Abstract
Despite the pathophysiological importance of neurohumoral activation in patients with heart failure (HF), the precise underlying mechanisms contributing to elevated vasopressin (VP) activation in HF remains unknown. Carbon monoxide (CO) is a gaseous neurotransmitter in the central nervous system that stimulates VP neuronal firing activity. Recently, we showed that the excitatory effect of CO on VP neurons in the hypothalamic paraventricular nucleus (PVN) was mediated by inhibition of nitric oxide (NO). Given that previous studies showed that VP neuronal activity is enhanced, whereas NO inhibitory signaling is blunted in HF rats, we tested whether an enhanced endogenous CO availability within the PVN contributes to elevated VP neuronal activity and blunted NO signaling in HF rats. We found that both haeme-oxygenase 1 (the CO-synthesizing enzyme) protein and mRNA expression levels were enhanced in the PVN of HF compared with sham rats (∼18% and ∼38%, respectively). We report that in sham rats, bath application of a CO donor (tricarbonyldichlororuthenium dimer) increased the firing activity of identified PVN VP neurons (P < .05), whereas inhibition of endogenous CO production (Tin-protoporphyrin IX [SnPP]) failed to affect neuronal activity. In HF rats, however, SnPP decreased VP activity (P < .05), an effect that was occluded by previous NO synathase blockade NG-nitro-larginine methyl ester. Finally, we found that SnPP increased the mean frequency of γ-aminobutyric acid inhibitory postsynaptic currents in VP neurons in HF (P < .05) but not sham rats. Our results support an enhanced endogenous CO excitatory signaling in VP neurons, which likely contributes to blunted NO and γ-aminobutyric acid inhibitory function in HF rats.
The paraventricular nucleus (PVN) of the hypothalamus is an important site in the central nervous system (CNS) for the integration of behavioral, cardiovascular and neuroendocrine homeostatic responses (1–3). Magnocellular neurosecretory cells (MNCs) of the PVN synthesize and release the neurohormone vasopressin (VP), which besides its classical antidiuretic function has been implicated in altered body fluid balance during pathological conditions, including heart failure (HF) (4, 5). Studies in animal models of HF support elevated VP neuronal activity along with chronically increased plasma VP levels (6–8), being a critical component of hypothalamic neurohumoral activation, a hallmark in this disease (5, 9–12). Despite the well-established contribution of neurohumoral activation and, in particular, elevated VP levels to the morbidity and mortality in HF patients (11, 13–15), relatively little is known about the precise central underlying mechanisms regulating VP release during HF.
MNC firing activity is directly controlled by the combined action of intrinsic membrane properties and synaptic inputs (16). Among the later, γ-aminobutyric acid (GABA), glutamate and the gaseous molecule nitric oxide (NO) are key hypothalamic neurotransmitters (17–19) that regulate MNC activity and VP release (20–25). Within the PVN, GABA is a dominant inhibitory neurotransmitter, tonically restraining firing activity of PVN MNCs (23). Moreover, NO has been shown to regulate body fluid homeostasis by inhibiting VP neuronal activity and systemic VP release (26, 27), an effect mediated largely by stimulation of local inhibitory GABAergic synaptic activity (20, 28–30). Importantly, a decreased expression (31–35) and function (36–40) of GABA and NO have been shown to contribute to increased neuronal activity and neurohumoral activation during HF (34, 40–42). Still, the precise mechanisms contributing to blunted NO-GABA inhibitory function in MNCs in HF remains to be elucidated.
We reported recently, that carbon monoxide (CO), another gaseous neurotransmitter in the CNS (43–45), acts as an excitatory gas molecule stimulating MNCs firing activity (28, 46). Endogenous CO is generated by haeme-oxygenase (HO) activity, which catalyzes haeme degradation to CO, free iron and biliverdin (44, 45). Three HO isoforms have been described so far: the inducible HO-1, the constitutive isoform HO-2, and HO-3, whose function is yet unknown (47). The expression HO-1 was previously shown to be up-regulated during stress conditions (48, 49), as well as in response to an osmotic challenge, particularly in MNCs (28, 46). Importantly, we recently showed that HO-1 and the neuronal NO synathase (nNOS), 1 of the 3 NOS isoforms enzymes found in the PVN (50), and known to be critically involved in the regulation of neurosecretory and sympathetic outflow from the PVN (25), are coexpressed in VP MNCs. Moreover, we found that the excitatory effect of endogenous CO on MNCs in rats submitted to an osmotic stimulation was largely mediated by inhibiting the NO-GABA signaling unit, resulting in the disinhibition of MNCs activity (28). Taken together, our data support a functional interaction between CO-NO-GABA in the control of VP MNCs activity. However, whether an altered CO signaling contributes to the previously reported blunted NO-GABA function in HF, and hence, increased neuronal activity and neurohumoral activation, is at present unknown.
Here, we investigated the role of the HO-1/CO system in identified VP MNCs of HF rats. Using a combination of immunohistochemistry, single-cell real-time PCR and patch-clamp electrophysiology, we evaluated CO-NO-GABA signaling in VP neurons of HF rats and tested the hypothesis that an exacerbated CO expression/function occurs in VP neurons of HF rats and that this occurs via blunted NO-GABA inhibitory signaling.
Materials and Methods
HF animals
Male Wistar heterozygous transgenic endogenously green-fluorescent protein (eGFP)-VP rats (5–6 wk old), in which VP neurons are endogenously green fluorescent (51), were used in this study. Founders of these rats colony, in which eGFP expression is driven by VP promoter, were kindly donated by Dr Yoichi Ueta (University of Occupational and Environmental Health, Kitakyushu, Japan). Rats were housed in rooms with constant temperature (22°C–24°C) under 12-hour light, 12-hour dark cycle and given free access to normal chow and water ad libitum. All the procedures used in this study were carried out and approved in agreement with Georgia Regents University Institutional Animal Care and Use Committee guidelines.
Ischemic HF was induced by coronary artery ligation as previously described (33, 52). Briefly, animals were anesthetized with isoflurane (4%) and intubated for mechanical ventilation. A left thoracotomy through the fifth intercostal space was performed and the heart exteriorized. The ligation was placed on the main diagonal branch of the left anterior descending coronary artery using a 6.0 monofilament propylene suture. The heart was placed to the preceding position and the chest cavity incision was closed. Buprenorphine (Bruprenex C3, 0.3 mg/kg sc; Butler Schein/NLS) was given immediately after surgery to minimize postsurgical pain. Sham animals underwent the same procedure, but the coronary artery was not occluded. All animals were used 6–7 weeks after surgery. Transthoracic echocardiography (Vevo 770 system; Visual Sonics) was performed 4 weeks after surgery under light anesthesia. The left ventricle internal diameter, as well as that of the left ventricle posterior and anterior walls, was obtained throughout the cardiac cycle from the short-axis motion-imaging mode. Automatic calculation using the parameters measured was obtained for ejection fraction and fractional shortening. Only animals that showed less than 40% of ejection fraction and less than 20% fractional shortening were considered as rats with functional HF. Plasma osmolarity did not differ between sham and HF rats (sham: 306.3 ± 3.0 mOsm/L; HF: 313.6 ± 1.0 mOsm/L, n = 16 and 10, respectively; P > .08).
