Abstract
The peripubertal period of development is a sensitive window, during which adverse experiences can increase the risk for presentation of cognitive and affective dysfunction throughout the lifespan, especially in women. However, such experiences in the context of a supportive social environment can actually ameliorate this risk, suggesting that resilience can be programmed in early life. Affective disorders and cognitive deficits commonly emerge during aging, with many women reporting increased difficulty with prefrontal cortex (PFC)-dependent executive functions. We have developed a mouse model to examine the interaction between peripubertal experience and age-related changes in cognition and stress regulation. Female mice were exposed to peripubertal chronic stress, during which they were either individually housed or housed with social interaction. One year after this stress experience, mice were examined in tasks to access their cognitive ability and flexibility in stress reactive measures. In a test of spatial memory acquisition and reversal learning where aged females normally display a decreased performance, the females that had experienced stress with social interaction a year earlier showed improved performance in reversal learning, a measure of cognitive flexibility. Because peripuberty is a time of major PFC maturation, we performed transcriptomic and biochemical analysis of the aged PFC, in which long-term changes in microRNA expression and in myelin proteins were found. These data suggest that stress in the context of social support experienced over the pubertal window can promote epigenetic reprogramming in the brain to increase the resilience to age-related cognitive decline in females.
The peripubertal period of development is a particularly sensitive window, during which adverse experiences such as stress are associated with an increased risk for affective and cognitive dysfunction in women across the lifespan (1–4). Interestingly, a supportive social environment during such experiences can ameliorate these risk factors, suggesting that resilience is a valuable characteristic that can be programmed early in life (5–9). Affective disorders and cognitive deficits commonly emerge during aging, with many women reporting increased difficulty with executive functions such as organization and planning, working memory, and sustained attention (10, 11). Stress during aging and cumulative lifelong stress is associated with poor cognitive performance in normally aging individuals (12–15). Similarly, early childhood abuse leads to deficits in verbal declarative memory in adult women with abuse-related posttraumatic stress disorder (4). Rodent studies support the hypothesis that early life adversity negatively impacts cognitive performance across the lifespan, including during aging (16–19).
The prefrontal cortex (PFC) undergoes critical maturation throughout the peripubertal period, as described in both humans and rodents (20–23). In humans, early adversity is associated with changes in brain structure, including reduced overall PFC volume, reduced PFC gray matter volume, and increased white matter volume (24–28). Animal models highlight potential mechanisms that may underlie the stress-induced changes in PFC structure and subsequent behaviors, including long-term changes to myelination and development of neurotransmitter systems (22, 29). Epigenetic mechanisms likely govern such broad regulation in the maturation of the PFC (29–31). For example, unique DNA methylation signatures have been identified in human PFC across the lifespan (32).
Therefore, to examine the interaction between peripubertal stress experience and age-related changes in cognition, we developed a novel mouse model, in which female mice were exposed to stress either in isolation or with concurrent social interaction and were then examined a year later for changes in PFC-related learning and cognitive tasks. Stress-mediated changes in gene expression patterns in the PFC of aged female mice were compared. We hypothesized that stress exposure in isolation would exacerbate aging-related cognitive decline, whereas social interaction would buffer this effect.
Materials and Methods
Animals
All mice used were virgin, mixed strain C57BL/6:129 bred in our facility. The combination of these two strains produces a hybrid vigor that provides a reproducible balance of stress reactivity, behavioral performance, and maternal care (33–36). All mice were housed in a 12-hour light, 12-hour dark cycle. Food (28.1% protein, 59.8% carbohydrate, and 12.1% fat; Purina Rodent Chow) and water were provided ad libitum. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Peripubertal chronic variable stress (CVS)
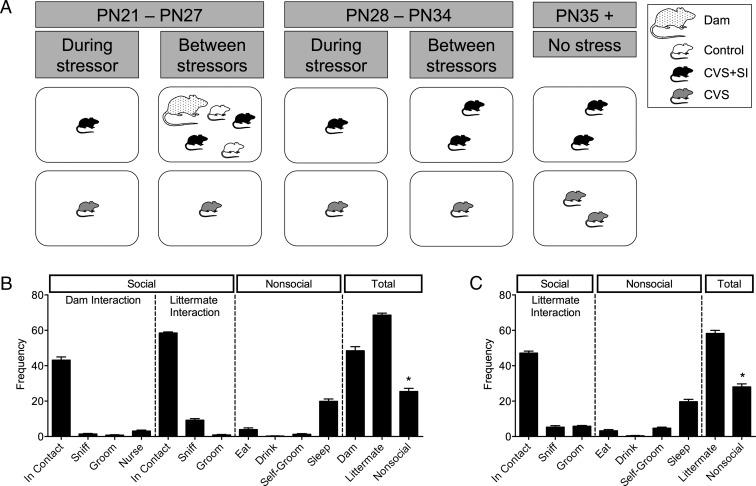
Administration of CVS was performed as described previously (37, 38) aside from the modifications listed here (Figure 1A). Subjects underwent 14 days of CVS starting on postnatal day (PN)21. One stressor was administered per day from the following list: 15-minute restraint in a 50-mL conical tube, 60 minutes of exposure to fox odor (1:5000, 2,4,5-trimethylthiazole; Acros Organics), multiple cage change (3×) in 1 day, 36 hours of constant light, exposure to novel object (marbles) overnight, novel 100-dB white noise overnight (Sleep Machine; Brookstone), and saturated bedding overnight. The order of the stressors was randomized between the first and second week of CVS. Within a litter, animals were randomly assigned to control (n = 34), CVS (n = 32), or CVS with social interaction (CVS+SI) (n = 33) groups. Animals in the CVS group were weaned into singly housed cages at the beginning of stress (PN21). After 2 weeks of stress, CVS animals were pair-housed with a same-sex, same-stress cage mate. CVS+SI individuals lived with the dam and control littermates (PN21–PN27) and then a same-sex, same-stress cage mate (PN28–PN34) between stressors, but these animals experienced each stressor while isolated in a separate cage. Control individuals were weaned at PN28. To evaluate the potential impact of stress on puberty onset, females (n = 6 per group/time) was assessed for the appearance of a vaginal opening (VO) at PN28 and PN35.
Figure 1.
Peripubertal stress increased social interaction in CVS+SI mice. A, Within each litter, females were assigned to control, CVS, or CVS+SI groups. Subjects underwent 14 days of stress starting at PN21. Females in the CVS group were weaned into singly housed cages at PN21. After the 2 weeks of stress, CVS animals were then pair-housed with same-stress group littermate. CVS+SI subjects remained with the dam and control littermates (PN21–PN27) and then a same-stress group cage littermate (PN28–PN34) between stressors, but these animals experienced each stressor while isolated in a separate cage. Similar to CVS mice, CVS+SI animals were pair-housed with a same-stress littermate after stress end. Control mice were also weaned into pair-housing at PN28. B, During the first week of stress, CVS+SI females were more likely to be observed exhibiting social than nonsocial behaviors, with the dam and littermates, during the first hour after return to the cage each day. C, Similarly, during the second week of stress, CVS+SI females were more likely to engage in social behaviors with the littermate than nonsocial behavior after their return to the cage. *, P < .05 compared with total dam interaction or total littermate interaction.
To investigate the influence of stress on social interaction behaviors in the CVS+SI mice when they were returned to the home cage after stress, CVS+SI females (n = 14) were observed for 1 hour. Unique shave patterns were used to identify the mice within each cage. Scan sampling was performed every 5 minutes for 1 hour. The observer noted the behavior of the subject in several categories, including social interaction with the dam, social interaction with littermates, and nonsocial behavior (Table 1).
Table 1.
