estradiol-17β 50 μg/mL) and placed sc in the back of the neck. This procedure was used to provide equivalent hormone level regardless of the genotype or sex of the mouse. After surgery, mice were housed individually. Ten to 14 days after surgery, mice were tested for one of the three behaviors. The observers who scored the behaviors were blind to the sex and genotype of the mice. All behavioral tests were conducted in the light portion of the light/dark cycle.
At the end of the experiment, all mice were anesthetized using sodium pentobarbital and euthanized. Brains were rapidly removed and frozen on dry ice. Using a cryostat, brains were cut in coronal sections (120 μm) onto slides. A tissue punch (1 mm) was used to dissect the complete amygdala and the prefrontal cortex using anatomical guidelines established by visually comparing slices with figures in the Mouse Brain Atlas (32). The amygdala was collected in two bilateral punches from eight sections corresponding to Figures 31–40. The prefrontal cortex was collected in two medial punches from prelimbic and infralimbic areas in Figures 14–20.
Fear conditioning
We used a modified fear conditioning protocol (33). Before testing, mice were moved, one at a time, into the testing room. On the first test day, the subject was placed in half of a mouse shuttle box (Med Associates; number ENV-010MC; each interior compartment was 20.3 cm × 15.9 cm × 21.3 cm) for 10 minutes to explore the novel environment and then returned to their home cage. Twenty-four hours later, the animals were returned to the same test chamber. Freezing behavior was observed at 10-second intervals for 2 minutes (baseline, trial 1). Freezing was defined as no movement (other than natural respiratory motions). Next, an 80-dB white noise was presented for 30 seconds and immediately followed by a 2-second, 0.35-mA foot shock. This pairing was repeated after a 2-minute intertrial interval (training). Each animal was returned to the home cage 30 seconds after the second shock. The next day, freezing behavior was recorded in the same environment for 5 minutes (context, trial 2). One to 2 hours later, the mice were introduced to the novel side of the shuttle box, which had been altered in the following way: a divider was placed on the diagonal to divide the chamber into a triangularly shaped area. Pieces of dark tape were affixed to the walls, the floor was covered with a solid piece of plastic, and a drop of orange extract on a cotton swab was suspended from the ceiling of the chamber, out of reach of the mouse. The animals were observed for a total of 6 minutes. During the first 3 minutes (preconditioned stimulus, trial 3), no auditory stimulus was presented. In the last 3 minutes, the auditory stimulus was presented (preconditioned stimulus, trial 4). Freezing behavior was recorded at 10-second intervals for the entire 6-minute testing period. The amount of time in context-dependent freezing was expressed as a percentage of baseline freezing: ([trial 2 freezing time − trial 1 freezing time]/total time) × 100%. Percentage time in cue-dependent freezing was calculated in the same way: ([trial 4 freezing time − trial 3 freezing time]/total time) × 100%. In total, data from 36 mice were used in the analysis (KO, n = 10 of each sex; WT males, n = 7; and WT females, n = 9).
Elevated plus maze
Mice were habituated to the testing room for at least 30 minutes. At the start of the test, each subject was placed in the center of the elevated plus maze (EPM) facing an open arm. Mice explored the maze for 10 minutes and videotaped from above. Between subjects, the maze was thoroughly cleaned with ethyl alcohol. The floors and walls of the EPM (ENV-560A; Med Associates, Inc) were black polypropylene. Each runway measured 6 cm wide × 35 cm long × 71.25 cm tall. The walls on the closed arms were 20.3 cm high. Observers, blind to the sex and genotypes of the mice, scored the time spent in each portion of the maze (open, closed, or center) as well as the number of crosses between each portion. In addition, because others have reported motor coordination differences between KO and WT mice (34), we scored the number of times each animal turned around in the open arms of the maze and the total amount of time spent turning. Time spent turning was subtracted from total time in the open arms. Group sizes were as follows: WT males, females, and KO males, n = 7; KO females, n = 9).
Social investigation
Mice were tested in a Plexiglas cage divided into three chambers: two equal-size end areas (31.5 × 25.5 cm each) and a smaller neutral section between them (10.5 × 25.5 cm). During the habituation phase, the end areas contained an empty holding cell (10.16 cm in diameter and 13.97 cm tall). Each mouse was placed in the center of the box and allowed to explore the entire box for 10 minutes. The subject was then restricted in the center while a gonad-intact adult male was placed under the holding cell (on one randomly selected side), and an ovariectomized, estrogen-implanted adult female (both were C57BL/6 WT mice) was placed on the opposite side. These stimulus mice were habituated to the holding cells in their home cages for at least 10 minutes before the test. During the test phase, the subjects were allowed to investigate the entire box for 10 minutes. Observers blind to sex and genotype scored the number of entrances into each side and the time spent investigating the stimulus mouse. Investigation was defined as the test mouse's nose touching the stimulus mouse through the bars or sniffing within 1 cm of the mouse. The test arena was carefully cleaned with ethanol between subjects. We tested the following numbers of subjects: WT males, n = 9; all other groups, n = 10.
Quantitative real-time PCR (qRT-PCR)
We selected genes that are either related to the behaviors we examined or are associated with calbindin neurons/signaling. We assesssed brain-derived neurotrophic factor (BDNF) signaling targets because BDNF has been shown to regulate calbindin (35). Because Calb1 is primarily expressed in inhibitory neurons, we examined two γ-aminobutyric acid (GABA)-related genes. We measured both nuclear estrogen receptors because estradiol regulates Calb1 expression. We also investigated the potential effects on three epigenetic regulators; Hdac3, Hdac4, and Mecp2. Other targets, Nr3c1, Crhr1, and Avp, were selected because they are involved in the behaviors we tested. Additionally, many of these genes have been shown to have sexually dimorphic expression in the brain.
The RN easy lipid tissue minikit (QIAGEN) was used to isolate total RNA according to the manufacturer's protocol. The quantity and quality of the RNA were determined using a NanoVue spectrophotometer. cDNA templates were prepared using an AffinityScript quantitative PCR cDNA synthesis kit (Agilent Technologies) according to the manufacturer's protocol. The StepOnePlus real-time PCR system (Applied Biosystems) was used to perform qRT-PCR. Either TaqMan probe or SYBR Green-based detection (Applied Biosystems) were used to detect PCR products of interest. The following TaqMan gene expression assays were used: estrogen receptor-α (Esr1, Mm00433149m1), estrogen receptor-β (Esr2, Mm00599821 m1), histone deacetylase 4 (Hdac4, Mm01299557_m1), methyl CpG binding protein 2 (Mecp2, Mm01193537 g1), and neurotrophic tyrosine kinase, receptor, type 2 (Ntrk2, Mm00435422_m1). All TaqMan assays were normalized to mouse β-actin (Actb, number 4352933E). Oligonucleotide primers (Table 1) were either designed for SYBR-Green based analysis using consensus sequences from the National Center for Biotechnology Information genomic alignment database or derived from prior publications and were synthesized by Invitrogen. The following primers were based on previously published constructs: Bdnf-exon IV (36); Calb1 (9); and Gabrb1 and Gad67 (37). All other primers were designed to span an exon-exon junction to prevent binding to genomic DNA. For all SYBR assays, samples were normalized to the endogenous control, β2-microglobulin (B2m).
Table 1.