Fluorescent immunohistochemistry, imaging acquisition, and analysis
Sham and HF rats (n = 7/group) were deeply anesthetized with sodium pentobarbital (100 mg/kg ip) and perfused transcardially with 0.01M PBS (150 mL at pH 7.4) followed by 4% paraformaldehyde (350 mL). Brains were removed and postfixed in 4% paraformaldehyde for 3 hours at 4°C. Fixed brains were cryoprotected at 4°C with 0.01M PBS containing 30% sucrose for 72 hours. Sequential coronal sections (30 μm in thickness) through the frozen hypothalamus, between bregma −1.33 and −2.12 (53), were cut with a Leica CM3050S cryostat in 3 series (1:3 series; 60-μm interval between each section within each group), stored at 4°C until used, and then incubated in a solution of 0.01M PBS with 0.1% Triton X-100, 0.04% NaN3, and 10% normal horse serum for 1 hour. Sections were then incubated for 24 hours in a cocktail of primary antibodies containing either a rabbit anti-HO-1 polyclonal antibody (SPA-895, 1:1000; StressGen), along with a mouse anti-NOS-brain (nNOS) monoclonal antibody (N2280, 1:400; Sigma-Aldrich) as previously described (28). Reactions with primary antibodies were followed by 4 hours of incubation in the presence of fluorescently labeled secondary antibody donkey antirabbit cyanine3-labeled (1:250) and donkey antimouse cyanine5-labeled (1:50) from Jackson ImmunoResearch. All samples from the different experimental groups were run together in parallel, and mounted on slides using the same volume of mounting media (for antibodies, please see Table 1).
Table 1.
Antibody Information
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog Number | Species Raised, Mono or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| HO-1 | Recombinant Hsp32 lacking the membrane spanning region | Anti-HO-1 | StressGen, SPA-895 | Rabbit, polyclonal | 1:1000 |
| nNOS | Clone NOS-B1 | Anti-NOS-neuronal | Sigma-Aldrich, N2280 | Mouse, monoclonal | 1:400 |
Stained sections were examined with a Zeiss LSM 510 Confocal Microscope System (Carl Zeiss). Fluorescent signal cross talk among the channels was avoided by setting image acquisition parameters with individually labeled section. Once laser acquisition settings were set, all sections from the different experimental groups were exposed to the same settings, for the same extension of time. Ten-twelve consecutive optical focal planes (2-μm interval) were acquired and a projection image was generated as a single section. All images from sham and HF groups were digitized with identical acquisition settings. To determine the degree of a single immunoreactive signal, or the degree of colocalization among signals, as well as the mean fluorescent signal intensity, a densitometry analysis based on a threshold paradigm (1.5 times the background threshold fluorescence) was used as previously described (31). Briefly, for each rat, 3 representative sections of the middle level of the PVN containing the lateral magnocellular (LM) subnucleus (bregma, −1.80), in which the vast majority of magnocellular VP neurons are densely grouped (2, 54, 55), were used for quantification. The LM subnucleus was readily identified by the “ball-shaped” structure visible after VP immunostaining (see also Figure 1) (54). The LM was then traced, and the density (ie, the percentage of the total traced region of interest area occupied by immunoreactive pixels) and the mean imunoreactive intensity (expressed in arbitrary units ranging from 0 [absolute black] to 255 [absolute white]) of the different signals (HO-1, nNOS, and VP) were calculated using ImageJ (1.47v; National Institutes of Health). The degree of colocalization signals between HO-1 and VP (or nNOS and VP) from each traced section were also calculated by identifying those pixels containing both immunoreactive signals, using an algorithm provided by an ImageJ plugin http://rsbweb.nih.gov/ij/plugins/colocalization.html. Colocalization results represent the proportion of double-immunoreactive density relative the total VP density, which are expressed as percentage. A mean value of 3 PVN sections, for each animal, was obtained as previously described (28), and the overall mean for each signal and experimental condition were used for statistical comparisons. All semiquantitative immunofluorescent analyses were carried out blindly.
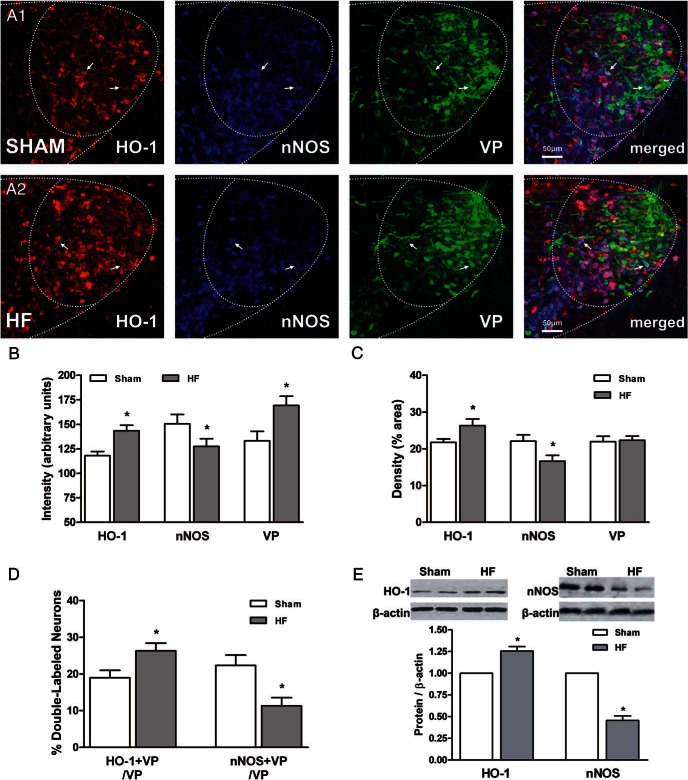
Figure 1.
nNOS and HO-1 protein expression in MNCs of the PVN in HF rats. A, Representative confocal photomicrographs of eGFP-VP neurons (green) in the LM subnucleus of the PVN displaying inducible HO-1 (red) and nNOS (blue) immunoreactivity in sham (A1) and HF (A2) rats. Last right panel, Merged images shown for better comparison. Arrows indicate examples of triple-labeled neurons. Scale bar, 50 μm. Bar graphs showing the mean immunoreactive intensity (B) and the mean immunoreactive density (ie, % area) of HO-1, nNOS, and VP density (C) in the traced LM subnucleus of the PVN in sham (open bars, n = 7) and HF (close bars, n = 7) rats. D, Summary of double-labeled HO-1+VP and nNOS+VP neurons (expressed as proportions of the number of VP neurons sampled within the LM subnucleus of the PVN). E, Summary of Western blotting data analysis of HO-1 and nNOS protein expression normalized to the endogenous control β-actin in the PVN of sham (open bar, n = 8) and HF (close bar, n = 8) rats. Above the mean bar graphs, representative HO-1 and nNOS bands protein are shown. Data are reported as the mean ± SEM. Student's unpaired t test. *, P < .05 vs sham.