CVS+SI Observation Ethogram
| Behavior | Description |
|---|---|
| Dam interaction | |
| In contact | Body is in contact with the body of the dam |
| Sniff | Dam is sniffing the pup |
| Groom | Dam is grooming the pup |
| Nurse | Dam is nursing the pup |
| Littermate interaction | |
| In contact | Body is in contact with the body of a littermate |
| Sniff | Pup is sniffing a littermate or being sniffed by a littermate |
| Groom | Pup is grooming a littermate or being groomed by a littermate |
| Nonsocial | |
| Eat | Pup is eating chow |
| Drink | Pup is drinking water |
| Self-groom | Pup is grooming itself |
| Sleep | Pup is sleeping |
Assessment of reproductive function
To investigate the potential for stress during the peripubertal window to impact reproductive function, a cohort of adult females (n = 20/group) was bred with naive males. Upon confirmation of a copulation plug (identified each morning within 1 h after lights turned on), females were established as pregnant and were immediately removed to their own cage. The presence of a copulation plug, a successful pregnancy, and litter characteristics (litter size, sex of offspring) at offspring weaning were recorded.
Examination of stress-related phenotype in aged mice
At 1 year of age, we examined the long-term effects of peripubertal CVS on spatial cognition and cognitive flexibility on a Barnes maze and responsiveness to the environment, as indicated by corticosterone response to acute restraint stress and prepulse inhibition (PPI) of acoustic startle. One cohort was tested on the Barnes maze, whereas a separate cohort was subject to corticosterone response to acute restraint stress testing followed one week later by PPI testing. All equipment was thoroughly cleaned with distilled water between each mouse.
Barnes maze
The Barnes maze is a test of spatial learning and memory and was performed here as described previously (35, 37, 38). The apparatus consisted of a white circular platform (90 cm in diameter) with 24 holes (5 cm in diameter) evenly distributed around the perimeter. An escape box (the “target”) was located under one of the holes. The disc was elevated 70 cm above the floor. Distinct visual cues (black and white checkerboard; 2 red circles; blue and white stripes, all 55.8 × 71.1 cm) remained in a fixed position 80 cm above the floor and 15 cm from the perimeter of the maze on 3 of the white walls in the room.
Training (trials 1–6)
Mice were trained for 2 trials per day for 3 days. Trials occurred at least 4 hours apart. A mouse was placed in the center of the maze at the beginning of each trial. The trial ended upon either entry into the target or after 4 minutes with no success. If a mouse failed to enter the target, the investigator gently guided the mouse into the escape box, and a latency of 240 seconds was assigned for that trial. A mild novel stimulus was introduced daily during training. During trials 1 and 2, a bright light (400 lx) was placed 35 cm above the maze. During trials 3 and 4, an 8-inch fan on the high setting was added, also 35 cm above the maze. During trials 5 and 6, a novel noise (100-dB alarm) was added.
Reversal (trials 7–9)
For reversal trials, the location of the target was rotated 180° to a hole directly across the disc from the training trial location. The stimuli were the same as in trials 5 and 6. The trial ended upon either entry into the target or after 4 minutes with no success. If a mouse failed to enter the target, the investigator gently guided the mouse into the escape box, and a latency of 240 seconds was assigned for that trial.
Young controls
To address the impact of age at 1 year on naive mice, a separate set of aged and young naive (16 wk old) females were tested in the Barnes maze as described above.
Scoring parameters
A video camera was used to record the behavior of the mice during all trials. Trials were scored for the following parameters using AnyMaze Software (Stoelting): latency to find the target (indicated by nose poke into the hole) and latency to escape the maze (all 4 paws in the target). The difference between time to find the target and time to climb into the target (ΔTC) was calculated. The rate at which individuals learned the task was calculated as the slope between 2 trials.
Corticosterone response to acute restraint stress
Plasma corticosterone was measured in response to a 15-minute restraint stress. Individuals were placed in a 50-mL conical tube during the 2–5 hours after lights-on. Tail blood (n = 10/group) was collected from a small nick at the end of the tail at 4 time points: the onset and completion of restraint (0 and 15 min, respectively), and 15 and 105 minutes after the completion of restraint (30 and 120 min, respectively). Samples were immediately mixed with 5 μL of 50mM EDTA and kept on ice until being centrifuged for 10 minutes at 5000 rpm. Plasma was collected and stored at −80°C until analysis. Corticosterone levels were determined by 125I-corticosterone radioimmunoassay (MP Biomedicals).
Prepulse inhibition
PPI of the acoustic startle response (ASR) was recorded in SR-LAB startle chambers (San Diego Instruments) as described previously (37, 38). All test sessions were conducted 1–6 hours after lights-off. PPI test sessions involved the following: 5 minutes of acclimation to background noise (65 dB), 5 consecutive acute habituation tones (40-ms duration; 120 dB), 10 repetitions of each of the following trial types: startle pulse only (40 ms; 120 dB), no stimulus (65-dB background), and prepulses +4, +8, or +16 dB above background (20 ms; 69, 73, or 81 dB) 100 milliseconds preceding the startle pulse. Intertrial intervals averaged 15 seconds. ASR was defined as the peak startle magnitude recorded during 65 consecutive 1-millisecond readings after the startle pulse onset. The percentage PPI for each prepulse intensity was calculated for each animal (n = 10/group) as ([1 − (average response to prepulse + startle)/(average response to startle only)] × 100) before group averages were compared. After recovery from PPI testing, females were killed and brains removed and frozen for examination in biochemical assays below.
Analysis of estradiol levels in aging
To examine the hormonal state at 1 year of age, 17β-estradiol (E2) levels were assayed. Animals were deeply anesthetized with Isoflurane and decapitated. Trunk blood was collected into tubes with 50 μL of 50mM EDTA and kept on ice until being centrifuged for 10 minutes at 5000 rpm. Plasma was collected and stored at −80°C until analysis. Estradiol levels were determined by 125I-E2 radioimmunoassay (MP Biomedicals).
PFC transcriptome analysis
At 1 year of age, a separate group of aged females (n = 8/group) was used for PFC transcriptome analysis. Females were deeply anesthetized with Isoflurane and decapitated. Brains were rapidly removed and immediately frozen on dry ice. Brains were stored at −80°C until micropunching. Whole brains were cryosectioned at −20°C and micropunched using a 1.0-mm Harris Uni-Core tissue puncher (Ted Pella). To collect PFC tissue, 4 punches (2/hemisphere) were made from 2 successive 300-μm sections from +1.98 to +1.38 mm anterior to bregma (39). Micropunches were immediately submerged in 500 μL of TRIzol reagent (Invitrogen), and the tube was frozen on dry ice and stored at −80°C until RNA isolation. RNA was isolated by miRNeasy kit (QIAGEN) and suspended in Ribonuclease-free water. Total RNA was sent to the University of Pennsylvania Path BioResource Molecular Profiling Core for whole-transcript Affymetrix GeneChip Mouse Gene 2.0 ST analysis.
Biochemical analyses
PFC quantitative real-time PCR (RT-PCR)
Total RNA isolated for microarray analysis was also used for RT-PCR. cDNA was transcribed using the High-Capacity cDNA reverse transcriptase kit (Applied Biosystems). Changes in glycogen synthase kinase 3β (Gsk3β) (NM_019827.6, Mm.394930), peroxisome proliferative-activated receptor, γ, coactivator 1α (Pparγc1α) (NM_008904.2, Mm.259072), myelin basic protein (Mbp) (NM_001025251.2, Mm.252063), proteolipid protein 1 (Plp1) (NM_001290561.1, Mm.1268), and platelet/endothelial cell adhesion molecule 1 (Pecam1) (NP_032842.2, Mm.343951) gene expression were measured by quantitative real-time PCR using TaqMan gene expression assays (Applied Biosystems). Samples were run in triplicate for the target genes and the endogenous control glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (NM_0080842.2, Mm99999915_g1) on the same 96-well plate. Analysis was performed by the comparative threshold cycle method, and expression levels were normalized to controls (37, 40).