Quantitative RT-PCR Primer Sequences
| Gene | Sequence (5′–3′) |
|---|---|
| Avp | F, TGCTCGCCAGGATGCTCAACAC |
| R, TTGCCGCCTCTGGGCAGTT | |
| B2M | F, GGCTCACACTGAATTCACCCCC |
| R, ACATGTCTCGATCCCAGTAGAC | |
| Bdnf, all exons | F, TTAAGCGGCTTCACAGGAG |
| R, CCTGCTGCCATGCATAAAAC | |
| Bdnf, exon IVa | F, CTCCGCCATGCAATTTCCAC |
| R, GCCTTCATGCAACCGAAGTA | |
| Calbb | F, ACTCTCAAACTAGCCGCTGCA |
| R, TCAGCGTCGAAATGAAGCC | |
| Crhr1 | F, CTGAACAGTGAGGTCCGCTC |
| R, GGCTCTGATGGAGTGCTT | |
| Gabrb1c | F, GCCATGGACTGGTTTATTGC |
| R, CCACGCATACCCTCTCTTGGTG | |
| Gad67c | F, GGGTTCCAGATAGCCCTGAGCGA |
| R, TGGCCTTGTCCCCTTGAGGCT | |
| Hdac3 | F, ATGACAGGACTGACGAGGCCGA |
| R, TGGGTGCTTCTGGCCTGCTGTA | |
| Nr3c1 | F, GGATGCCATTATGGGGTGC |
| R, TCGTTTTTCGAGCTTCCAGG |
Validation experiments were conducted to test for equally efficient target and endogenous control gene amplification, and primers were between 90% and 110% efficient for all amplifications. In each qRT-PCR reaction using SYBR-Green, primers were verified for a single PCR product of expected size with the disassociation melting curve stage. For TaqMan and SYBR-Green-based detection, target and endogenous control genes were measured in triplicate for each cDNA sample during each real-time run to avoid intersample variance. All genes of interest were analyzed with Step One software (Applied Biosystems) using the comparative cycle thresholds method.
Serum corticosterone
Blood was collected from awake, adult male mice of both genotypes (n = 6 WT and n = 7 KO). These mice had been tested in the EPM at least 2 weeks prior to blood collection. Each mouse was sampled twice: once for a baseline measurement and again after 15 minutes of restraint stress. Samples were collected at least 1 week apart and the order of the conditions was counterbalanced by genotype. Samples were obtained by submandibular punctures using a 4-mm Goldenrod lancet during the first hour of the light period. This blood collection method is rapid (less than 2 min) and requires no anesthesia (38). Samples were centrifuged at 12 000 rpm for 10 minutes, and serum was collected and frozen until assay. We assessed the serum concentrations of corticosterone using an enzyme immunoassay kit (DetectX corticosterone immunoassay kit; Arbor Assays). Control serum was assayed in duplicate and interpolated from the standard curve, which was fitted with a 4PLC nonlinear regression in GraphPad Prism 6 for Mac. The sensitivity of the kit was 18.6 pg/mL; the limit of detection was 16.9 pg/mL. The average coefficient of variance was 3.89%.
Statistics
All data were analyzed using NCSS Software (2000). Data points greater than 2 SD from the mean were tested using Grubb's outlier test. For data analyses two-way or repeated-measures ANOVAs were used, as appropriate, to assess the contributions of genotype and sex. Paired comparisons were conducted using Fisher's least significant differences multiple comparison tests.
Results
Male KO mice display reduced conditioned fear in response to an auditory cue
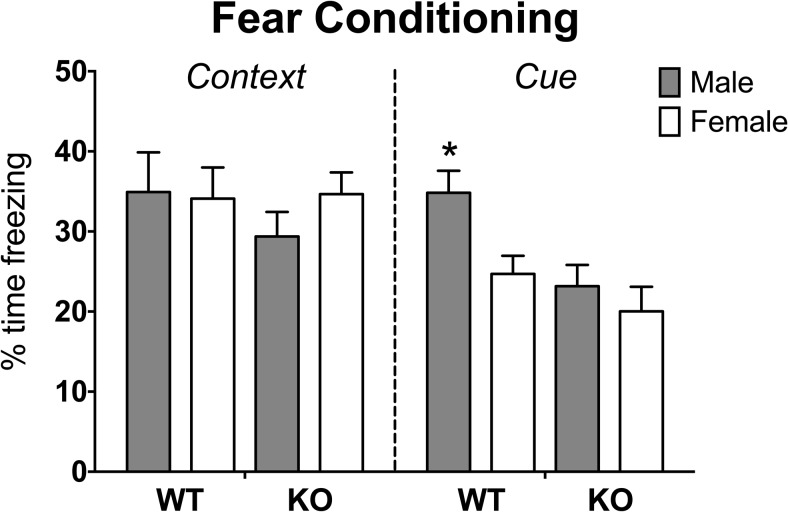
A repeated-measures ANOVA revealed an interaction between sex and genotype (F[1,95] = 5.73, P < .025) for freezing time across trials 1–4 and an interaction between trial and genotype (F[1,95] = 3.21, P < .03). The genotype-by-sex interaction was caused by male KO mice, which displayed less freezing behavior than male WT or female KO mice over all trials (P < .05). The genotype difference was noted only on the cued conditioned stimulus trial (trial 4; Figure 1). To further investigate this interaction, we ran repeated measures analysis on the baseline and cued conditioned stimulus trials. Again, a significant interaction between sex and genotype was indicated along with interactions between sex and trial and between genotype and trial (F[1,95] = 4.13, 5.73, and 8.67, respectively, P < .05 or less). A main effect of trial was also noted (F[1,95] = 343.19, P < .0001). The sex-by-genotype interaction was caused by a difference between KO and WT males, whereby KO males displayed significantly less freezing than did WT males (P < .05). The interaction between sex and trial was caused by a sex difference on the conditioned stimulus trial; males froze more than females (P < .05). The genotype-by-trial effects were noted on the final trial in which KO mice were less reactive to the tone than WT mice (P < .05).
Figure 1.
Impaired retention of cued fear memory in Calb1 KOs. Mean ± SEM percentage of time spent freezing for context-specific and cue-specific fear conditioning expressed as a percentage of baseline freezing: (context trial freezing − baseline trial freezing/total time) × 100% and (cue trial freezing − baseline trial freezing/total time) × 100%. *, Male WT significantly different from all other groups (P < .05).
