An increasing body of literature suggests that perinatal exposure to low doses of bisphenol A (BPA) has lasting effects on brain development and/or behavior in rodents (1, 2). Concerns about the occurrence of similar effects in humans are based on the parallel increase of neurobehavioral conditions in humans that appear in rodents exposed to BPA (3), and on epidemiological studies (4). In their recent article, Franssen et al (5) demonstrated opposite dose-dependent effects of BPA on the neuroendocrine maturation of female rats exposed during early life from postnatal day (PND)1 to PND15. In animals exposed to a 0.025-μg BPA/kg bw (body weight)/d dose, neuroendocrine maturation related to puberty was found to be delayed, with opposite effects observed in animals exposed to a 5000-μg BPA/kg bw/d dose. Modulation of inhibitory γ-aminobutiric acid (GABA)ergic neurotransmission was found to be associated with the delayed maturation of GnRH secretion through increased GABAergic tone for the 0.025-μg BPA/kg bw/d dose; opposite effects were observed for the higher dose.
These new findings prompted us to reexamine in more detail our results obtained for male mice exposed to very low doses of BPA from gestational day 8 to PND16 (maternal exposure to 0-, 0.025-, 0.25-, or 25-μg BPA/kg bw/d) (6). In this 2013 study, we used untargeted 1H-nuclear magnetic resonance (NMR) metabolomics to explore the metabolites present in a set of tissues, including male brains at PND21. All brain metabolites were included in a partial least square-discriminant analysis model, which demonstrated extremely significant differences between the 4 groups. GABA was one of the major metabolites responsible for intergroup differences. To better understand the mechanisms by which BPA disrupts brain development, we reanalyzed our raw data from 2013 with the purpose of 1) challenging the hypotheses by Franssen et al, and 2) seeking additional hypotheses that could explain the disruption of GABAergic pathways. We addressed the former by means of a 2 by 2 group comparison and the latter by analyzing the genome-scale metabolic network based on our 2013 1H-NMR brain metabolomics data, and by extracting the most prominent pathways (eg, subnetworks) modulated by BPA exposure.
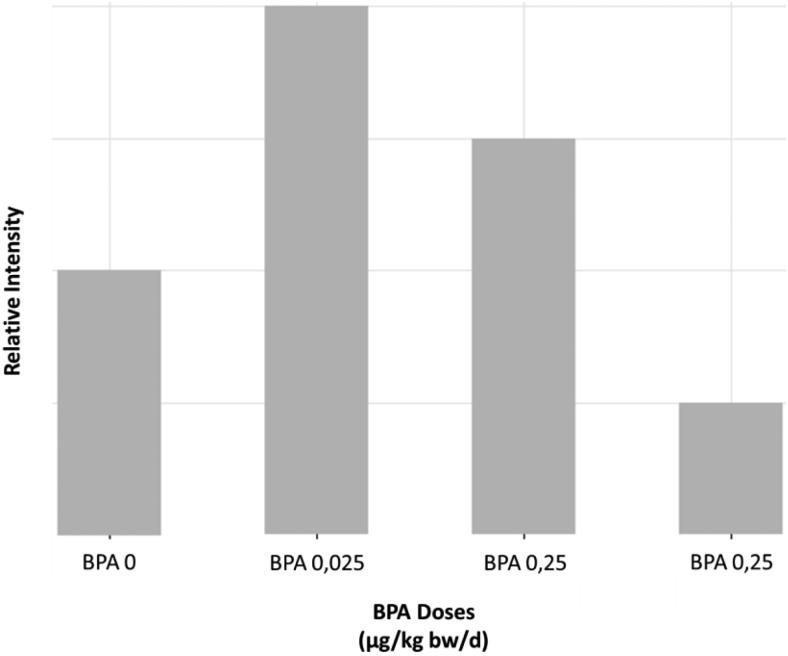
In the first place, our results clearly support the conclusions by Franssen et al about GABA being a key determinant of BPA effects, despite species, sex, and exposure scenario differences (perinatal in our study, strictly postnatal in the Franssen et al work). In our study, GABA was significantly increased in whole-brain extracts from the 0.025-μg BPA/kg low-dose group compared with controls (vehicle). No comparisons could be carried out for the 5000-μg/kg dose, not addressed in Cabaton et al (6). However, untargeted metabolomics already highlighted a decrease of GABA at the highest dose tested in Cabaton et al, namely 25-μg BPA/kg compared with controls (Figure 1). In other words, the lower GABAergic tone observed by Franssen et al at 5000-μg BPA/kg in specific brain regions was already obvious at 25-μg BPA/kg in a perinatal scenario when using NMR-based metabolomics on whole-brain extracts.
Figure 1.