Protein biochemistry by Western blotting
Whole brains from aged females (n = 10/group) were cryosectioned at −20°C and micropunched using a 1.0-mm Harris Uni-Core tissue puncher (Ted Pella). To collect PFC tissue, 4 punches (2/hemisphere) were made from 2 successive 300-μm sections from +1.98 to +1.38 mm anterior to bregma (39). Tissue from the prelimbic (PL) PFC and infralimbic (IL) PFC were collected and processed separately, based on the landmarks in the mouse brain atlas. The subregions were pooled across 2 animals, resulting in n = 5/group for each subregion. Micropunches were immediately submerged in 50-μL cold radioimmune precipitation assay buffer, and the protein was extracted and stored at −80°C. After quantification by Bradford assay, protein was used to probe for MBP and PLP on separate blots (Table 2). A total of 7.5-μg protein was loaded per lane for gel electrophoresis onto a NuPAGE 10% Bis-Tris gel (Life Technologies). After running and transfer of proteins, protocols differed for MBP and PLP. For MBP, the membrane was blocked with Odyssey blocking buffer (Li-Cor). The membrane was probed with rabbit anti-MBP (1:1000; Abcam), followed by incubation in IRDye800-conjugated donkey antirabbit secondary (1:20 000; Li-Cor). The membrane was then stripped and reprobed with rabbit anti-GAPDH (1:1000; Abcam), followed by incubation in IRDye800-conjugated donkey antirabbit secondary (1:20 000; Li-Cor). For PLP, the membrane was blocked with 5% milk. Membranes were simultaneously probed with rat hybridoma anti-PLP (1:1000; generous gift of Dr Judy Grinspan) and mouse anti-α-tubulin (1:8000; Sigma-Aldrich), followed by incubation in IRDye800-conjugated goat antirat secondary (1:20 000; Li-Cor) and IRDye680 goat antimouse secondary (1:10 000; Li-Cor). Within each sample, MPB values were normalized to GAPDH, and PLP values were normalized to α-tubulin for quantitative analysis.
Table 2.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Antibody Source | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| MBP | Anti-MBP | Abcam, ab40390 | Rabbit; polyclonal | 1:1000 |
| PLP | Anti-PLP | Dr Judy Grinspan, University of Pennsylvania | Rat; hybridoma | 1:1000 |
| GAPDH | Anti-GAPDH | Abcam, ab9485 | Rabbit; polyclonal | 1:1000 |
| α-Tubulin | Anti-α-tubulin | Sigma-Aldrich, T5168 | Mouse; monoclonal | 1:8000 |
Cytochrome c oxidase (CCO) assay
Whole brains from aged females that experienced Barnes maze testing (n = 7–8/group) were used to assess mitochondrial function via a CCO assay. Females were deeply anesthetized with Isoflurane and decapitated. Brains were rapidly removed and immediately frozen on dry ice. Brains were stored at −80°C until micropunching. Whole brains were cryosectioned at −20°C and micropunched using a 1.0-mm Harris Uni-Core tissue puncher (Ted Pella). To collect PFC tissue, 4 punches (2/hemisphere) were made from 2 successive 300-μm sections from +1.98 to +1.38 mm anterior to bregma (39). Punches from successive sections were stored and analyzed separately to provide an average value for each subject. Punches were placed into a prechilled tube, and the tube was frozen on dry ice and stored at −80°C until CCO assay. Tissue was thawed and incubated for 5 minutes in 25 μL of 25mM potassium phosphate buffer and 2 μL of 10% lauryl maltoside. After incubation, samples were centrifuged at 14 000 rpm for 5 minutes at 4°C, and the supernatant was moved to a new tube. In a cuvette, 0.5-mL potassium phosphate buffer and 25-μL reduced cytochrome c was loaded before adding 15 μL of sample. Optical density was measured at 550 nm for 1 minute, and rate of oxidation was calculated using Cary-Win kinetics software.
Statistical analysis
An investigator blind to treatment group conducted all data collection and analysis. CVS+SI social interaction behavioral observation data and litter characteristics were analyzed by one-way ANOVA or t test where appropriate. χ2 test was used to analyze VO data and the reproductive data on presence of copulation plug and pregnancy. Barnes maze measures were analyzed by one-way repeated measures ANOVA with trial as a repeated measure and peripubertal stress as the between-subjects factor. Total corticosterone was analyzed by one-way repeated measures ANOVA with time as a repeated measure. PPI was analyzed by one-way repeated measures ANOVA with prepulse intensity as a repeated measure. Mauchly's test of sphericity was applied to all repeated measures ANOVAs, and Huynd-Feldt corrections were applied where appropriate. Area under the curve for corticosterone, plasma E2 levels, gene expression, protein levels, and CCO activity were analyzed by one-way ANOVA. For each analysis, values greater than 2 SDs away from each group mean were considered outliers and were excluded from analysis. Main effects and interactions were further analyzed with Tukey's honest significant difference post hoc testing. The significance level was set at P < .05. Statistical analyses were performed using JMP11 Pro (SAS) software. All data are reported as mean ± SEM.
Microarray data were analyzed in the R environment for Mac (version 3.1.2) using packages oligo and limma (41–43). To identify genes that were significantly different, the Benjamini Hochbert false discovery rate correction was applied and an adjusted P < .05 was used. Database of Annotation, Visualization, and Integrated Discovery functional annotation clustering was used for determination of gene clusters that were significantly enriched within a gene set based on gene ontology terms, with an enrichment score more than 1.3, equivalent to a P < .05. Gene set enrichment analysis (GSEAv2.0.7; Broad Institute) was used to analyze enrichment of predicted microRNA targets. The miRWalk database, which identifies putative microRNA target sequences in mRNA 3′-untranslated region commonly predicted by miRWalk, miRDB.org, miRanda, and TargetScan algorithms, was used to determine predicted targets of significantly changed microRNAs (44). Heat maps were generated using Gene Pattern (Broad Institute).
Results
CVS+SI observations
During the first week of stress, CVS+SI females were returned to the cage with the dam and control littermates between stressors (Figure 1A). As predicted, during the first hour in the cage after stress exposure, CVS+SI females were significantly more likely to engage in social behaviors compared with nonsocial behaviors (F2,39 = 145.4, P = .0001) (Figure 1B) when returned to the cage, both with the dam (P = .0001) and littermates (P = .0001). The same pattern was observed during the second week of stress (Figure 1C), when CVS+SI females were more likely to engage in social behaviors with the littermate compared with nonsocial behaviors (t(26) = 12.63, P = .0001).
Pubertal onset
Peripubertal stress produced no effect on VO appearance when compared with unstressed control subjects (control, 3 out of 7 with VO; CVS, 2 out of 6 with VO; CVS+SI, 5 out of 8 with VO; χ2 [2, n = 21] = 1.26, P = .53). All females had a VO at PN35 (Table 3).
Table 3.
Percent of Females With Vaginal Opening
| Control | CVS | CVS+SI | |
|---|---|---|---|
| PN28 | 42.8 | 33.3 | 62.5 |
| PN35 | 100 | 100 | 100 |
Reproductive function
Peripubertal stress did not alter any aspect of reproductive function that was measured (Table 4). The presence of a copulation plug did not differ based on peripubertal stress (control, 11 of 20 plug; CVS, 12 of 20 plug; CVS+SI, 11 out of 20 plug; χ2 [2, n = 60] = 0.14, P = .93). Similarly, successful pregnancy, defined as birthing a live litter, was not affected by peripubertal stress (control, 10 of 20 pregnant; CVS, 9 of 20 pregnant; CVS+SI, 8 of 20 pregnant; χ2 [2, n = 34] = 0.40, P = .82). Neither average litter size (F2,24 = 0.42, P = .66) nor % males within the litter (F2,24 = 2,58, P = .10) were altered by peripubertal stress.
Table 4.
Assessment of Reproductive Function
| Control | CVS | CVS+SI | |
|---|---|---|---|
| % plugged | 55.0 | 60.0 | 55.0 |
| % pregnant | 50.0 | 45.0 | 40.0 |
| Litter characteristics | |||
| Average size | 7.4 ± 0.4 | 7.7 ± 0.8 | 6.9 ± 0.5 |
| % male | 52.9 ± 5.4 | 42.8 ± 5.1 | 62.7 ± 7.7 |
% plugged indicates the percent of females found with a copulation plug within 3 nights of mating.