Calb1 KO mice show less anxiety-like behavior on the elevated plus maze
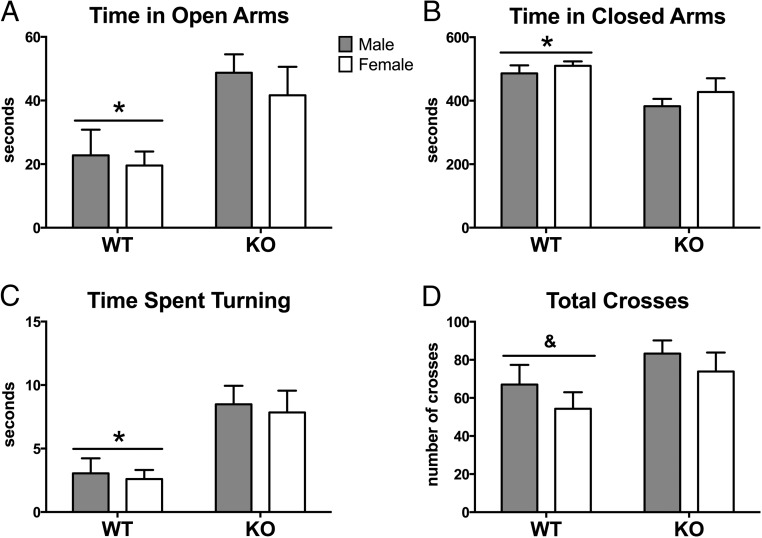
Calb1 KO mice spent more time than WT mice exploring the open arms of the EPM (F[1,29] = 9.78; P < .004; Figure 2A). KO mice spent more time turning around in the open arms (F[1, 29] = 14.40, P < .001) (Figure 2C) and made more turns than WT mice (F[1, 29] = 9.67, P < .005). The genotype effect on time spent exploring the open arms mice remained significant, even after we subtracted the amount of time spent turning around from total time in the open arms (F[1, 29] = 10.81; P < .004). There were no effects of sex nor were there any sex-by-genotype interactions. Additionally, WT mice spent more time in the closed arms than KO animals (F[1, 29] = 10.67; P < .003; Figure 2B), but no other effects or interactions were noted. No significant effect of sex was found in the total number of crosses (open, closed, and center areas) made during the test. However, there was a trend toward a genotype effect (F[1, 29] = 3.9 P = .06): KO mice made more crosses in the maze than WT mice (Figure 2D).
Figure 2.
Decreased anxiety-like behavior in Calb1 KO mice on the elevated plus maze. Mean ± SEM time (seconds) spent in the open arms (A), in the closed arms (B), and turning around in the open arms (C) during the 10-minute (600 sec) test. Total crosses between open, closed, and center portions of the maze are represented in panel D. *, Significant main effect of genotype (P < .05); &, genotype trend (P = .06).
Male Calb1 KO mice display enhanced investigation of novel mice
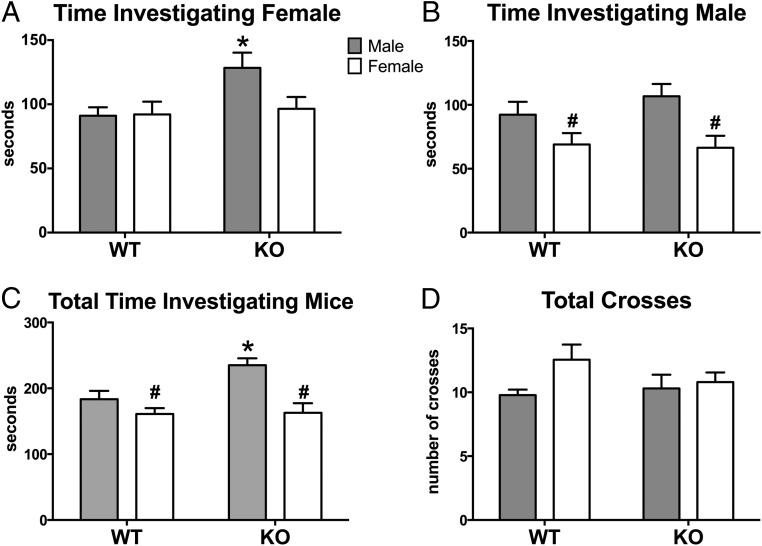
The duration of social interactions during the social preference test proved to differ by genotype and/or sex. Analyses revealed main effect of genotype, with KO mice spending more time than WT mice investigating the female stimulus mouse (F[1,35] = 4.58, P < .04; Figure 3A). There was also an overall effect of sex, in which females of both genotypes investigated the male stimulus mouse less than males of both genotypes (F[1,35] = 11.23, P = .002; Figure 3B); however, no interaction was noted. No group showed a significant sexual preference, calculated by subtracting time spent with male from time spent with the female, nor were there differences between the groups for this measure. Interestingly, the time spent investigating both stimulus mice of either sex (male and female) was significantly affected by sex and genotype, and we noted an interaction between these factors (F[1,35] = 16.04, 5.13, 4.46; P < .0003, 0.03, and 0.05 respectively). The effects were caused by the male KO mice, which spent more time with the stimulus animals than females or WT males (P < .05; Figure 3C). Lastly, the total number of entries into each chamber was not significantly different by genotype, nor was there a sex-by-genotype interaction (Figure 3D). There was a trend for a sex difference in which females were more active than males (F[1,35] = 3.74, P = .06).
Figure 3.
Increased investigation during the social preference task by male KOs. Mean ± SEM time (seconds) spent investigating female stimulus mouse (A), male stimulus mouse (B), and total time with either stimulus (C) are shown. Total number of transitions between different compartments of the three-chambered box are represented in panel D. #, Significant main effect of sex (P < .05; *, significantly different from all other groups (P < .05).
Sexually dimorphic genes in the prefrontal cortex and amygdala
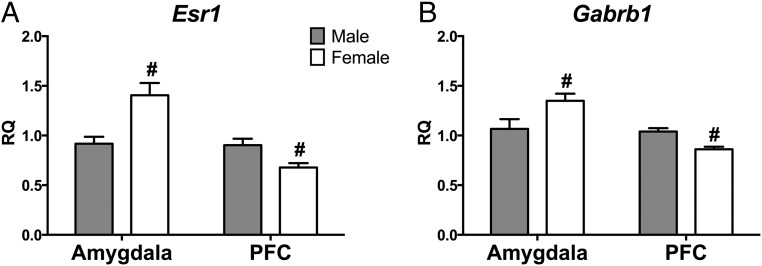
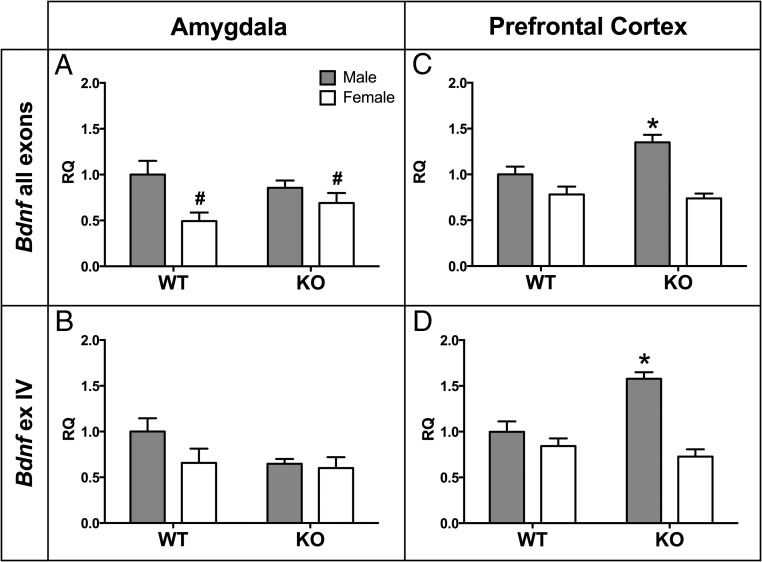
Consistent with our previous research (9), WT females expressed more Calb1 mRNA than males in the prefrontal cortex (t [12] = 2.85, P < .02) but not in the amygdala (t [13] = 0.54, P = .60). We also noted region-specific, sexually dimorphic expression of estrogen receptor-α (Esr1), GABA-A receptor subunit-β (Gabrb1), and methyl CpG binding protein 2 (Mecp2). In the amygdala, all three genes were more highly expressed in females than males, regardless of genotype (Esr1: F[1,29] = 12.7, P < .001; Gabrb1: F[1,31] = 4.92, P < .034; Mecp2: F[1,28] = 4.93, P < .035; Figure 4A). In contrast, the opposite pattern was true in the prefrontal cortex: males had higher expression than females (Esr1: F[1,27] = 8.59, P < .007; Gabrb1: F[1,30] = 17.03, P < .00001; Mecp2: no sex difference; Figure 4B). There was a significantly higher expression of brain-derived neurotrophic factor (Bdnf) in the amygdala of WT males than in WT females (F[1,29] = 8.17, P < .009; Figure 5A). There were no significant effects noted in the expression of Bdnf exon IV in the amygdala (Figure 5B).