Western blotting
Brains from anesthetized sham and HF rats (n = 8 each group) were dissected, fast frozen in isopentane, and sliced into 3 coronal sections of 300 μm at the PVN level. PVN was bilaterally punched using an 18-gauge needle (Harris Micropunch; Sigma-Aldrich), and samples were immediately frozen on liquid nitrogen. After collection, samples were homogenized in ice-cold radioimmunoprecipitation buffer containing fresh protease inhibitor cocktail (Sigma-Aldrich) followed by protein extraction. Bradford method was used to measure protein concentration in the samples using bovine serum albumin as standard. A total of 50 μg of total protein from each sample were separated on an SDS-PAGE 8% polyacrilamide gel and electrophoretically transferred to a nitrocellulose membrane. Nonspecific binding was blocked with 5% nonfat dry milk in Tris-buffered saline solution with Tween 0.1% for 1 hour at room temperature. The membrane was then incubated overnight at 4°C with a mouse anti-NOS-brain (nNOS) monoclonal antibody (1:400, N2280; Sigma-Aldrich), or rabbit anti-HO-1 polyclonal antibody (1:1000, SPA-895; StressGen) diluted in 5% nonfat milk in Tris-buffered saline solution with Tween 0.1%. Membrane was washed and subsequently incubated with a donkey antimouse or antirabbit horseradish peroxidase secondary antibody (1:1000; Santa Cruz Biotechnology, Inc). A chemiluminescent assay kit (Supersignal, Thermo Scientific) was used to detect immunoreactive bands, and the intensity of all bands was estimated by densitometry analysis using software (Un-Scan-IT Gel 6.1; Silk Scientific). All densitometry measurements were normalized using an antibody against β-actin (1:20 000, A3854; Sigma-Aldrich) as a loading control and expressed as unit of change. Data were collected in a series of 2 or 3 experiments and pulled together for statistical analyses.
Quantitative real-time PCR
PVN mRNA expression
Sham and HF rats (n = 8/group) were deeply anesthetized with sodium pentobarbital and the whole brains were fast collected and immediately frozen. The brains were then fixed in a cryostat (Leica CM3050S), and PVN microdissections (900 μm) were obtained according to coordinates from Paxinos and Watson atlas (PVN, −1.33 to −2.12 from bregma) (53). Using a stainless steel punch needle of 1.5-mm diameter, bilateral PVN samples were collected and transferred to a microtube with RNAlater reagent (Ambion) and stored at 4°C for a maximum of 24 hours as previous described (56). Total RNA was isolated from each sample using TRIzol reagent (Invitrogen), according to the protocol provided by the manufacturer. RNA concentration was determined by NanoDrop Spectrophotometer (Thermo Scientific), and only 250 ng of RNA in each sample were used for cDNA synthesis using iScript cDNA Synthesis kit (Bio-Rad) according to the manufacturer protocol. PCR amplification was performed using a mixture containing: 1 μL of 10μM sense primer, 1 μL of 10μM antisense primer, 10 μL of 2× master mix buffer (Power SYBR Green Master Mix; Applied Biosystems), 2 μL of the cDNA template, and 6 μL of diethylpyrocarbonate (DEPC) water.
Single-cell RT-PCR
The cytoplasm of patched sham (n = 12 cells) and HF (n = 14 cells) VP neurons were individually pulled into a pipette containing 2-μL DEPC water. The content was immediately transferred in a tube with 1 μL of ribonuclease inhibitor (Applied Biosystems) and frozen in liquid nitrogen as previous described (57). Reverse transcriptase reaction for cDNA was carried out with iScript cDNA Synthesis kit (Bio-Rad) according to manufacturer protocol. PCR amplification was performed with a fraction of cDNA as a template. The mixture of PCR contained: 1 μL of 10μM sense primer, 1 μL of 10μM antisense primer, 10 μL of 2× master mix buffer (Power SYBR Green Master Mix; Applied Biosystems), 5 μL of the cDNA template, and 3 μL of DEPC water.
The following primers were used in both PVN and single-cell PCR: VP sense 5′-CCTCACCTCTGCCTGCTACTT-3′ and antisense 5′-GGGGGCGATGGCTCAGTAGAC-3′; HO-1 sense 5′-CTGGAAGAGGAGATAGAGCGAA-3′ and antisense 5′-TCTTAGCCTCTTCTGTCACCCT-3′; nNOS 5′-TTCCGAAGCTTCTGGCAAC-3′ and antisense 5′-GGATGGCTTTGAGGACATC-3′; glyceraldehyde 3-phosphate dehydrogenase 5′-TTCAACGGCACAGTCAAGG-3′and antisense 5′-TGGTTCACACCCATCACAAA-3′; β-actin sense 5′-GACCCAGATCATGTTTGAGACCTT-3′ and antisense 5′-CACAGCCTGGATGGCTACGTA-3′. All primers were synthesized by Integrated DNA Technologies. Quantitative real-time PCR was performed using Applied Biosystems Fast 7500 Real-Time PCR System. The annealing temperature in the thermal cycler was 60°C and 40 cycles were performed. Each PCR was performed in duplicate. Water instead of cDNA was used as a negative control. Housekeeping genes (β-actin and glyceraldehyde 3-phosphate dehydrogenase) were run for each cDNA sample. Determination of gene transcript in each sample was obtained by the ΔΔCT method. For each sample, the threshold cycle (CT) of mRNA was measured and normalized to the average of the housekeeping genes (ΔCT = CT unknown − CT housekeeping genes). The fold change of mRNA in the unknown sample (HF) relative to control group (sham) was determined by 2−ΔΔCT, where ΔΔCT = ΔCT unknown − ΔCT sham. Data are shown as a relative mRNA expression to the sham group.