Barnes maze
Peripubertal stress did not alter spatial learning
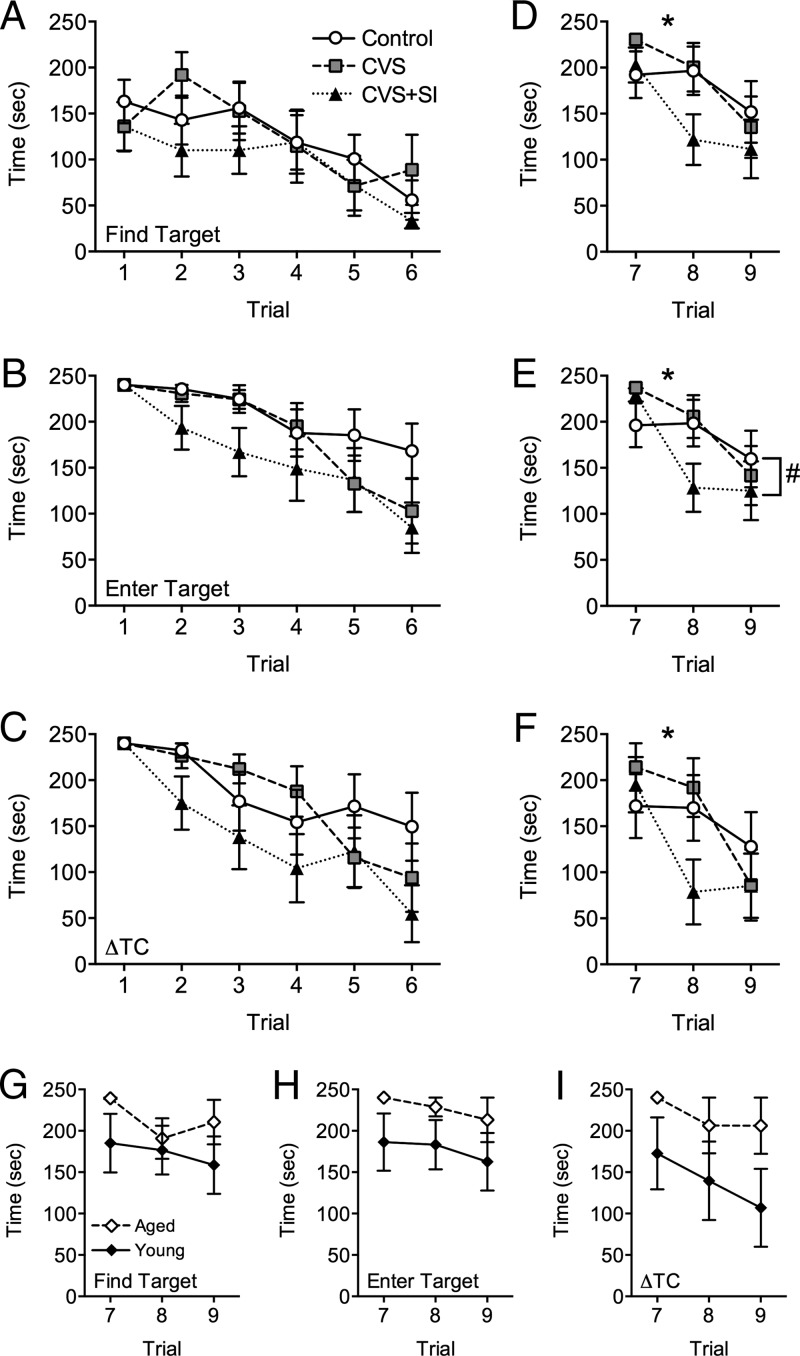
Spatial learning was not significantly affected by peripubertal stress (Figure 2). Although there was a main effect of trial on both the latency to find the target (F5,130 = 7.82, P = .0001) (Figure 2A) and the latency to enter the target (F3.8,98.1 = 19.43, P = .0001) (Figure 2B), there was no interaction of trial with peripubertal stress (find target: F10,130 = 0.78, P = .65; enter target: F7.5,98.1 = 1.24, P = .28) or main effect of peripubertal stress (find target: F2,26 = 0.72, P = .50; enter target: F2,26 = 1.77, P = .19). Similarly, the ΔTC significantly decreased across trials (F4.4,114.9 = 17.72, P = .0001) (Figure 2C), although there was no effect of peripubertal stress (F2,26 = 1.54, P = .23) or trial × peripubertal stress interaction (F8.8,114.9 = 1.47, P = .17).
Figure 2.
Females that experience peripubertal stress in the context of social interaction show improved cognitive flexibility in aging. Surprisingly, there was no effect of peripubertal stress experience in 1-year-old female mice on (A) the latency to find the target, (B) the latency to completely enter the target box, or (C) the ΔTC. However, females experiencing peripubertal stress with social interaction (CVS+SI) showed a significant improvement in their rate of learning during reversal training, as indicated by a steeper slope between trials 7 and 8 for (D) the latency to find the new target, (E) the latency to completely enter the target box in the new location, and (F) the ΔTC. Notably, there was also an interaction between trial and stress on the latency to completely enter the target box during reversal (E), with CVS+SI females entering the box more quickly than control females. In a separate comparison of young and 1-year aged naive females, there was an expected pattern for young females to perform reversal learning more quickly than aged females, as indicated by (G) the latency to find the new target, (H) the latency to completely enter the target box in the new location, and (I) the ΔTC. *, P < .05 on the rate of learning from trial 7 to trial 8; #, P < .05 between control and CVS+SI.
Peripubertal stress with social interaction improved reversal learning
Peripubertal stress significantly impacted behavioral flexibility, as indicated by the latency to learn during the reversal phase of the Barnes maze (Figure 2). Compared with control females, CVS+SI females learned the new location of the target box more quickly. There was a trial × peripubertal stress interaction on the latency to enter the target (F4,52 = 2.67, P = .042) (Figure 2E), such that CVS+SI females entered the target more quickly than control females (P = .0063), although there was no difference between control and CVS females (P = .36), whereas there was a trend for CVS+SI and CVS females to differ (P = .052). Further, the rate of intertrial learning between trials 7 and 8, as indicated by the slope of the line, was different for latency to find the target (F2,26 = 4.36, P = .023), latency to enter the target (F2,26 = 6.72, P = .0044), and ΔTC (F2,26 = 3.52, P = .044). Post hoc analyses revealed that CVS+SI females had a significantly more negative slope, which indicates faster learning, compared with control females for latency to find the target (P = .018), latency to enter the target (P = .0036), and ΔTC (P = .048). There was no effect of stress (find target: F2,26 = 1.06, P = .36; ΔTC: F2,26 = 0.79, P = .46) or stress × trial interaction (find target: F4,52 = 1.81, P = .14; ΔTC: F4,52 = 2.23, P = .079) on the latency to find the target (Figure 2D) and the ΔTC (Figure 2F), although there was an effect of trial (find target: F2,52 = 12.87, P = .0001; ΔTC: F2,52 = 9.93, P = .0002).
Reversal learning was not significantly different in a separate cohort of 1-year-old naive females compared with naive young controls (Figure 2, G–I). There was no effect of age (F1,13 = 1.33, P = .27), trial (F2,26 = 0.97, P = .39), or age × trial interaction (F2,26 = 0.50, P = .61) on the latency to find the target. Similarly, there was no effect of age (F1,13 = 2.39, P = .15), trial (F2,26 = 0.75, P = .48), or age × trial interaction (F2,26 = 0.02, P = .98) on the latency to enter the target. Finally, there was no effect of age (F1,13 = 2.73, P = .12), trial (F1.5,20.0 = 1.29, P = .28), or age × trial interaction (F1.5,20.0 = 0.14, P = .82) on the ΔTC. These findings are likely due to the high variability in the young naive females.