Figure 4.
Sexually dimorphic expression of Esr1 and Gabrb1 in amygdala and PFC independent of genotype. Relative quantity (RQ) estrogen receptor (Esr1) (A) and GABA receptor subunit-β (Gabrb1) (B) mRNA in two brain areas: amygdala and PFC. WT and KO RQ values are combined by sex. Gray bars, Mean ± SEM WT male + KO male, white bars, Mean ± SEM WT female + KO female; #, significant main effect of sex within brain region (P < .05.).
Figure 5.
Elevated Bdnf expression in male Calb1 KO PFC but not amygdala. Mean ± SEM relative quantity (RQ) Bdnf (all exons) mRNA are expressed in the amygdala (A) and prefrontal cortex (C). RQ values of Bdnf exon IV mRNA in the amygdala (B) and prefrontal cortex (D) are shown. #, Significant main effect of sex (P < .05); *, significantly different from all other groups (P < .05).
Genes affected by Calb1 KO in the amygdala and PFC
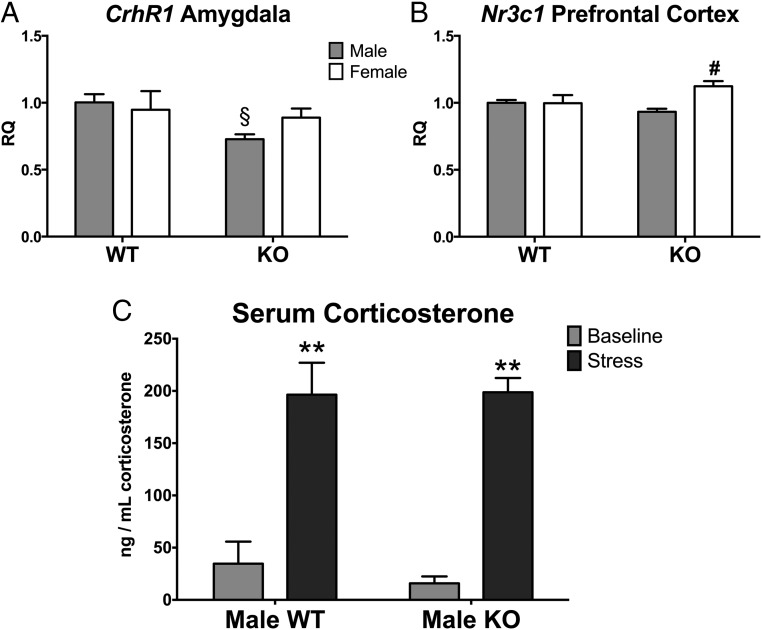
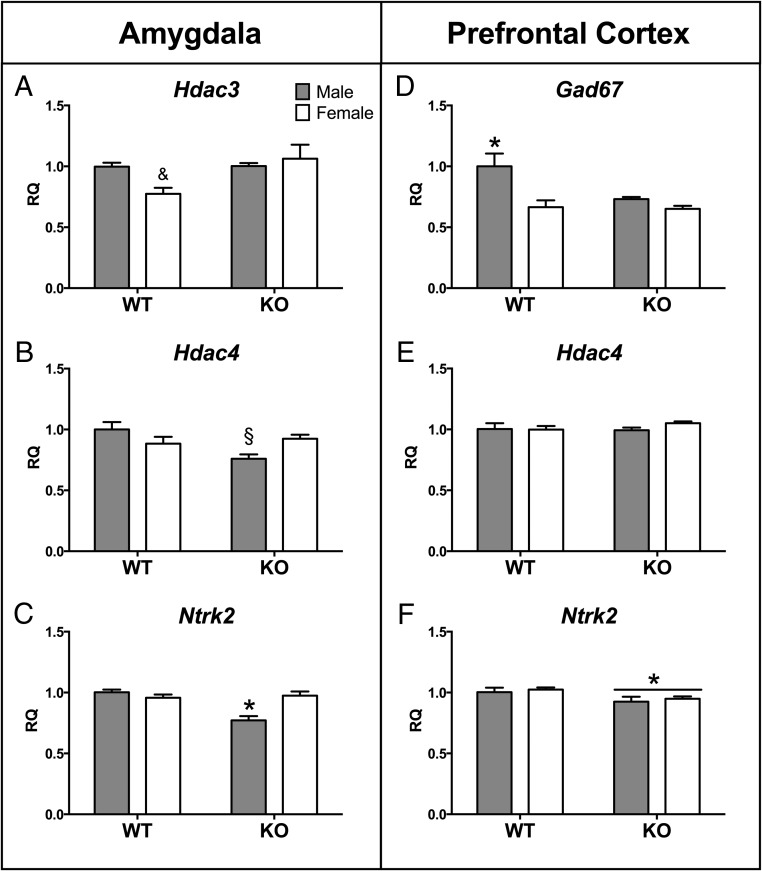
In the amygdala, there was a significant main effect of genotype (KO < WT) on CRH receptor 1 (Crhr1: F[1,30] = 4.69, P < .04; see Figure 7A), histone deacetylase 4 (Hdac4: F[1,30] = 4.48, P < .04; Figure 6B), and neurotrophic tyrosine kinase receptor type 2 (Ntrk2: F[1,31] = 12.08, P < .002; Figure 6C). However, genotype effects on Hdac4 and Ntrk2 predominated in the males as indicated by significant sex by genotype interactions for both genes [Hdac4 [F]1,30] = 8.76, P < .006] and Ntrk2 [F]1,31]=16.54, P < .00001]). Moreover, post hoc tests revealed an effect on Crhr1 limited to males in which WT males had higher levels than KO males (P < .05).
Figure 7.
Calb1 KO affects stress-related genes in the brain but not serum corticosterone levels in males. Mean ± SEM relative quantity (RQ) values of the CRH receptor 1 (Crhr1) in the amygdala (A) and glucocorticoid receptor (Nr3c1) in the prefrontal cortex (B). C, Serum corticosterone levels in WT and KO males at baseline (gray bars) and 15 minutes after a 15-minute restrain stressor (dark gray bars). §, Significantly different from WT male group (P < .05); #, significantly different from KO male group (P < .05); **, significant main effect of stress (P < .05).
Figure 6.
Genotype-dependent expression changes in amygdala and PFC. Mean ± SEM relative quantity (RQ) values for expression of histone deacetylase 3 (Hdac3) in the amygdala (A); histone deacetylase 4 (Hdac4) in the amygdala (B) and PFC (E); neurotrophic tyrosine kinase receptor 2 (Ntrk2) in the amygdala (C) and in PFC (F). &, Trend for a sex-by-genotype interaction: female knockout greater than female wild type (P = .06); §, significantly different from same-sex control group (P < .05); *, significantly different from all other groups (P < .05).