Relative intensity of GABA in the brain of PND21 mice perinatally exposed to 0-, 0.025-, 0.25-, or 25-μg BPA/kg bw/d (based on 1H-NMR semiquantitative spectral data from Cabaton et al [6]).
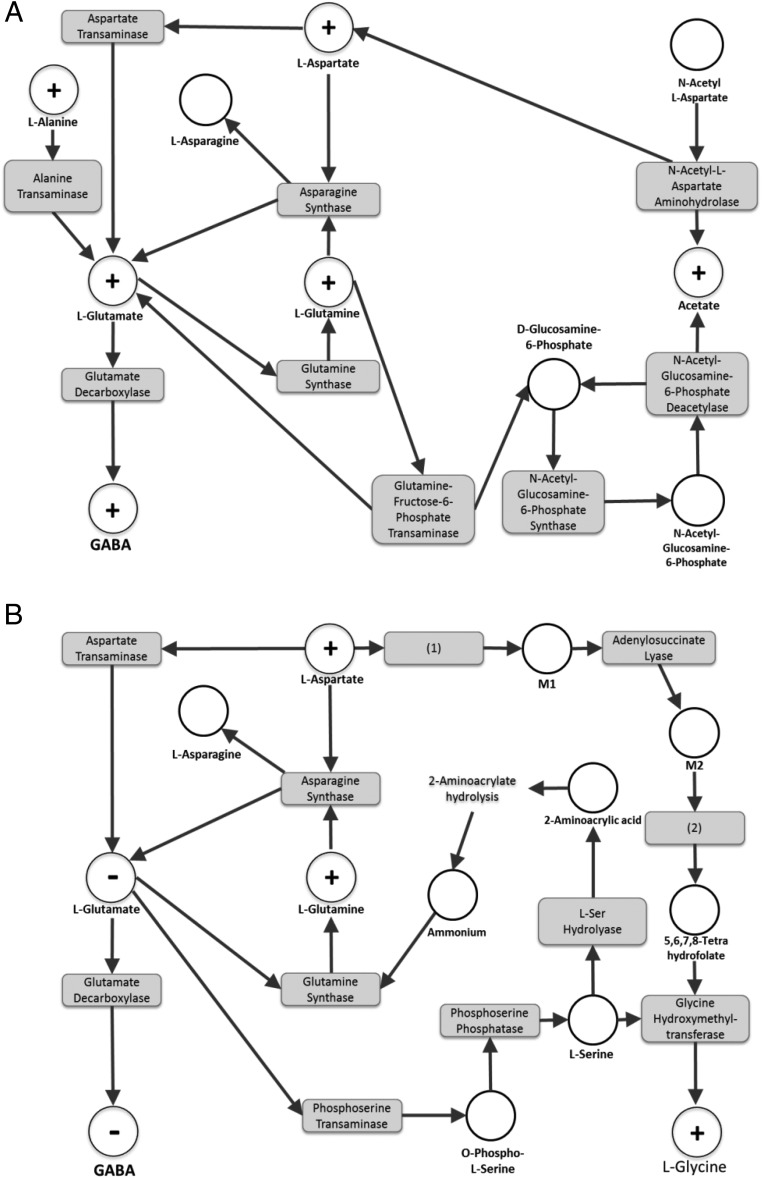
In a second step, based on our raw data from brain extracts, metabolic networks analysis was performed for the lowest and highest BPA doses used in Cabaton et al. The discriminant metabolites were examined using MetExplore, the open access webserver we developed for mapping, visualization, and mining of omics data (www.metexplore.fr) (7). The subnetworks of interest relating to 0.025-μg BPA/kg bw/d animals (vs controls) and 25-μg BPA/kg bw/d animals (vs controls) are displayed in Figure 2, A and B, respectively.
Figure 2.
Metabolic subnetwork extractions obtained using the webserver MetExplore (www.metexplore.fr) showing the metabolic pathways modulated by BPA perinatal exposure in PND21 mice: (A) 0.025-μg/kg bw/d vs control and (B) 25-μg/kg bw/d vs control. The “+” dots represent the identified metabolites that were increased, the “−” dots represent the identified metabolites that were decreased, and the white dots represent the metabolites predicted to be involved in the corresponding biochemical pathway. The gray boxes are the enzymes catalyzing the reactions linking substrates and products. Metabolites: M1, (S)-2-[5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido]succinate; M2, 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide. Enzymes: (1), phospho-ribosyl-amino-imidazole-succino-carboxamide synthase; (2), phospho-ribosyl-amino-imidazole-succino-carboxamide formyltransferase.