Sensorimotor gating was not changed by peripubertal stress
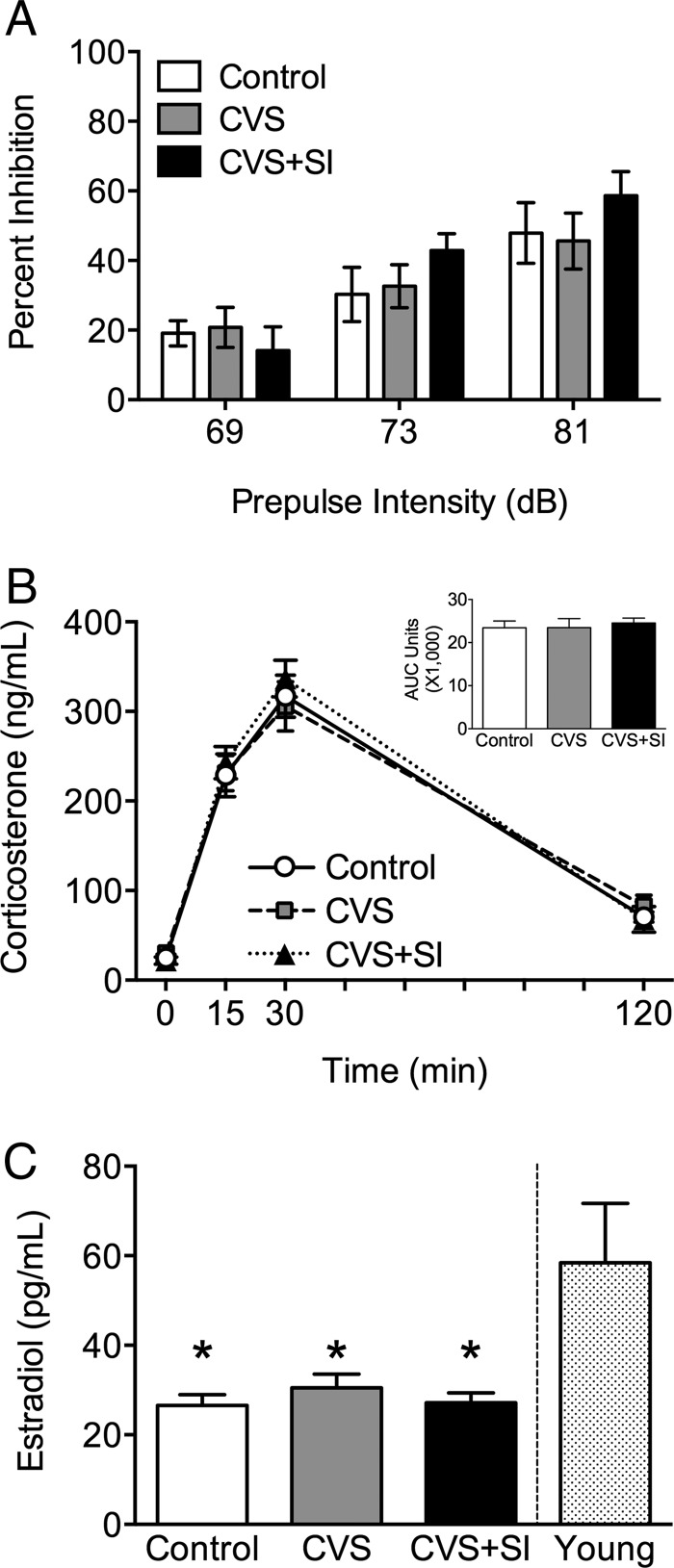
Peripubertal CVS did not impact baseline startle reactivity, as indicated by ASR (F2,25 = 1.63, P = .21) in aged female mice. For PPI (Figure 3A), there was a main effect of prepulse intensity (F2,50 = 43.71, P = .0001), such that increased prepulse intensity resulted in increased PPI. However, there was no interaction between prepulse intensity and peripubertal stress (F4,50 = 1.90, P = .12).
Figure 3.
Peripubertal stress does not alter stress reactivity in aging. There was no effect of peripubertal stress on stress reactivity in aged female mice as measured in (A) PPI, or (B) corticosterone response to an acute restraint stress. C, As evidence of aging, 1-year aged females from all treatment groups showed reduced circulating E2 levels compared with young adult females. These physiological indicators of how aged females respond to the challenge of the learning task suggest that differences detected in reversal learning were not driven by sensorimotor gating, stress responsiveness, or differing levels of circulating estradiol. *, P < .05 compared with young mice.
Peripubertal stress did not impact hypothalamic-pituitary-adrenal (HPA) axis responsiveness
In 1-year-old female mice, peripubertal stress did not have a long-term effect on corticosterone response to acute restraint stress (Figure 3B). There was a significant main effect of time on corticosterone levels in response to a 15-minute restraint stress (F1.6,38.8 = 251.0, P = .0001) that did not interact with peripubertal stress (F3.2,38.8 = 0.80, P = .51). Similarly, there was no effect of peripubertal stress on area under the curve values for total corticosterone (F2,24 = 0.02, P = .98).
Age-related reduction in estradiol is not changed by peripubertal stress
A reduction in plasma estradiol was associated with age, although there was no effect of peripubertal stress on estradiol levels (Figure 3C). There was an effect of group on plasma estradiol (F3,65 = 8.05, P = .0001), and post hoc analysis revealed a difference between young control females and aged controls (P = .0001), aged CVS females (P = .001), and aged CVS+SI females (P = .0001). There was no significant effect of peripubertal stress on the age-related decline in plasma estradiol levels (P > .05 for all comparisons).
Peripubertal stress with social interaction alters the PFC transcriptome
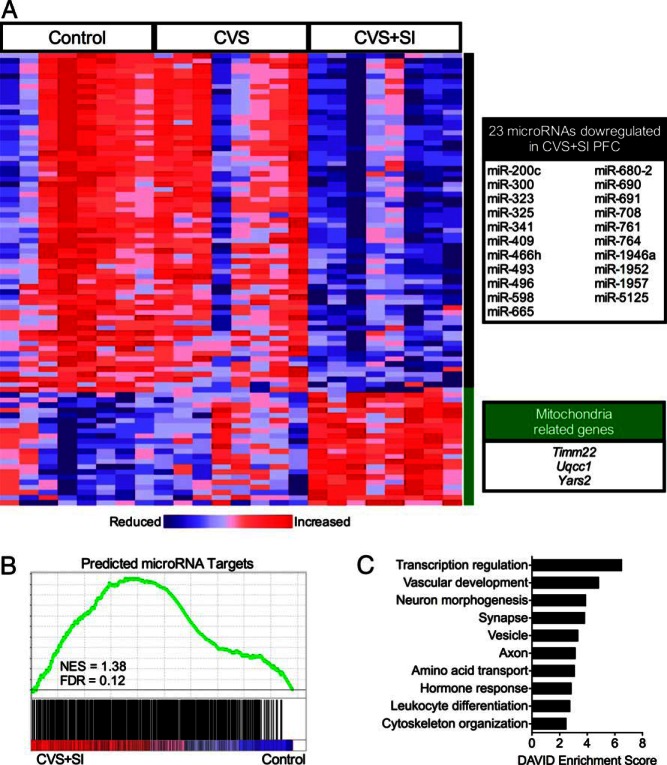
To examine long-term programming of the PFC by peripubertal experience, we evaluated patterns of gene expression by microarray. Following multiple comparisons correction, there were 88 differentially expressed genes when the CVS+SI transcriptome was compared with that of control mice, where 65 genes were down-regulated and 23 genes were up-regulated in the CVS+SI subjects (Figure 4A). Of the genes that were down-regulated in CVS+SI PFC, a striking 23 were microRNAs. Surprisingly, there were no differentially expressed genes when CVS and control subjects were compared. Because the canonical function of microRNAs is to down-regulate target mRNA, we examined enrichment of predicted microRNA targets in the transcriptome of control and CVS+SI subjects. As predicted based on the canonical function of microRNAs, gene set enrichment analysis showed that the transcriptome of CVS+SI was significantly enriched for predicted targets of the 23 down-regulated microRNAs compared with the transcriptome of controls (Figure 4B). Functional annotation clustering of the predicted microRNA targets suggests that the down-regulation of these microRNAs may have an important and cognition-relevant function in the PFC. Predicted microRNA targets cluster into categories including transcription regulation, vascular development, neuron morphogenesis, synapse, vesicle, and axon (Figure 4C). Further, several genes related to mitochondrial function, including Timm22, Uqcc1, and Yars2, were up-regulated in CVS+SI PFC. Altogether, these microarray findings suggest possible buffering of processes relevant to age-related cognitive decline, such as myelin production, mitochondrial function, and vascularization.