Similar to the amygdala results, the male KO group also drove several genotype effects in the prefrontal cortex. Male KO mice expressed significantly more Bdnf in the PFC compared with WT males as indicated by a significant sex-by-genotype interaction (F[1,29] = 6.52, P < .017; Figure 5C). More specifically, exon IV of Bdnf was increased in KO compared with WT mice (F[1,29] = 5.97, P < .02) and a significant interaction between sex and genotype (F[1,29] = 14.29, P < .0008) limited the KO effect to males (Figure 5D). Expression of the GABA synthesis enzyme glutamate decarboxylase (Gad67) in the PFC was also significantly reduced in KO males as compared with WT males, but female KOs were unaffected (P < .05; Figure 6D). Unlike the amygdala, however, the expression of Hdac4 was unaffected by genotype or sex (Figure 6E). In contrast, gene expression was reduced in the KO mice of both sexes for the BDNF receptor gene, Ntrk2 (F[1,30] = 5.97, P < .021; Figure 6F).
Only two of the genes investigated showed significant main effects driven by female KOs. In the prefrontal cortex, we noted a significant sex effect in glucocorticoid receptor (Nr3c1) expression in which the females were greater than males (F[1,28] = 6.13, P < .02). However, a significant interaction between sex and genotype (F[1,28] = 6.52, P < .02) showed that the sex effect was specific for KO mice: female KOs had higher expression than male KOs only (P < .05; Figure 7B). Histone deacetylase 3 (Hdac3) expression was also significantly higher in the amygdala of KO as compared with WT mice (F[1,31] = 4.18, P < .05; Figure 6A). Hdac3 expression also tended to be higher in females compared with males; however, the interaction between sex and genotype was not statistically significant (F[1,31] = 3.93, P = .06). Finally, there were no significant genotype or sex effects on the expression of vasopressin (Avp) and estrogen receptor-β (Esr2) in either brain area (P > .05; Table 2).
Table 2.
Statistical Summary of qPCR Results From Amygdala and Prefrontal Cortex
| Gene | Amygdala |
Prefrontal Cortex |
|||||
|---|---|---|---|---|---|---|---|
| Sex Effect | Genotype Effect | Sex-Genotype Interaction | Sex Effect | Genotype Effect | Sex-Genotype Interaction | ||
| Avp | Vasopressin | NS | NS | NS | NS | NS | NS |
| Bdnf (all exons) | Brain-derived neurotrophic factor | M > F, P < .008 | NS | NS | M > F, P < .00 001 | NS | MKO > MWT, FWT, FKO |
| Bdnf exon IV | Brain-derived neurotrophic factor | NS | NS | NS | M > F, P < .00 001 | KO > WT, P < .02 | MKO > MWT, FWT, FKO |
| Calb1 (WT only) | Calbindin | NS | NA | NA | F > M, P < .007 | NA | NA |
| Crhr1 | CRH receptor 1 | NS | WT > KO, P < 0.04 | NS | NS | NS | NS |
| Esr1 | Estrogen receptor-α | F > M, P < .001 | NS | NS | M > F, P < .007 | NS | NS |
| Esr2 | Estrogen receptor-β | NS | NS | NS | NS | NS | NS |
| Gabrb1 | GABA A receptor, subunit-β | F > M, P < .034 | NS | NS | M > F, P < .0003 | NS | NS |
| Gad67 | Glutamate decarboxylase 67 | NS | NS | NS | M > F, P < .005 | WT > KO, P < .045 | MWT > FWT, FKO, MKO |
| Hdac3 | Histone deacetylase 3 | NS | KO > WT, P < .05 | NS trend FKO > FWT | NS | NS | NS |
| Hdac4 | Histone deacetylase 4 | NS | WT > KO, P < .043 | MWT > MKO | NS | NS | NS |
| Mecp2 | Methyl CpG binding protein 2 | F > M, P < .035 | NS | NS | NS | NS | NS |
| Ntrk2 | Neurotrophic tyrosine kinase receptor 2 | F > M, P < .015 | WT > KO, P < .001 | MKO < MWT, FWT, FKO | NS | WT > KO, P < .02 | NS |
| Nr3c1 | Glucocorticoid receptor | NS | NS | NS | F > M, P < .02 | NS | FKO > MKO |
Abbreviations: F, female; M, male; NA, not applicable; NS, not significant. Fisher's least significant differences test was used for all interactions (P < 0.05).
Stress response is not affected by genotype
As expected, serum corticosterone concentrations significantly increased immediately after restraint stress (repeated measures ANOVA: F[1,25] = 46.68, P = .00003). Corticosterone concentrations were unaffected by genotype, nor was there a stress by genotype interaction (F[1,25] = 0.82, 0.18; P > .05, respectively; Figure 7C). Additionally, there was no effect of trial (ie, stressed on first or second trial) on serum corticosterone concentrations (F[1,25] = 0.97, P > .05).
Discussion
We hypothesized that loss of calbindin, via dysfunction of calcium signaling in neurons would influence affective and social behaviors. Calbindin is present in specific prefrontal cortical and limbic brain regions that underlie emotional and cognitive behavior (5, 8, 30, 39, 40). We also predicted that, because Calb1 is sexually dimorphic in the mouse prefrontal cortex, the elimination of Calb1 would likewise influence sexual dimorphism in some of the behaviors we tested (9, 12). In one of the three behaviors we tested, fear conditioning, we found that sex differences in WT male mice were not present in the KO mice. In another behavior, social preference, sex differences were present in the KO mice that were not noted in WT animals. It is worth noting that we have previously observed a sex difference in WT C57 mice using a Y-maze to test partner preferences between males and females in gonadectomized and hormone-treated adults (12, 41). We noted a significant decrease in anxiety-like behavior on the elevated plus maze in KO mice of both sexes. Thus, KO mice tended to show less anxiety-like behavior and increased exploratory behavior in the other tests we performed. For example, in the social preference test, KO males spent more time interacting with the stimulus mice than the other groups, which suggests a reduction in anxiety-like behavior. Moreover, in the cued portion of the fear conditioning task, KO mice of both sexes displayed less freezing behavior than the WT males, also indicative of a reduction of anxiety-like behavior in Calb1 KO mice.
Because an earlier study reported an increase in pain threshold in Calb1 KO mice (42), we tested reaction times to shock, and all mice reacted the same, with very short latencies. It has been previously documented that Calb1 KO mice do not have auditory impairments, and we therefore assumed that they heard the tone we conditioned to the shock (43). Alternatively, the genotype effect may be due to the loss of a calbindin-dependent sex difference in fear behavior. Freezing behavior in response to an auditory cue tended to be higher in WT males than in WT females, despite the fact that all groups of mice were gonadectomized and treated with estradiol. Therefore, ruling out adult differences in circulating gonadal hormones, the normal sex difference in fear behavior may depend on the presence of calbindin.
In the elevated plus maze, Calb1 KO mice of both sexes spent more time exploring the open arms and less time in the closed arms as compared with WT mice. These data indicate that reduced anxiety-like behavior is associated with the loss of calbindin. Due to potential differences in motor coordination (34), we predicted that KO mice would take longer to turn in the open arms of the maze than WT mice. After correcting for the time spent turning around in the open arms, the genotype effect remained significant. Given that the Calb1 KO mice may have difficulty maneuvering in the EPM, it is notable that they select to spend more time in open arms and less time in the secure closed arms of the maze as compared with WT mice.