Patch-clamp electrophysiology
Conventional whole-cell patch-clamp recordings (30) from identified PVN eGFP-VP neuron were obtained using a combination of fluorescence illumination and infrared differential interference contrast video microscopy (Figure 2B1). Briefly, rats were anesthetized with sodium pentobarbital and perfused thought the heart with cold artificial cerebrospinal fluid (aCSF) (120mM NaCl, 2.5mM KCl, 1.25mM NaH2PO4, 1mM MgSO4, 2mM CaCl2, 26mM NaHCO3, 20mM glucose, 2mM pyruvic acid, and 0.4mM ascorbic acid in pH 7.4, 295 mOsm), in which NaCl was replaced by an equimolar amount of sucrose. Coronal hypothalamic slices containing the PVN were cut (210 μm) using a Leica VT1000S vibroslicer and placed in a holding clamber containing aCSF for at least 30 minutes until used. For electrophysiological recordings, slices were transferred to a submersion-type recording chamber, continuously perfused (∼2 mL min−1 at 30°C–32°C) with a standard aCSF bubbled with a gas mixture of 95% O2 and 5% CO2. Patch pipettes (4–6 MΩ) were pulled from thin-walled (outer diameter, 1.5 mm; inner diameter, 1.7 mm) borosilicate glass (GC150T-7.5; Clark) on a horizontal electrode puller (P-97; Sutter Instruments). Electrical recordings were obtained using an Axopatch 700A (Axon Instruments) amplifier. The series resistance was monitored throughout the experiment, and data were discarded if the series resistance during recordings increased by 2-fold.
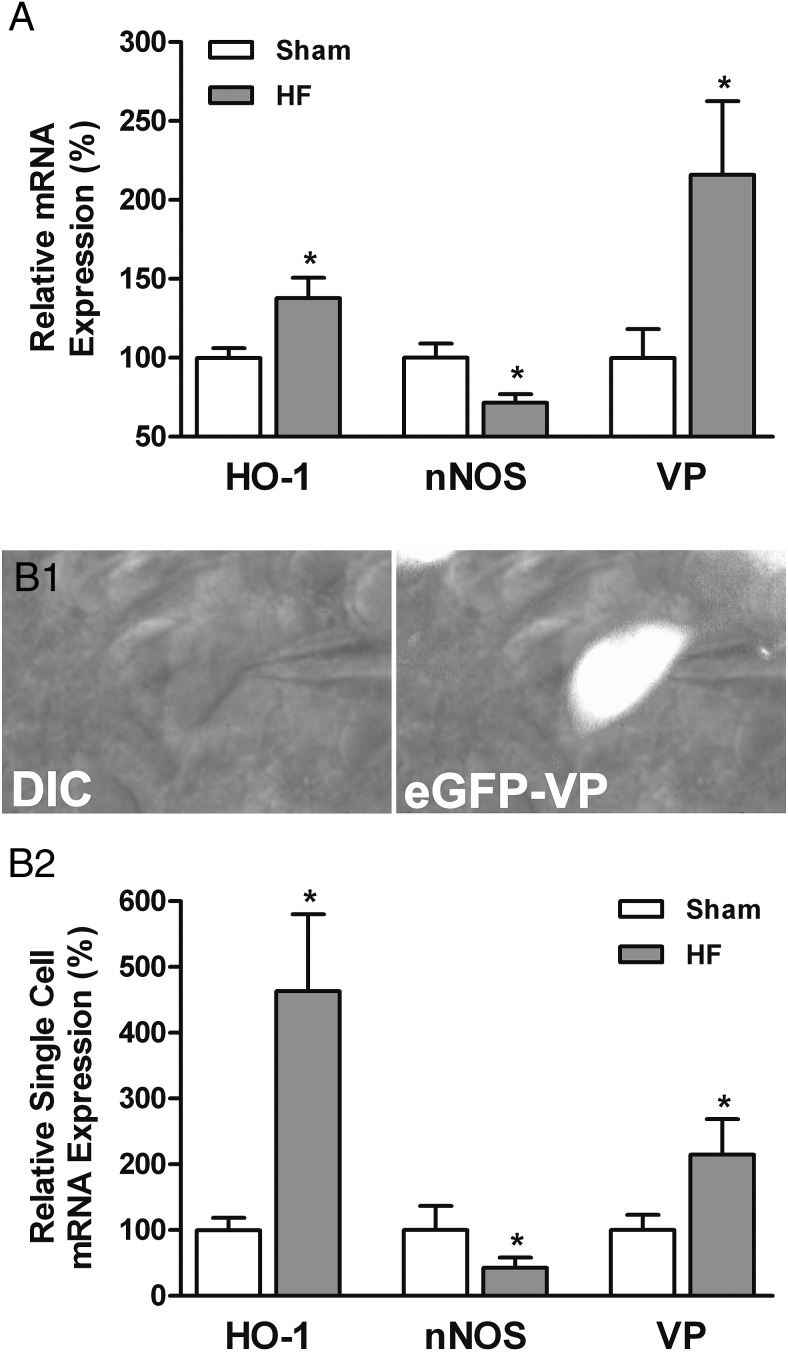
Figure 2.
HO-1, nNOS, and VP mRNA expression in PVN MNCs in HF rats. A, Bar graphs summarizing the qRT-PCR data analysis of HO-1, nNOS, and VP mRNA expression in the PVN of sham (open bar, n = 8) and HF (close bar, n = 8) rats. B1, Representative high magnification image showing an identified PVN eGFP-VP neuron under an infrared differential interference contrast (IR-DIC) (left) and DIC combined with fluorescence illumination (right). Note the presence of the patch pipette in the images showing that identified VP neurons were utilized to perform single-cell RT-PCR and patch-clamp recordings protocols. Scale bar, 10 μm. B2, Summary of single-cell RT-PCR data analysis showing the relative mRNA expression of HO-1, nNOS, and VP in identified PVN VP neurons of sham (open bar, n = 12) and HF (close bar, n = 14) rats. Data are reported as the mean ± SEM. Student's unpaired t test. *, P < .05 vs sham.