Figure 4.
Peripubertal stress in the context of social support reprograms microRNAs in the PFC in aged female mice. A, Heat map illustrating significant differences in gene expression in the PFC of CVS+SI females, where data are expressed as relative levels within each gene, such that blue indicates a reduction in expression and red indicates an increase in expression. Genes that were significantly down-regulated in CVS+SI PFC transcriptome included 23 microRNAs (black box), whereas genes that were significantly up-regulated in CVS+SI PFC transcriptome included several mitochondria-related genes (green box). B, As expected by the canonical function of microRNAs, GSEA using a list of predicted targets of the 23 significantly decreased microRNAs demonstrated that expression of the predicted targets was significantly more enriched in the PFC of CVS+SI females compared with controls. C, Functional annotation clustering of the predicted mRNA targets suggested key functions of these genes in brain signaling and organization. NES, normalized enrichment score; FDR, false discovery rate.
Biochemical analysis of the PFC suggests long-term changes in the myelin system
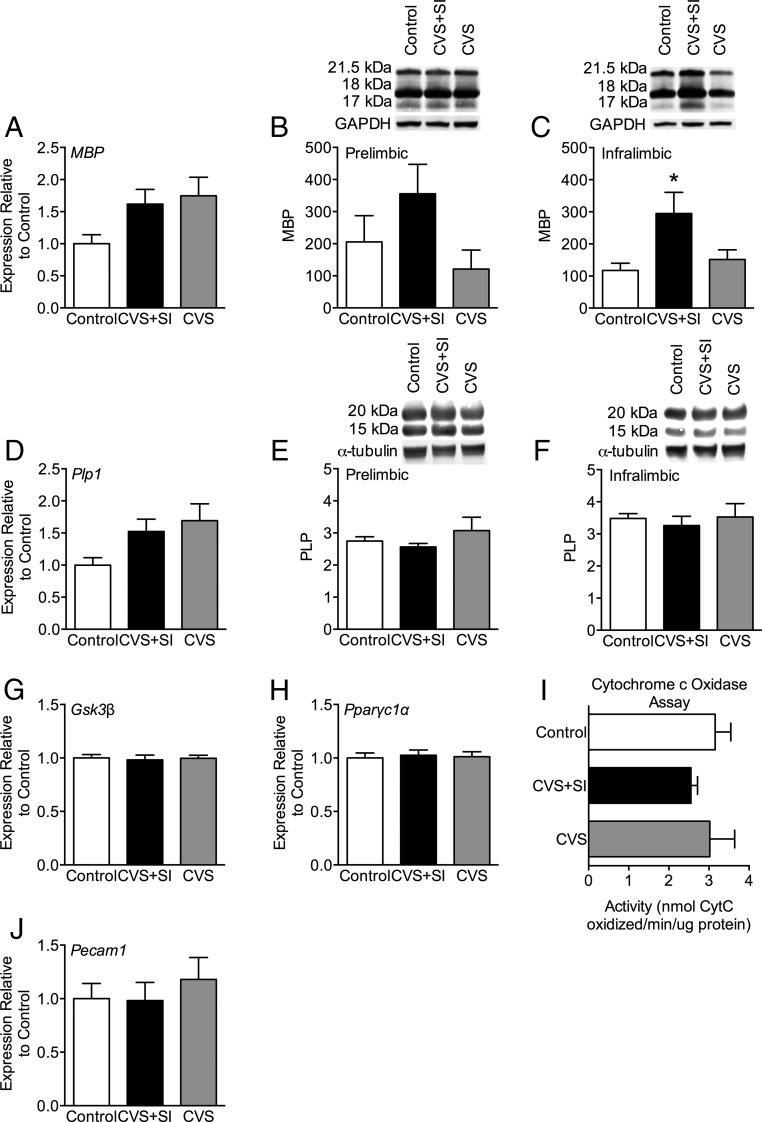
Based on the transcriptome findings and known processes linked to age-related cognitive performance, we examined gene expression changes in aged females (Figure 5). Specifically, we assessed gene expression in the PFC for indicators of myelination, MBP and Plp1; as indicators of mitochondrial function, Gsk3β and Pparγc1α; and as an indicator of vascularization, Pecam1. Although there was a trend for both MBP (F2,20 = 2.76, P = .087) and Plp1 (F2,20 = 2.96, P = .075) gene expression to be increased in females that experienced peripubertal stress (Figure 5, A and D), this did not reach significance. Because MBP has several isoforms (14, 17, 18, and 21.5 kDa), we further interrogated potential differences in myelination via Western blot analysis. For MBP, there was a significant increase in the 17-kDa isoform in the IL cortex of the PFC in CVS+SI females compared with controls (F2,11 = 4.15, P = .045, Tukey P = .055) (Figure 5C). CVS females did not differ in the 17-kDa isoform compared with either control (P = .87) or CVS+SI females (P = .10). There was no effect of peripubertal stress on the 17-kDa isoform in the PL cortex of the PFC (F2,11 = 2.22, P = .15) (Figure 5B). There was no significant effect of peripubertal stress on the 18- or 21.5-kDa isoforms in either the PL (21.5 kDa: F2,11 = 0.59, P = .57; 18 kDa: F2,11 = 0.04, P = .96) or IL (21.5 kDa: F2,11 = 0.68, P = .52; 18 kDa: F2,11 = 0.34, P = .71). Analysis of PLP protein levels revealed no effect of peripubertal stress in the PL (21.5 kDa: F2,11 = 0.96, P = .41; 15 kDa: F2,11 = 0.07, P = .94) (Figure 5E) or the IL (21.5 kDa: F2,11 = 0.22, P = .81; 15 kDa: F2,11 = 0.04, P = .96) (Figure 5F).
Figure 5.
Peripubertal stress in the context of social interaction programs long-term changes in PFC MBP. A, MBP gene expression suggested a nonsignificant trend for increased myelin in the PFC of females that experienced peripubertal stress. Western blot analysis of the (B) PL and (C) IL regions of the PFC revealed that CVS+SI females had increased MBP protein of the 17-kDa isoform in the IL PFC compared with controls. D, No significant differences were found for PLP gene expression in the PFC. Western blotting confirms the gene expression findings, in that there were no differences in the 20-kDa isoform of PLP in the (E) PL or (F) IL regions of the PFC. Peripubertal stress did not program changes in indicators of mitochondrial function in the aged PFC, including gene expression for (G) Gsk3β and (H) Pparγc1α, or in (I) CCO activity. J, No significant differences in Pecam1 gene expression were detected. *, P < .05 on Tukey post hoc test between control and CVS+SI.
There was no effect of peripubertal stress on the expression of Gsk3β (F2,20 = 0.07, P = .93) or Pparγc1α (F2,20 = 0.07, P = 0. 93) in the PFC of aged females (Figure 5, G and H). Further, functional assessment of mitochondrial activity via CCO assay (Figure 5I) revealed no effect of peripubertal stress on the ability of mitochondria to oxidize cytochrome c (F2,18 = 0.53, P = .60). Together, this suggests that mitochondrial function in the female aged PFC is not impacted by peripubertal experience. Finally, there was no significant effect of peripubertal stress on expression of Pecam1 in the PFC of aged females (F2,21 = 0.39, P = 0. 68) (Figure 5J).
Discussion
The developmental years surrounding puberty onset and progression are a time during which adverse experiences are more likely to precipitate cognitive and affective disturbance throughout life, especially in women (1–4). However, a supportive social environment can buffer against such negative outcomes of peripubertal adversity, and instead program resiliency or “grit” (5–9). These resiliency factors are especially critical during aging, when cognitive deficits commonly emerge in animals and humans (4, 12–19). The PFC is a key brain region in age-related affective and cognitive disturbances and is undergoing critical maturation throughout puberty, such that early adversity is associated with permanent disruption of PFC structure and function in clinical and preclinical studies (20–29). Therefore, we examined the impact of peripubertal stress on age-related changes in cognition and stress regulation in female mice exposed to stress either in isolation or with concurrent social interaction.