We did not observe sex differences in any aspect of EPM behavior for either genotype, unlike sex differences observed in most studies when gonad-intact mice are tested (44–46). Thus, our data suggest that differences in circulating gonadal hormones between adult males and females produce the reported sex differences in anxiety-like behavior on the EPM. However, research using gonadectomized, testosterone, or blank-implanted four-core genotype mice indicates that sex chromosome complement also affects anxiety-like behavior. In the elevated plus maze, mice with two X chromosomes spent significantly more time in the open arms than XY mice, regardless of gonadal sex (47). However, using the four core genotype mice, we have shown that XX mice have more calbindin in the PFC than do XY mice (9). Thus, we believe sex differences noted here are caused by sex chromosome compliment.
To unravel the molecular underpinnings of the behavioral results described above, we used quantitative PCR to quantify gene expression changes in amygdala and prefrontal cortex tissue harvested from WT and KO gonadectomized adult mice of both sexes. Three distinct classes of the genes examined were impacted by Calb1 knockout. These include genes associated with BDNF signaling, GABA neurotransmission, and histone deacetylation. These genes are associated with the behaviors we examined. In the PFC, the BDNF receptor Ntrk2 was significantly down-regulated in both male and female KO animals. The differences between male Calb1 KO mice and the other groups drove the sex-specific genotype effects we observed in the expression of Ntrk2 in the amygdala (male KO less than all groups) and Bdnf/Bdnf exon IV in PFC (male KO greater than all groups). Numerous studies have shown a causal link between BDNF and calbindin. For example, BDNF induced the expression of Calb1 mRNA and protein in cultured hippocampal and cortical neurons (35, 48). Additionally, Bdnf KO mice have reduced Calb1 expression in the hippocampus and cortex despite a normal density of GABAergic neurons (49). However, to our knowledge, we are the first to demonstrate reciprocity between the two genes by showing changes in Bdnf-Ntrk2 gene expression in the amygdala and PFC as a result of calbindin knockout.
The latter finding could offer another explanation as to why male KO mice displayed decreased freezing behavior during the cued fear conditioning trial. The acquisition of conditioned fear is dependent on normal BDNF signaling through NTRK2 in the amygdala (50–52). Changes in Bndf expression in the PFC also align with our other behavioral results. Overexpression of Bdnf in forebrain neurons decreased anxiety-like behavior of mice on the EPM, similar to the anxiolytic phenotype of the Calb1 KO mice (53). We also noted that social investigation in male Calb1 KO mice is increased as compared with WT males, an effect that parallels the genotype effects on Bdnf expression in the PFC. These data are supported by a study in mice, which showed that increased affiliative behavior during a social interaction test is associated with significantly higher expression of Bdnf in the frontal cortex of mice (54). Bdnf IV transcription is regulated by Ca2+ influx through voltage-sensitive calcium channels (Cav1 L-type CA2+) that are activated in cortical neurons (55). Calbindin colocalizes with these calcium channels in mouse cortex (35, 48), which suggests one possible route by which calbindin could regulate the expression of Bdnf. However, it remains to be determined whether the effects of Calb1 KO on NTRK2 are due to the loss of calbindin itself or secondary effects caused by feedback regulation of the receptor by BDNF (56).
Expression of the GABA synthesis enzyme, glutamate decarboxylase 67 (Gad67), was reduced in the prefrontal cortex of male Calb1 KO mice compared with WT controls. We found a sex difference in the WT mice, in which the males were greater than females; this was attenuated by the loss of calbindin. This expression pattern parallels the changes we observed in the social preference task. KO males spent significantly more time investigating the stimulus mice during the test than any other group. Similarly, decreased Gad67 expression in the medial prefrontal cortex has been associated with concomitant changes in behavior in a social interaction task (57). Others have reported connections between Gad67 and social behavior, such as social odor preference (conspecific urine vs water), sociability (novel mouse vs novel object), and social preference (familiar mouse vs novel mouse) (58, 59). In the prefrontal cortex of schizophrenic individuals, the expressions of Gad67 and Ntrk2 are positively correlated (60). The authors suggest that decreased Ntrk2 may underlie a dysfunction of GABAergic neurons in schizophrenia. Ntrk2 signaling may also mediate the effect we observed in the PFC of Calb1 KO mice because Ntrk2 and Gad67 expression were both decreased compared with controls.
In the amygdala, two classes of histone deacetylases were affected in response to a loss of calbindin. Class I and IIa histone deacetylases (HDACs) have opposite effects on learning and memory. Class I HDACs, such as HDAC3, tend to impair memory and cognition, whereas HDAC4, a class IIa HDAC, improves learning (61). Male Calb1 KO mice showed reduced levels of Hdac4 and increased expression of Hdac3 in the amygdala. Similarly, cued fear conditioning in rats results in increased histone acetyl-transferase activity in the amygdala, and treatment with HDAC3 reduces fear memory and conditioning (62). Conditional forebrain only Hdac4 knockout animals exhibit reduced fear in a context-dependent fear conditioning paradigm and also spend more time exploring the open arms of the EPM (65). Likewise, Calb1 KOs show decreased anxiety and reduced fear and express less Hdac4. In addition, because the amygdala is required for cue-dependent associative fear memory (66), it follows that we observed HDAC expression changes only in the amygdala and not the PFC. Synaptic activity-dependent calcium signaling affects the nuclear export of HDAC4, which may explain calbindin's role in regulating HDAC activity and expression (67). We speculate that the synergistic interaction of the two HDACs in the amygdala are part of a network of genes that underlie the impaired retention of cued fear and decreased anxiety in Calb1 KOs.
Changes in corticotropin releasing hormone (CRH) receptor (Crhr1) expression may also partially explain the fear and anxiety-like phenotypes of calbindin knockouts. Male Calb1 KO mice express significantly less Crhr1 in the amygdala than WT males. CRH signaling in the amygdala is a crucial regulator of fear memory. Activation of CRHR1 receptors in the basolateral amygdala enhances fear memory consolidation (68), whereas antagonizing CRHR1 in the central amygdala attenuates stress-induced freezing behavior (69). Genetic variations in the Crhr1 gene have even been shown to affect fear acquisition behavior in humans (70). Additionally, Crhr1 in the amygdala and forebrain is known to play a critical role in anxiety-related behaviors, which serves to further explain the anxiolytic phenotype of Calb1 KO mice (71–73). We also noted gene expression changes in another regulator of the stress and anxiety response, the glucocorticoid receptor (Nr3c1). Female Calb1 KO mice express significantly more Nr3c1 in the prefrontal cortex than male KO mice, whereas there was no sex difference in WT mice. Based on the genotype-dependent effects on expression of Crhr1 and Nr3c1 in the amygdala and prefrontal cortex, respectively, we hypothesized that the hypothalamic-pituitary-adrenal axis (HPA) would be affected by the loss of calbindin. For example, mice lacking Crhr1 have a decreased stress-induced corticosterone release and atrophy of the adrenal gland (73). However, in this first-pass assessment of the HPA axis, we did not note any differences in serum corticosterone levels at baseline or poststress between male Calb1 KO and WT mice. Therefore, we suggest that the significant behavioral differences between genotypes are due to gene expression changes within the limbic system and not via a secondary effect of stress hormones.