Current-clamp recordings
The pipette internal solution contained 130mM K+-gluconic acid, 20mM KCl, 10mM HEPES, 0.5mM EGTA, 0.9mM Mg-ATP, and 0.3mM Na-GTP. The pH was slowly titrated to 7.25–7.30 and the osmolality was 285 mOsm. As previous described, firing activity was recorded in the current-clamp mode (28), and the effects of HO inhibitor Tin-protoporphyrin IX (SnPP) (20μM; Porphyrin Products) and CO donor tricarbonyldichlororuthenium dimer (CORM) (30μM; Sigma-Aldrich) were tested (5-min bath application). In another group of VP neurons, the effects of SnPP and CORM were tested in cells that were preincubated (30 min) with a combination of NOS blocker (NG-nitro-larginine methyl ester [LNAME], 2mM; Sigma-Aldrich) and NO scavenger (2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide [carboxy-PTIO], 100μM; Alexis Biochemicals). Finally, in the last group of cells, the effects of CORM were tested in VP neurons that were preincubated (10 min) with a GABAA receptor blocker (picrotoxin, 50μM; Sigma-Aldrich). Due to the variability in basal firing activity, and the fact that in some instances recorded neurons were silent, neurons were subjected to DC current injection through the pipette in an attempt to bring the membrane potential near or at spike threshold (−50 to −45 mV). The amount of DC current injection between groups was similar (range, 7–10 pA). Firing rate (defined as the number of spikes/s) was measured during a basal period (3-min period before drug application) and during an effect period (5 min) that started 1 minute after drug administration.
GABAA-mediated inhibitory postsynaptic currents (IPSCs)
For voltage-clamp experiments, patch pipettes were filled with a high Cl−-containing solution: 140mM KCl, 10mM HEPES, 0.9mM Mg-ATP, 20mM sodium phosphocreatine, 0.3mM Na-GTP, and 10mM EGTA. Briefly, spontaneous IPSCs were recorded as outward currents while holding the membrane at −55 mV, as previously described (23, 39). The direct effects of the HO inhibitor SnPP (20μM) and the CO donor CORM (30μM) on GABA-mediated IPSCs were tested (5-min bath application). The detection threshold was set at 50 pA. Frequency rate of IPSCs (defined as the number of spikes/s) was analyzed during a basal period (3-min period before drug application) and during an effect period (5 min) that started 1 minute after drug administration.
Neurons were considered responsive if a change in firing rate or IPSCs frequency of at least ± 10% was observed between basal period and drug administration. Current and voltage output were filtered at 2 kHz and digitized at 10 kHz (16-bit resolution) in conjunction with pClamp 8 software (Digidata 1440A; Axon Instruments). All data were analyzed by using MiniAnalysis software (Synaptosoft, Inc).
Statistical analysis
Results are expressed as mean ± SEM. Data were analyzed using Student's paired and unpaired t tests, as well as one-way ANOVA, followed by Bonferroni post hoc tests, as indicated. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc). P < .05 was considered statistically significant.
Results
Altered nNOS and HO-1 mRNA and protein expression in VP neurons of the PVN in HF rats
It is well demonstrated by our laboratory and others that chronic HF leads to a reduction of nNOS expression and therefore diminished endogenous NO signaling in magnocellular neurosecretory neurons of the PVN (31–35). Given also that we recently showed that PVN VP neurons have the ability to synthesize both NO and CO (28), we aimed to determine here whether expression of the CO-synthesizing enzyme HO-1 was also altered in HF. To this end, we performed immunofluorescence and Western blotting using specific antibodies raised against HO-1 and nNOS. As previously reported, we found a significant decrease in the degree of nNOS immunoreactive expression (revealed both as a decrease in mean immunoreactivity intensity and density in the LM subnucleus of the PVN) (P < .05 in both cases, unpaired t test) (Figure 1, A–C). Conversely, we found a significant increase in the level of HO-1 immunoreactive expression (revealed also both as an increase in the mean immunoreactivity intensity and density in the LM subnucleus of the PVN) (P < .05 in both cases, unpaired t test) (Figure 1, A–C). Similar results were observed by Western blot analysis, where the total amount of nNOS expression in the PVN of HF rats was reduced (−45.5 ± 0.1%), whereas HO-1 expression was increased (+25.5 ± 0.1%), when compared with sham rats (P < .05 in both cases, unpaired t test) (Figure 1E).
We recently showed that VP neurons in the PVN express both nNOS and HO-1 (28). We found increased VP immunoreactive intensity in the PVN of HF rats compared with sham rats (P < .05) (Figure 1B). Moreover, we found that the degree of VP neurons expressing nNOS in HF rats was significantly smaller than that observed in the PVN of sham animals (P < .05, unpaired t test) (Figure 1D). Conversely, a significantly higher incidence of HO-1 immunoreactivity was observed in VP neurons in HF rats compared with sham (P < .05, unpaired t test) (Figure 1D).
To determine whether changes in nNOS, HO-1 and VP protein levels during HF corresponded with changes in gene expression, we performed quantitative RT-PCR. In a first set of studies, we measured mRNA expression from whole punched PVN tissue. As shown in Figure 2A, we found increased HO-1 (+37.7 ± 13.0%) and VP (+115.9 ± 46.7%) mRNA expression in PVN punches from HF rats compared with sham rats (P < .05 in both cases, unpaired t test). Conversely, a decrease in nNOS mRNA expression (−28.4 ± 5.5%, P < .05, unpaired t test) was observed in HF rats. To determine whether changes in nNOS and HO-1 mRNA occurred in VP neurons, we performed single-cell RT PCR in identified VP neurons (Materials and Methods). As shown in Figure 2B1, after establishing a whole-cell patch configuration on eGFP-VP cells in PVN slices, their cytoplasm content was aspirated into the patch pipette. As observed in whole PVN samples, we found HO-1 (+363.3 ± 116.8%) and VP (+114.5 ± 54.2%) mRNA expressions to be significantly increased in identified VP neurons in HF rats compared with sham rats (P < .05 in both cases, unpaired t test), whereas nNOS mRNA expression was diminished (−57.4 ± 15.4%, P < .05, unpaired t test) (Figure 2B2). Taken together, these studies support a diminished nNOS and increased HO-1 mRNA and protein expression in VP neurons of HF rats.