To assess the effect of peripubertal experience on cognition during aging, 1-year-old female mice were tested for both spatial learning, a hippocampal dependent task, and reversal learning, an executive function largely dependent on the PFC, on a modified Barnes maze. Surprisingly, peripubertal stress alone did not significantly alter Barnes maze performance in aging compared with aged controls. However, mice that had experienced stress with concurrent social support (CVS+SI) actually performed better than control aged mice, specifically in learning the reversal task faster, similar to what we see when comparing young adults with aged females in this test. These results are intriguing as the cognitive flexibility required for tasks such as reversal learning is associated with PFC function (45–48). These findings are actually consistent with previous studies showing that some amount of adversity, or adversity under more favorable circumstances such as social support or a protective gene polymorphism, provides a measure of grit in coping with later life challenges (8, 49–51). Our findings provide a unique perspective on this relationship, because they highlight the important link between experience during the pubertal window and cognitive health during aging.
In this particular paradigm, separating CVS+SI females during the stressors likely leads to increased parental care or increased social play when the mice are reintroduced to the social environment following each stressor, factors which are known to alter stress responding (52–55). In support of this hypothesis, we found that CVS+SI females were more likely to engage in social than nonsocial behaviors upon the poststress return to the cage. Although surprising that peripubertal stress alone did not produce lasting significant effects on learning in this task, given that mice at this age (1 y) are commonly compared with “late middle aged” humans, later aging time points may yield differences in this group. Alternatively, it is possible that there was an effect of peripubertal stress that was not long-lasting due to the mild nature of our chronic stress model. Importantly, to include early neglect as a part of the stressor experience, CVS females were weaned one week earlier (PN21) than control and CVS+SI mice. The addition of the stress of this earlier weaning likely poses a significant contribution to the programming of the PFC. We also examined the potential for peripubertal stress to impact reproductive function but detected no differences in pregnancy or litter outcomes when mice were bred as adults. Similarly, in our comparison of gonadal hormones in aging, there was a similar decrease in estradiol across all groups of aged mice signaling the onset of reproductive senescence.
To confirm that differences in Barnes maze performance were not related to stress reactivity, we examined the HPA axis stress response and PPI in these aged mice. We found that peripubertal stress had no effect on corticosterone levels in response to an acute restraint stress or in sensorimotor gating and baseline startle reactivity. This suggests that stress experience did not program a long-term change in stress circuitry in the outcomes examined in these mice. This was somewhat surprising, especially given that the HPA axis is known to mature over the pubertal window and is sensitive to gonadal hormone levels, which are changing during both puberty and aging (56–61). However, these findings point to the potential specificity of the effect of CVS+SI on reversal learning and executive function, and for long-term changes in preventing processes involved in aging in the PFC.
Because the PFC is critical for cognitive flexibility, is undergoing maturation during the peripubertal window when stress experience occurred, and is a brain region known to be significantly impacted during aging, we completed a transcriptomics analysis of micropunches from the PFC. Consistent with our behavioral findings, stress in the context of social interaction resulted in long-term reprogramming of gene expression in the PFC. Although there were no differentially expressed genes between control and CVS females, there were 88 genes that were significantly different between control and CVS+SI groups. Of the genes that were down-regulated in CVS+SI females compared with controls, a large portion (23 genes; ∼35%) were microRNAs. microRNAs are small, noncoding RNAs that regulate posttranscriptional gene expression by affecting the stability or translational efficiency of specific mRNA targets (62). A single microRNA can target more than a hundred different mRNA targets, and more than 45 000 conserved microRNA binding sites have been annotated in the 3′-untranslated region of 60% of human genes (63). This suggests that microRNAs represent a mode of regulation capable of enacting far-reaching programmatic effects, and are a critical epigenetic gene expression regulatory mechanism (64, 65). The identification of microRNAs is consistent with other literature showing epigenetic regulation of the PFC in the aging female brain (66, 67). In agreement with the canonical function of microRNAs in decreasing mRNA and protein of target transcripts, we found that the PFC transcriptome of CVS+SI aged females was significantly enriched for predicted targets of the 23 microRNAs that were down-regulated in the PFC in these mice. When these predicted gene targets were grouped by functional annotation analysis, several interesting functional categories were revealed that were relevant to age-related cognitive decline and overall PFC function, including myelination, mitochondrial function, and vascularization. Normal aging is associated with a regression of dendrites of pyramidal neurons and a loss of synapses in the PFC in humans, nonhuman primates, and rodents, and some of these measures have been directly related to cognitive impairment (68–72). Aging is also accompanied by white matter degeneration in humans, which has been correlated with cognitive capacity in healthy aging populations (73, 74). Therefore, to further probe the effect of peripubertal stress to program long-term PFC function, we examined these relevant targets from our PFC microarray analysis.
Based on the behavioral and PFC transcriptomic outcomes, we hypothesized that CVS+SI females would have increased expression of genes in the PFC that are known to be decreased with aging. For 2 critical myelin proteins, MBP and PLP, there was a nonsignificant trend to suggest that peripubertal experience may have increased gene expression in the PFC. We further probed this finding by examining protein levels of these myelinating proteins, especially as MBP has multiple isoforms that are predictive of functional differences. Here, we examined the subregions of the PFC, the IL and PL cortices, separately, as these closely related subregions have well-established differences in circuitry and response to stress (75, 76). As hypothesized, we found that the 17-kDa MBP isoform was significantly increased in the IL PFC from CVS+SI females. Unlike the more abundant and well-studied MBP isoforms, which are protein products that eventually reside within the layers of the myelin sheath, the 17-kDa isoform is found predominantly in the nucleus of oligodendrocytes, and is more closely associated with remyelination and may be an indicator of increasing oligodendrocyte cell numbers in our mice (77–80). Peripubertal PFC maturation is a critical period for significant increases in myelination, such that social isolation during this window results in long-term deficits (22). These data suggest that stress in the context of social support experienced over the pubertal window can buffer against age-related changes in PFC myelination. In summary, our findings demonstrate that exposure to peripubertal stress with concurrent social interaction promotes a novel buffering against aging-related cognitive decline. Further, these studies support previous studies in humans and animal models demonstrating that the peripubertal window represents a period of brain maturation, during which the PFC is sensitive to long-term reprogramming by experience (22, 24–26, 28, 29). Although these studies do not identify a definitive mechanism for the long-term reprogramming of the PFC related to the beneficial outcomes in aging, the identification of 23 microRNAs as significantly changed a year after the stress experience suggests the involvement of an upstream epigenetic mechanism programmed during puberty. This pubertal PFC programming likely interacts with normal hormonal changes to alter cognitive functioning during aging.
Acknowledgments
We thank Jessica Fluharty for technical assistance, Dr Daniel Beiting for assistance with transcriptomics analysis, Dr Satish Srinivasan for assistance with CCO assay, and Dr Judy Grinspan and Micah Romer for providing the PLP antibody and assistance with the protocol.
This work was supported by National Institutes of Health Grants MH073030, MH091258, MH087597, MH099910, and MH104184.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ASR
- acoustic startle response
- CCO
- cytochrome c oxidase
- CVS
- chronic variable stress
- CVS+SI
- CVS with social interaction
- E2
- 17β-estradiol
- Gapdh
- glyceraldehyde-3-phosphate dehydrogenase
- Gsk3β
- glycogen synthase kinase 3β
- HPA
- hypothalamic-pituitary-adrenal
- IL
- infralimbic
- Mbp
- myelin basic protein
- Pecam1
- platelet/endothelial cell adhesion molecule 1
- PFC
- prefrontal cortex
- PL
- prelimbic
- Plp1
- proteolipid protein 1
- PN
- postnatal day
- Pparγc1α
- peroxisome proliferative-activated receptor, γ, coactivator 1α
- PPI
- prepulse inhibition
- ΔTC
- difference between time to find the target and time to climb into the target
- VO
- vaginal opening.