We found several sex differences in gene expression in the amygdala and PFC. First, we replicated our previous work by showing that Calb1 expression is sexually dimorphic and biased to females in the prefrontal cortex (9). As expected, we did not find a sex difference in Calb1 expression in the amygdala. Estrogen receptor-α (Esr1) mRNA was sexually dimorphic in the PFC; expression in males was significantly greater than in females, regardless of genotype, and the opposite was true in the amygdala. This finding is in contrast to other studies that reported a sex difference in the opposite direction in Esr1 expression in postnatal day 10 prefrontal cortex (74) and no sex difference in the PFC of postnatal day 21–25 mice (9). Early postnatal and prepubertal mice have very low levels of circulating sex hormones and have yet to undergo hormonal reorganization of the brain during puberty. This may explain why our current results using adult estradiol-implanted gonadectomized mice differ from those studies.
GABA-A receptor subunit-β (Gabrb1) expression was also sexually dimorphic in opposing directions in both brain areas: males had higher expression than females in the PFC and the opposite was true for the amygdala. This sex difference is interesting, considering the important role of Gabrb1 in sexually dimorphic disorders, like autism (75, 76). We also noted a sex difference in expression of methyl CpG binding protein 2 (Mecp2) in the amygdala. Females expressed higher levels of this X-chromosome gene associated with the neurodevelopmental disorder, Rett syndrome, than did males. Others have reported a similar sex difference in Mecp2 expression in the postnatal day 1 rat amygdala (77), which indirectly supports our result by suggesting that the sex difference is organized by early estradiol exposure. The same group also showed that sexually dimorphic Mecp2 expression in the amygdala is required for the development of sexually dimorphic behaviors in later life (78). We were able to detect significant sex differences in neuronal gene expression despite equalized estradiol levels across sexes, which suggests an organizational effect of steroid hormones/receptors and/or sex chromosome complement rather than an activational effect of sex hormones (9, 79).
A number of previous studies have indirectly examined the relationship between calbindin and affective and social behavior via environmental manipulations. For example, early maternal deprivation decreases the density of calbindin-positive GABAergic neurons in the paraventricular region of the hypothalamus of periadolescent rats. These animals interact more with an unfamiliar conspecific and demonstrated less anxiety-like behavior compared with controls (80). Likewise, in juvenile Octodon degus, a rodent species that shows biparental care, the absence of the sire in the nest causes brain region- and age-specific changes in Calb1 expression in the hippocampus, cortex, and bed nucleus of the stria terminalis, areas that regulate stress and anxiety (63, 81). Finally, early social isolation reduces the amount of calbindin in prefrontal cortex neurons in male rats (64). The authors suggested that a reduction of calbindin in GABAergic interneurons compromised their ability to interact normally with medial PFC pyramidal neurons, which connect to subcortical limbic networks underlying fear and anxiety. Therefore, these behaviors would be disrupted if calbindin is reduced.
To our knowledge, this is the first set of studies to directly examine how calbindin affects affective and social behaviors in rodents. In this study, we chose a more direct route by using Calb1 KO mice. Moreover, we chose to focus on global, as opposed to a region-specific, KO because Calb1 is expressed ubiquitously across multiple brain regions that are intimately involved in regulating emotional behaviors including fear, anxiety, and sociability. We reported significant genotype- and sex-dependent gene expression effects of Calb1 knockout in two of these brain areas: the prefrontal cortex and amygdala. We also demonstrated that loss of calbindin significantly alters fear, anxiety, and social behaviors in adult mice, thereby directly linking calbindin with affective behaviors.
Acknowledgments
This work was supported by National Institutes of Health Grant R21NS055218 (to E.F.R.). E.P.H. was supported by Grant T32GM008328.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BDNF
- brain-derived neurotrophic factor
- CRH
- corticotropin releasing hormone
- EPM
- elevated plus maze
- GABA
- γ-aminobutyric acid
- GABAergic
- γ-aminobutyric acid secretion
- GAD67
- glutamate decarboxylase 67
- HDAC
- histone deacetylase
- KO
- knockout
- NTRK2
- neurotrophic tyrosine kinase receptor type 2
- PFC
- prefrontal cortex
- qRT-PCR
- quantitative real-time PCR
- WT
- wild type.
References
- 1. Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. [DOI] [PubMed] [Google Scholar]
- 2. Kojetin DJ, Venters RA, Kordys DR, Thompson RJ, Kumar R, Cavanagh J. Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat Struct Mol Biol. 2006;13:641–647. [DOI] [PubMed] [Google Scholar]
- 3. Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell Mol Life Sci. 2009;66:275–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baimbridge KG, Miller JJ, Parkes CO. Calcium-binding protein distribution in the rat brain. Brain Res. 1982;239:519–525. [DOI] [PubMed] [Google Scholar]
- 5. DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. [DOI] [PubMed] [Google Scholar]
- 6. Druga R. Neocortical inhibitory system. Folia Biol (Praha). 2009;55:201–217. [DOI] [PubMed] [Google Scholar]
- 7. Hof PR, Glezer, Conde F, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. [DOI] [PubMed] [Google Scholar]
- 8. Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. [DOI] [PubMed] [Google Scholar]
- 9. Abel JM, Witt DM, Rissman EF. Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology. 2011;93:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J Comp Neurol. 2010;518:3618–3629. [DOI] [PubMed] [Google Scholar]
- 14. Sakai T, Oshima A, Nozaki Y, et al. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. [DOI] [PubMed] [Google Scholar]
- 15. Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. [DOI] [PubMed] [Google Scholar]
- 16. Riascos D, de Leon D, Baker-Nigh A, et al. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer's disease. Acta Neuropathol. 2011;122:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abraham H, Richter Z, Gyimesi C, et al. Degree and pattern of calbindin immunoreactivity in granule cells of the dentate gyrus differ in mesial temporal sclerosis, cortical malformation- and tumor-related epilepsies. Brain Res. 2011;1399:66–78. [DOI] [PubMed] [Google Scholar]
- 18. Abraham H, Veszpremi B, Kravjak A, Kovacs K, Gomori E, Seress L. Ontogeny of calbindin immunoreactivity in the human hippocampal formation with a special emphasis on granule cells of the dentate gyrus. Int J Dev Neurosci. 2009;27:115–127. [DOI] [PubMed] [Google Scholar]
- 19. Fatemi SH, Halt AR, Realmuto G, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. [DOI] [PubMed] [Google Scholar]
- 21. Giraldez-Perez RM, Avila MN, Feijoo-Cuaresma M, et al. Males but not females show differences in calbindin immunoreactivity in the dorsal thalamus of the mouse model of fragile X syndrome. J Comp Neurol. 2013;521:894–911. [DOI] [PubMed] [Google Scholar]
- 22. Real MA, Simon MP, Heredia R, de Diego Y, Guirado S. Phenotypic changes in calbindin D28K immunoreactivity in the hippocampus of Fmr1 knockout mice. J Comp Neurol. 2011;519:2622–2636. [DOI] [PubMed] [Google Scholar]