Excitatory CO effects on firing activity of PVN vasopressinergic neurons are mediated by NO signaling mechanisms during HF disease
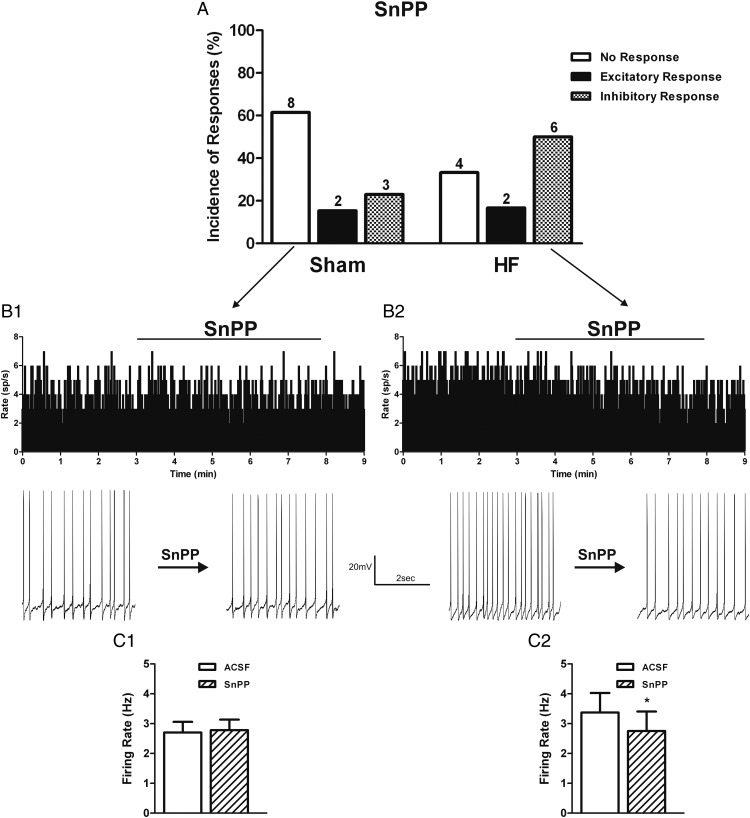
We recently showed that in control rats, although NO acts as a major inhibitory signal on MNCs (20, 29, 30), CO exerts an excitatory effect (28, 46), which was in part dependent on decreasing NO availability (28, 58). To determine whether the changes in nNOS and HO-1 expression during HF translated into altered NO-CO bioavailability, and consequently affected VP neuronal activity, we obtained electrophysiological recordings from identified PVN VP neurons in sham and HF rats. We firstly evaluated the effects of the HO inhibitor SnPP. In sham rats, we found that the majority (8/13, 61.5%) of VP neurons were unresponsive to SnPP (20μM) (baseline firing: 2.7 ± 0.4 Hz; SnPP firing: 2.8 ± 0.4 Hz; nonsignificant (NS), paired t test). Only 2/13 (15.4%) and 3/13 (23.8%) of VP neurons showed either an excitatory or and inhibitory response to SnPP (Figure 3, A B1, and C1). Conversely, half (6/12, 50.0%) of VP neurons in HF rats showed a significant decrease in the firing rate after SnPP application (baseline firing: 3.4 ± 0.7 Hz; SnPP firing: 2.8 ± 0.7 Hz; P < .05, paired t test) (Figure 3, A, B2, and C2). The remaining VP neurons in HF rats showed no response in 4/12 (33.3%) or and excitatory response in 2/12 (16.7%).
Figure 3.
HO inhibition decreases PVN VP neuronal activity in HF rats. A, Summary graphs showing the incidence of PVN VP neuronal responses to the HO inhibitor SnPP (20μM) in sham (n = 13) and HF (n = 12) rats. Representative examples showing the most predominant effect of SnPP on the electrical activity (average in 1-s bins) of PVN VP neurons in sham (B1) and HF (B2) rats. Expanded firing traces are shown before and during SnPP bath application. Graphs summarizing the mean effects of HO inhibition on the firing activity of VP neurons in sham (C1) and HF (C2) rats. Data are reported as the mean ± SEM. Student's paired t test. *, P < .05 vs aCSF group.
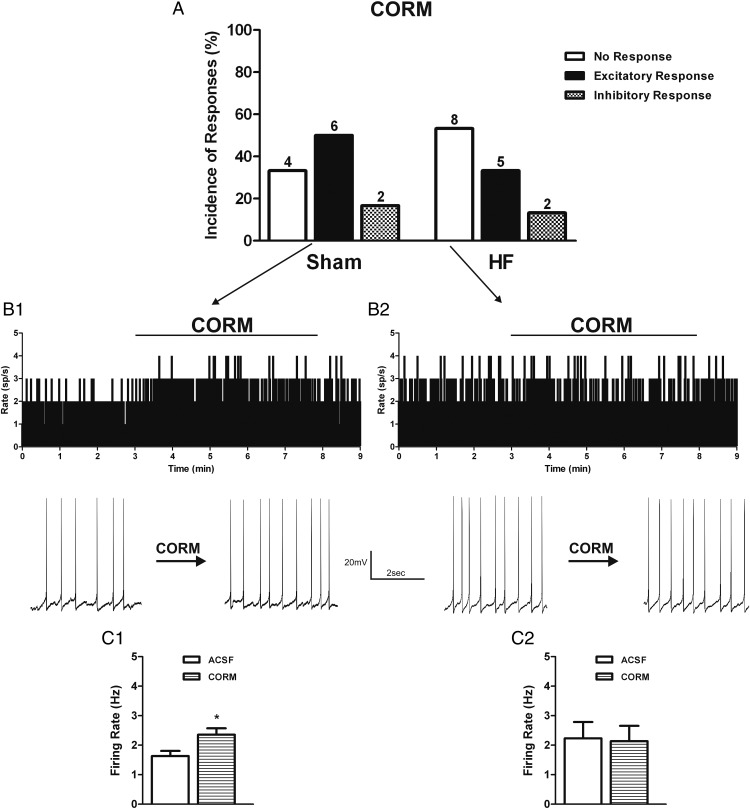
We also tested the effects of increasing CO bioavailability using the CO donor CORM. We found that in VP neurons in sham rats, bath application of CORM (30μM) increased the firing activity of 50.0% (6/12) of tested neurons (baseline firing: 1.6 ± 0.2 Hz; CORM firing: 2.4 ± 0.2 Hz; P < .05, paired t test). The remaining VP neurons showed either no response (4/12, 33.3%) or were inhibited (2/12, 13.3%) by CORM (Figure 4, A, B1, and C1). Different from what we observed in sham rats, the majority (8/15, 53.3%) of VP neurons in HF were unresponsive to CORM application (baseline firing: 2.2 ± 0.6 Hz; CORM firing: 2.1 ± 0.1 Hz; NS, paired t test), with the remaining cells showing either an excitatory (5/15, 33.3%) or an inhibitory (2/15, 13.3%) response (Figure 4, A, B2, and C2). These results support higher level of endogenous CO levels and activity in HF rats, contributing to a predominantly excitatory effect in VP neurons in this condition. The elevated endogenous CO levels may have contributed to a partially blunted response of VP neurons to exogenous CO in HF rats.
Figure 4.
Exogenous CO donor increases PVN vasopressinergic neuronal activity in sham rats. A, Summary graphs showing the incidence of PVN VP neuronal responses to the CO donor CORM (30μM) in sham (n = 12) and HF (n = 15) rats. Representative examples showing the most predominant effect of CORM on the electrical activity (average in 1-s bins) of PVN VP neurons in sham (B1) and HF (B2) rats. Expanded firing traces are shown before and during CO donor bath application. Graphs summarizing the mean effects of CORM on the firing activity of VP neurons in sham (C1) and HF (C2) rats. Data are reported as the mean ± SEM. Student's paired t test. *, P < .05 vs aCSF group.