L-glutamate is the primary substrate for GABA synthesis, which is carried out by brain glutamate decarboxylases. Both L-glutamate and GABA were found to change in a similar way: increased in 0.025-μg BPA/kg exposed mice and decreased in 25-μg BPA/kg exposed mice. For this reason, and because there is only 1 report so far (8) suggesting regulation of the glutamate decarboxylase activity by BPA (at 40 μg/kg, in rats), it is unlikely that this enzymatic pathway would play a major role in the observed alterations of GABA levels. Rather, we clearly favor the hypothesis that the concentration of L-glutamate itself is the determining parameter in all of these studies.
Network analysis based on the Cabaton et al study provides further support for BPA-driven GABA modulation by highlighting the active pathways of L-glutamate consumption and production.
For L-glutamate consumption, an enhanced depletion of L-glutamate ultimately leading to the production of the final metabolite Glycine was demonstrated for the highest BPA dose of 25-μg BPA/kg. Glycine was significantly increased in the brain of these animals (Figure 2B). This observation could be explained by the modulation of 2 metabolic pathways, the most direct one being the production of L-serine. No existing data currently documents alteration of this specific pathway by BPA. However, up-regulation of the phosphoserine transaminase gene by estrogen was previously shown in vitro in female rat trigeminal ganglia, consistent with an enhanced consumption of L-glutamate (9). This pathway may participate in lowering glutamate (and consequently GABA) levels via increased phosphoserine transaminase activity.
For L-glutamate production, for 0.025-μg BPA/kg exposed animals (Figure 2A), the study by Cabaton et al demonstrated that at least 2 direct substrates of L-glutamate, namely the metabolites L-alanine (through the L-alanine transaminase pathway) and L-aspartate (through the aspartate transaminase pathway) were significantly higher in BPA exposed animals than in controls. Also, the latter metabolite is unequivocally involved in the regulation of L-glutamate (and consequently, of GABA) in the high-dose group (25-μg BPA/kg bw/d) (Figure 2B).
All these features strongly suggest that the modulation of transaminases involved in the synthesis and consumption of L-glutamate are key factors involved in the effects of BPA on neuroendocrine maturation highlighted by Franssen et al. Although data supporting this hypothesis based on experimental studies involving xeno-estrogens are lacking, it should be stressed that estradiol and ageing were previously found to decrease transaminase activity in rat brains (10), which is consistent with Franssen et al's conclusions for their high-dose BPA group, eg, an accelerated maturation of rats through diminished inhibitory GABAergic neurotransmission. It is hypothesized on the basis of network modeling that an opposite situation may take place for lower doses of BPA. BPA undoubtedly impacts brain development, and it is likely that part of its effects occur at the level of brain transaminases.
Our data together with those of Franssen et al suggest that the pathways of biosynthesis, action and metabolism of excitatory and inhibitory amino acids may be a fundamental component of BPA disruption of neurodevelopment. Finally, given that the brain is a heterogeneous organ, it is counterintuitive that BPA could sufficiently affect the content of the metabolites implicated in the synthesis of neurotransmitters such as GABA and glutamate, so that the observed effect would remain significant even in extracts of the whole organ. This suggests that BPA modulates transaminase activity in a generic way, as suggested for estrogens (10).
Acknowledgments
We thank the editorial contributions by Cheryl Schaeberle.
This work was supported in part by the National Institute of Environmental Health Sciences Award R01ES08314 and by the PhenoMeNal project, European Commission, Horizon 2020 Programme, Grant 654241. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
For related article see page 1740
- BPA
- bisphenol A
- bw
- body weight
- GABA
- γ-aminobutiric acid
- NMR
- nuclear magnetic resonance
- PND
- postnatal day.
References
- 1. Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. [DOI] [PubMed] [Google Scholar]
- 3. vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mustieles V, Pérez-Lobato R, Olea N, Fernández MF. Bisphenol A: human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. [DOI] [PubMed] [Google Scholar]
- 5. Franssen D, Gerard A, Hennuy B, Donneau AF, Bourguignon JP, Parent AS. Delayed neuroendocrine sexual maturation in female rats after a very low dose of Bisphenol A through altered GABAergic neurotransmission and opposing effects of a high dose. Endocrinology. 2016;1740–1750. [DOI] [PubMed] [Google Scholar]
- 6. Cabaton NJ, Canlet C, Wadia PR, et al. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect. 2013;121:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cottret L, Wildridge D, Vinson F, et al. MetExplore: a web server to link metabolomic experiments and genome-scale metabolic networks. Nucleic Acids Res. 2010;38:W132–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou R, Chen F, Chang F, Bai Y, Chen L. Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. J Psychiatr Res. 2013;47:1535–1544. [DOI] [PubMed] [Google Scholar]
- 9. Puri V, Puri S, Svojanovsky SR, et al. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26:33–42. [DOI] [PubMed] [Google Scholar]
- 10. Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol. 2005;40:295–302. [DOI] [PubMed] [Google Scholar]