- 23. Tessier CR, Broadie K. The fragile X mental retardation protein developmentally regulates the strength and fidelity of calcium signaling in Drosophila mushroom body neurons. Neurobiol Dis. 2011;41:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kook SY, Jeong H, Kang MJ, et al. Crucial role of calbindin-D28k in the pathogenesis of Alzheimer's disease mouse model. Cell Death Differ. 2014;21:1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan HH, Chen RJ, Zhu YH, Peng CL, Zhu XR. The neuroprotective effect of overexpression of calbindin-D(28k) in an animal model of Parkinson's disease. Mol Neurobiol. 2013;47:117–122. [DOI] [PubMed] [Google Scholar]
- 26. Dumas TC, Powers EC, Tarapore PE, Sapolsky RM. Overexpression of calbindin D(28k) in dentate gyrus granule cells alters mossy fiber presynaptic function and impairs hippocampal-dependent memory. Hippocampus. 2004;14:701–709. [DOI] [PubMed] [Google Scholar]
- 27. Molinari S, Battini R, Ferrari S, et al. Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proc Natl Acad Sci USA. 1996;93:8028–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kriegsfeld LJ, Mei DF, Yan L, et al. Targeted mutation of the calbindin D28K gene disrupts circadian rhythmicity and entrainment. Eur J Neurosci. 2008;27:2907–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westerink RH, Beekwilder JP, Wadman WJ. Differential alterations of synaptic plasticity in dentate gyrus and CA1 hippocampal area of Calbindin-D28K knockout mice. Brain Res. 2012;1450:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 1997;94:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. [DOI] [PubMed] [Google Scholar]
- 33. Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. [DOI] [PubMed] [Google Scholar]
- 34. Barski JJ, Hartmann J, Rose CR, et al. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23:3469–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fiumelli H, Kiraly M, Ambrus A, Magistretti PJ, Martin JL. Opposite regulation of calbindin and calretinin expression by brain-derived neurotrophic factor in cortical neurons. J Neurochem. 2000;74:1870–1877. [DOI] [PubMed] [Google Scholar]
- 36. Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol. 2012;4:188–200. [PMC free article] [PubMed] [Google Scholar]
- 37. Gilabert-Juan J, Castillo-Gomez E, Perez-Rando M, Molto MD, Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp Neurol. 2011;232:33–40. [DOI] [PubMed] [Google Scholar]
- 38. Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY). 2005;34:39–43. [DOI] [PubMed] [Google Scholar]
- 39. Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. [DOI] [PubMed] [Google Scholar]
- 40. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. [DOI] [PubMed] [Google Scholar]
- 41. Kauffman AS, Park JH, McPhie-Lalmansingh AA, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egea J, Malmierca E, Rosa AO, et al. Participation of calbindin-D28K in nociception: results from calbindin-D28K knockout mice. Pflugers Arch. 2012;463:449–458. [DOI] [PubMed] [Google Scholar]
- 43. Airaksinen L, Virkkala J, Aarnisalo A, Meyer M, Ylikoski J, Airaksinen MS. Lack of calbindin-D28k does not affect hearing level or survival of hair cells in acoustic trauma. ORL J Otorhinolaryngol Relat Spec. 2000;62:9–12. [DOI] [PubMed] [Google Scholar]
- 44. Rodgers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol Behav. 1993;54:729–736. [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Dong F, Yang Y, Wang Y, Wang R, Shen X. Sex-specific effects of long-term exposure to bisphenol-A on anxiety- and depression-like behaviors in adult mice. Chemosphere. 2015;120:258–266. [DOI] [PubMed] [Google Scholar]
- 46. Rilett KC, Friedel M, Ellegood J, MacKenzie RN, Lerch JP, Foster JA. Loss of T cells influences sex differences in behavior and brain structure. Brain Behav Immun. 2015;46:249–260. [DOI] [PubMed] [Google Scholar]
- 47. Seney ML, Chang LC, OH H, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. 2013;4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci. 1993;13:3394–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heldt SA, Zimmermann K, Parker K, Gaval M, Weinshenker D, Ressler KJ. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34:2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Musumeci G, Sciarretta C, Rodriguez-Moreno A, et al. TrkB modulates fear learning and amygdalar synaptic plasticity by specific docking sites. J Neurosci. 2009;29:10131–10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weidner KL, Buenaventura DF, Chadman KK. Mice over-expressing BDNF in forebrain neurons develop an altered behavioral phenotype with age. Behav Brain Res. 2014;268:222–228. [DOI] [PubMed] [Google Scholar]
- 54. Branchi I, Curley JP, D'Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25:294–303. [DOI] [PubMed] [Google Scholar]
- 56. Haapasalo A, Sipola I, Larsson K, et al. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277:43160–43167. [DOI] [PubMed] [Google Scholar]
- 57. Basta-Kaim A, Fijal K, Slusarczyk J, et al. Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience. 2015;287:78–92. [DOI] [PubMed] [Google Scholar]
- 58. Zhang K, Hill K, Labak S, Blatt GJ, Soghomonian JJ. Loss of glutamic acid decarboxylase (Gad67) in Gpr88-expressing neurons induces learning and social behavior deficits in mice. Neuroscience. 2014;275:238–247. [DOI] [PubMed] [Google Scholar]
- 59. Sandhu KV, Lang D, Muller B, et al. Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice. Genes Brain Behav. 2014;13:439–450. [DOI] [PubMed] [Google Scholar]
- 60. Hashimoto T, Bergen SE, Nguyen QL, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet. 2013;14:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. [DOI] [PubMed] [Google Scholar]
- 63. Gos T, Schulkin J, Gos A, Bock J, Poeggel G, Braun K. Paternal deprivation affects the functional maturation of corticotropin-releasing hormone (CRH)- and calbindin-D28k-expressing neurons in the bed nucleus of the stria terminalis (BNST) of the biparental Octodon degus. Brain Struct Funct. 2014;219(6):1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pascual R, Zamora-Leon P, Catalan-Ahumada M, Valero-Cabre A. Early social isolation decreases the expression of calbindin D-28k and dendritic branching in the medial prefrontal cortex of the rat. Int J Neurosci. 2007;117:465–476. [DOI] [PubMed] [Google Scholar]
- 65. Kim MS, Akhtar MW, Adachi M, et al. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. [DOI] [PubMed] [Google Scholar]
- 67. Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. [DOI] [PubMed] [Google Scholar]
- 70. Heitland I, Groenink L, Bijlsma EY, Oosting RS, Baas JM. Human fear acquisition deficits in relation to genetic variants of the corticotropin releasing hormone receptor 1 and the serotonin transporter. PLoS One. 2013;8:e63772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gray JM, Vecchiarelli HA, Morena M, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Muller MB, Zimmermann S, Sillaber I, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. [DOI] [PubMed] [Google Scholar]
- 73. Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. [DOI] [PubMed] [Google Scholar]
- 74. Wilson ME, Westberry JM, Trout AL. Estrogen receptor-α gene expression in the cortex: sex differences during development and in adulthood. Horm Behav. 2011;59:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ma DQ, Whitehead PL, Menold MM, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAα4, α5, β1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–178. [DOI] [PubMed] [Google Scholar]
- 78. Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E. Sex chromosome complement regulates expression of mood-related genes. Biol Sex Differ. 2013;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu JH, Yang ZB, Wang H, Tang FR. Co-localization of L-type voltage dependent calcium channel α1D subunit (Ca[v]1.3) and calbindin (CB) in the mouse central nervous system. Neurosci Lett. 2014;561:80–85. [DOI] [PubMed] [Google Scholar]
- 81. Braun K, Seidel K, Weigel S, Roski C, Poeggel G. Paternal deprivation alters region- and age-specific interneuron expression patterns in the biparental rodent, Octodon degus. Cereb Cortex. 2011;21:1532–1546. [DOI] [PubMed] [Google Scholar]