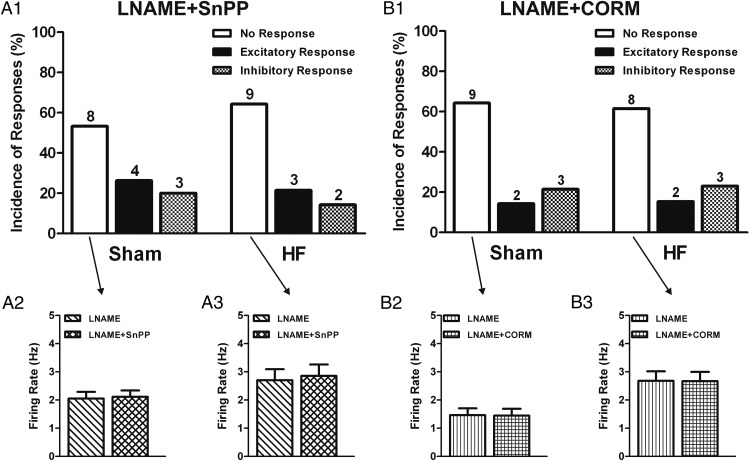
We recently showed that the excitatory effects of CO in VP neurons in control rats were partially mediated by inhibition of NO bioavailability (28). To determine whether the same mechanism was involved HF rats, given the already diminished nNOS expression and NO availability (31–35), we repeated experiments in the presence of the NOS blocker LNAME. In sham rats, we found that responses to SnPP were similar to control aCSF conditions, ie, a majority of nonresponsive cells (8/13, 53.3%; baseline firing: 2.1 ± 0.2 Hz; LNAME+SnPP firing: 2.1 ± 0.2 Hz; NS, paired t test) was observed (Figure 5, A1 and A2). Conversely, we found that in HF rats, previous application of LNAME almost completely prevented the inhibitory effect of SnPP. Thus, only 2/14 (14.3%) of tested neurons showed an inhibitory response to CO (compared with 50% in control conditions), with most VP neurons being unresponsive (9/14, 64.3%; baseline firing: 2.7 ± 0.4 Hz; LNAME+SnPP firing: 2.9 ± 0.4 Hz; NS, paired t test) to the HO inhibitor (Figure 5, A1 and A3).
Figure 5.
CO effects on PVN VP neuronal activity in HF rats are dependent on NO signaling. A1, Summary of the incidence of PVN VP neuronal responses to the HO inhibitor SnPP (20μM) in slices pretreated with the NOS inhibitor LNAME (2mM) plus an NO scavenger (carboxy-PTIO) (100μM) in sham (n = 15) and HF (n = 14) rats. Graphs summarizing the mean effects of HO inhibition in the presence of LNAME+carboxy-PTIO on the firing activity of VP neurons in sham (A2) and HF (A3) rats. B1, Summary of incidence of PVN VP neuronal responses to the CO donor CORM (30μM) in slices pretreated with LNAME plus NO scavenger in sham (n = 14) and HF (n = 13) rats. Graphs summarizing the mean effects of CO donor in the presence of LNAME+carboxy-PTIO on the firing activity of VP neurons in sham (B2) and HF (B3) rats. Note that in the absence of NO, CO actions were blunted. Data are reported as the mean ± SEM. Student's paired t test.
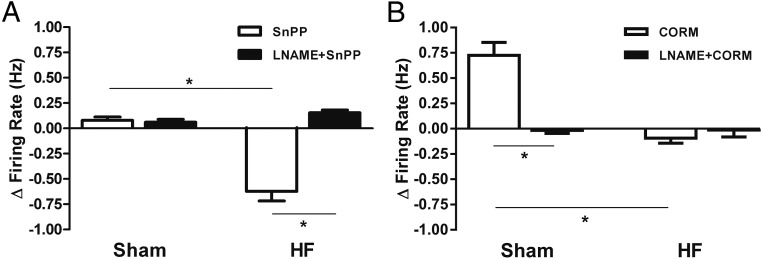
We also tested the effects of the CO donor in the presence of NOS blockade. In sham rats, LNAME blunted the excitatory effect of CORM (2/14, 14.3%, compared with 50% in control conditions) with most VP neurons becoming unresponsive (9/14, 64.3%; baseline firing: 1.5 ± 0.2 Hz; LNAME+CORM firing: 1.5 ± 0.2 Hz; NS, paired t test) (Figure 5, B1 and B2). As observed in control conditions, most VP neurons in HF rats in the presence of LNAME still remain unresponsive (8/13, 61.5%; baseline firing: 2.7 ± 0.3 Hz; LNAME+CORM firing: 2.7 ± 0.3 Hz; NS, paired t test) (Figure 5, B1 and B3). For better comparison of all main effects (excluding minority effects) across animals and treatments, we plotted together results expressed as Δ changes in firing rate (Figure 6, A and B). Taken together, these studies support that the enhanced excitatory effect of endogenous CO in HF rats is largely dependent on interactions with NO.
Figure 6.
Summary data comparing the actions of endogenous and exogenous CO on PVN VP neuronal activity in the different experimental conditions. A, Graphs summarizing the main effect of HO inhibition on the Δ firing rate of PVN VP neurons in the presence or absence of LNAME+carboxy-PTIO in sham and HF rats. B, Graphs summarizing the main effect of a CO donor on the Δ firing rate of PVN VP neurons in the presence or absence of LNAME+carboxy-PTIO in sham and HF rats. Note that inhibition of endogenous NO availability prevented the effects of CO manipulations. Data are reported as the mean ± SEM. One-way ANOVA, followed by Bonferroni post hoc tests. *, P < .05 as indicated.