References
- 1. Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. [DOI] [PubMed] [Google Scholar]
- 2. Janssen I, Krabbendam L, Bak M, et al. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand. 2004;109(1):38–45. [DOI] [PubMed] [Google Scholar]
- 3. Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. [DOI] [PubMed] [Google Scholar]
- 4. Bremner JD, Vermetten E, Afzal N, Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2004;192(10):643–649. [DOI] [PubMed] [Google Scholar]
- 5. Wingo AP, Wrenn G, Pelletier T, Gutman AR, Bradley B, Ressler KJ. Moderating effects of resilience on depression in individuals with a history of childhood abuse or trauma exposure. J Affect Disord. 2010;126(3):411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrenn GL, Wingo AP, Moore R, et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. J Natl Med Assoc. 2011;103(7):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schulz A, Becker M, Van der Auwera S, et al. The impact of childhood trauma on depression: does resilience matter? Population-based results from the Study of Health in Pomerania. J Psychosom Res. 2014;77(2):97–103. [DOI] [PubMed] [Google Scholar]
- 8. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101(49):17316–17321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu RT. A developmentally informed perspective on the relation between stress and psychopathology: when the problem with stress is that there is not enough. J Abnorm Psychol. 2015;124(1):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause. 2011;18(5):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. [DOI] [PubMed] [Google Scholar]
- 12. Rosnick CB, Small BJ, McEvoy CL, Borenstein AR, Mortimer JA. Negative life events and cognitive performance in a population of older adults. J Aging Health. 2007;19(4):612–629. [DOI] [PubMed] [Google Scholar]
- 13. Peavy GM, Salmon DP, Jacobson MW, et al. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166(12):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peavy GM, Jacobson MW, Salmon DP, et al. The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord. 2012;26(3):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall AC, Cooper NR, Segrave R, Geeraert N. The effects of long-term stress exposure on aging cognition: a behavioral and EEG investigation. Neurobiol Aging. 2015;36(6):2136–2144. [DOI] [PubMed] [Google Scholar]
- 16. Vallée M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11(8):2906–2916. [DOI] [PubMed] [Google Scholar]
- 17. Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Sapolsky RM. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9(5–6):731–734. [DOI] [PubMed] [Google Scholar]
- 18. Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–648. [DOI] [PubMed] [Google Scholar]
- 19. Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behav Brain Res. 2005;156(2):289–296. [DOI] [PubMed] [Google Scholar]
- 20. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. [DOI] [PubMed] [Google Scholar]
- 21. Perrin JS, Hervé PY, Leonard G, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28(38):9519–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staffend NA, Mohr MA, DonCarlos LL, Sisk CL. A decrease in the addition of new cells in the nucleus accumbens and prefrontal cortex between puberty and adulthood in male rats. Dev Neurobiol. 2014;74(6):633–642. [DOI] [PubMed] [Google Scholar]
- 24. Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44(13):799–807. [DOI] [PubMed] [Google Scholar]
- 25. Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. [DOI] [PubMed] [Google Scholar]
- 26. Chaney A, Carballedo A, Amico F, et al. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J Psychiatry Neurosci. 2014;39(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheffield JM, Williams LE, Woodward ND, Heckers S. Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res. 2013;143(1):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niwa M, Jaaro-Peled H, Tankou S, Seshadri S. Adolescent stress–induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blum K, Febo M, Smith DE, et al. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J Child Adolesc Psychopharmacol. 2015;25(4):286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westberry JM, Wilson ME. Regulation of estrogen receptor α gene expression in the mouse prefrontal cortex during early postnatal development. Neurogenetics. 2012;13(2):159–167. [DOI] [PubMed] [Google Scholar]
- 32. Numata S, Ye T, Hyde TM, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90(2):260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McEuen JG, Beck SG, Bale TL. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J Neurosci. 2008;28(33):8169–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91(1):55–65. [DOI] [PubMed] [Google Scholar]
- 36. Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–414. [DOI] [PubMed] [Google Scholar]
- 37. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33(21):9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal anti-inflammatory treatment. Endocrinology. 2014;155:2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 4th ed San Diego, CA: Academic Press, Inc; 2012. [Google Scholar]
- 40. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 41. Smyth GK. Limma: linear models for microarray data. In: Statistics for Biology and Health. New York, NY: Springer; 2005:397–420. [Google Scholar]
- 42. Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing; 2014. [Google Scholar]
- 44. Dweep H, Sticht C, Pandey P, Gretz N. miRWalk–database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44(5):839–847. [DOI] [PubMed] [Google Scholar]
- 45. Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain Behav. 2015;14(1):4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 2008;187(2):405–410. [DOI] [PubMed] [Google Scholar]
- 47. Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111(5):920–936. [DOI] [PubMed] [Google Scholar]
- 48. Salazar RF, White W, Lacroix L, Feldon J, White IM. NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behav Brain Res. 2004;152(2):413–424. [DOI] [PubMed] [Google Scholar]
- 49. Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev. 2011;35(7):1466–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker KJ, Buckmaster CL, Lindley SE, Schatzberg AF, Lyons DM. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. Int J Behav Dev. 2012;36(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banks DM, Weems CF. Family and peer social support and their links to psychological distress among hurricane-exposed minority youth. Am J Orthopsychiatry. 2014;84(4):341–352. [DOI] [PubMed] [Google Scholar]
- 52. Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm Behav. 2007;52(3):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Champagne DL, Bagot RC, van Hasselt F, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28(23):6037–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mustoe AC, Taylor JH, Birnie AK, Huffman MC, French JA. Gestational cortisol and social play shape development of marmosets' HPA functioning and behavioral responses to stressors. Dev Psychobiol. 2014;56(6):1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 2011;210(3):391–398. [DOI] [PubMed] [Google Scholar]
- 57. Lui P, Padow VA, Franco D, et al. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol Behav. 2012;107(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–132. [DOI] [PubMed] [Google Scholar]
- 59. Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80(6):387–393. [DOI] [PubMed] [Google Scholar]
- 60. Kudwa AE, McGivern RF, Handa RJ. Estrogen receptor β and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol Behav. 2014;129:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor α within the hypothalamus. Neuroscience. 2009;159(2):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 63. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. [DOI] [PubMed] [Google Scholar]
- 66. Rao YS, Mott NN, Wang Y, Chung WC, Pak TR. MicroRNAs in the aging female brain: a putative mechanism for age-specific estrogen effects. Endocrinology. 2013;154(8):2795–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor β expression in the rat cortex during aging. Neuroreport. 2011;22(9):428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152(4):970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8(8):671–684. [DOI] [PubMed] [Google Scholar]
- 70. Shimada A, Tsuzuki M, Keino H, et al. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol Appl Neurobiol. 2006;32(1):1–14. [DOI] [PubMed] [Google Scholar]
- 71. de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10(4):1261–1269. [DOI] [PubMed] [Google Scholar]
- 72. Dumitriu D, Hao J, Hara Y, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30(22):7507–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuznetsova KA, Maniega SM, Ritchie SJ, et al. Brain white matter structure and information processing speed in healthy older age [published online ahead of print August 9, 2015]. Brain Struct Funct. doi:10.1007/s00429-015-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maniega SM, Valdés Hernández MC, Clayden JD, et al. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015;36(2):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Riga D, Matos MR, Glas A, et al. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. [DOI] [PubMed] [Google Scholar]
- 77. Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Akiyama K, Ichinose S, Omori A, Sakurai Y, Asou H. Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J Neurosci Res. 2002;68(1):19–28. [DOI] [PubMed] [Google Scholar]
- 79. Pedraza L. Nuclear transport of myelin basic protein. J Neurosci Res. 1997;50(2):258–264. [DOI] [PubMed] [Google Scholar]
- 80. Pedraza L, Fidler L, Staugaitis SM, Colman DR. The active transport of myelin basic protein into the nucleus suggests a regulatory role in myelination. Neuron. 1997;18(4):579–589. [DOI] [PubMed] [Google Scholar]