CO diminished GABA-mediated IPSCs in PVN VP neurons
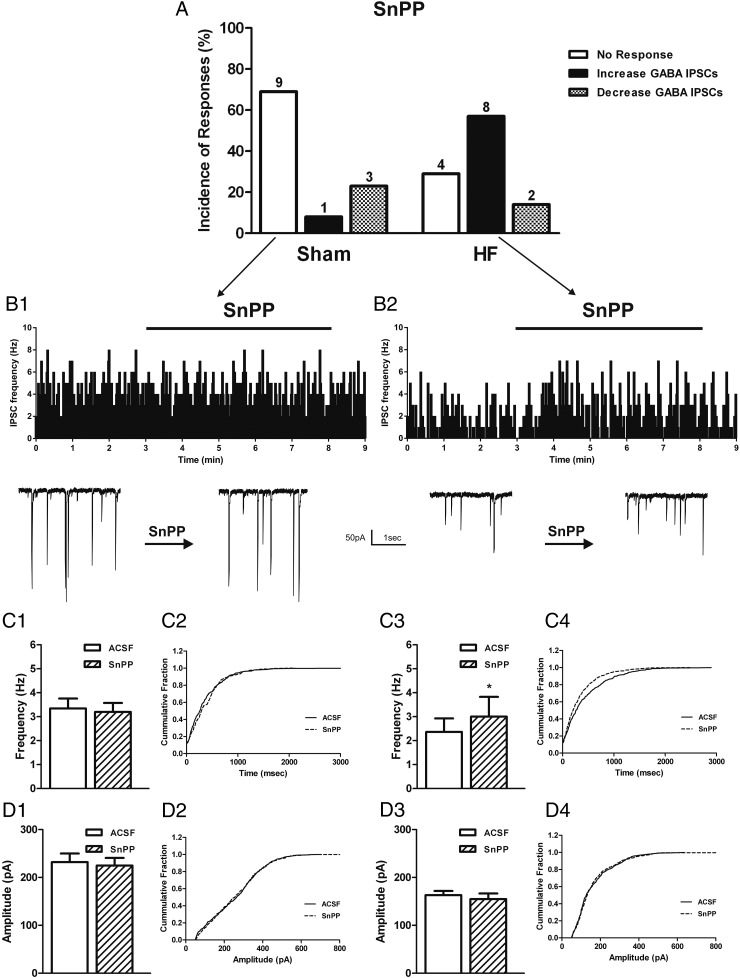
We have previously shown that NO inhibitory effects on VP neurons are largely mediated by incrementing local GABAergic inhibitory synaptic activity (20, 29, 30). Thus, given that our present results indicate that the elevated endogenous CO excitatory effect during HF was dependent on NO, we evaluated whether GABAergic activity was also involved in mediating CO effects during HF. Thus, to investigate whether CO affected GABAergic synaptic function, we recorded GABA IPSCs from identified VP neurons. In sham rats, we found that SnPP did not alter the mean frequency (baseline frequency: 3.3 ± 0.4 Hz; SnPP frequency: 3.2 ± 0.4 Hz; NS, paired t test) or amplitude (baseline amplitude: 232.2 ± 18.0 pA; SnPP amplitude: 224.9 ± 16.1 pA; NS, paired t test) of GABA-mediated IPSCs in most recorded VP neurons (9/13, 69.0%) (Figure 7, A, B1, C1, C2, D1, and D2). Conversely, SnPP in HF rats significantly increased the mean frequency of IPSCs (baseline frequency: 2.4 ± 0.6 Hz; SnPP frequency: 3.0 ± 0.8 Hz; P < .05, paired t test), without changing IPSCs mean amplitude (baseline amplitude: 163.1 ± 8.7 pA; SnPP amplitude: 154.9 ± 11.8 pA; NS, paired t test) in most VP neurons (8/14, 57.0%) (Figure 7, A, B2, C3, C4, D3, and D4).
Figure 7.
HO inhibition increases GABA-mediated IPSCs in PVN VP neurons in HF rats. A, Summary of the incidence of PVN VP neurons showing changes in GABA-mediated IPSCs in response to the HO inhibitor SnPP (20μM) in sham (n = 13) and HF (n = 14) rats (A). Representative examples showing the most predominant effect of SnPP on the IPSC frequency (average in 1-s bins) of PVN VP neurons in sham (B1) and HF (B2) rats. Samples of expanded GABA currents are shown before and during SnPP bath application. Graphs summarizing the mean effect of HO inhibition on the IPSCs frequency and amplitude of VP neurons in sham (C1, D1) and HF (C3, D3) rats. Cumulative distribution histograms of IPSC frequency (C2, D2) and amplitude (C4, D4) before and during SnPP application. Data are reported as the mean ± SEM. Student's paired t test. *, P < .05 vs aCSF group.
Discussion
In the present study, we aimed to determine whether CO expression and signaling was altered in the PVN of HF rats, contributing in turn to blunted NO-GABA function and altered VP neuronal activity during this disease state. We tested the hypothesis that an enhanced constitutive CO production contributes to elevated VP neuronal activity during HF, and that this results from a CO-mediated inhibition of NO-GABA function. The main findings obtained in this study were: 1) the expression of HO-1 (the CO-synthesizing enzyme), mRNA, and protein in VP neurons of the PVN is enhanced in HF rats; 2) exogenous CO application increased the firing activity of VP neurons only in sham rats, whereas inhibition of endogenous CO production decreased VP firing activity only in HF rats; 3) the excitatory effects mediated by CO during HF were significantly blunted when endogenous NO production or GABAergic inhibitory transmission were inhibited; and 4) endogenous CO diminished GABA-mediated IPSCs in PVN VP neurons during HF. Taken together, the increased HO-1 mRNA and protein expression, along with an enhanced effect of the HO inhibitor in HF rats, support an enhanced CO excitatory signaling in VP neurons during HF and that CO acts by inhibiting NO-GABA-mediated signaling pathways. We propose that elevated endogenous CO levels contribute to blunted NO-GABA inhibitory function during HF, being thus an important mechanism contributing to neurohumoral activation in this disease.
Endogenously produced by the activity of HO, CO is a gaseous neurotransmitter in the CNS (43–45) that has been shown to increase MNCs firing activity during osmotic stress (28, 46). This and previous studies indicate that the expression of the inducible HO isoform type 1 (HO-1) is found only in specific groups of neurons, particularly within hypothalamic PVN MNCs (28, 46, 48, 49, 59). Moreover, known as heat shock protein 32, HO-1 expression was shown to be up-regulated during a variety of stressful conditions (48, 49), including osmotic stimulation, hypertension, oxidative stress, and inflammation (28, 45, 46, 48, 60). Interestingly, oxidative stress and inflammation in the PVN have been described as critical processes contributing to neurohumoral activation during HF (36, 61–65). In this study, our results from a combination of immunofluorescence, Western blotting and RT-PCR, both at the whole PVN and single VP level, showed that HO-1 protein and mRNA expression are increased in the PVN of HF rats. However, to what extend oxidative stress or inflammatory mediators are critical factors inducing changes in PVN HO-1 expression remains to be determined.
The increased HO-1 mRNA and protein expression during HF would suggest an increased bioavailability and actions of CO during this condition. In fact, our electrophysiological experiments showed that blockade of endogenous CO production decreased firing activity in most VP neurons in HF, but not in sham rats, supporting an enhanced endogenous CO-excitatory drive of VP firing activity in HF. The diminished firing activity of VP neurons after HO-1 blockade we observed in HF is in accordance with our previous findings showing that HO-1 inhibition also diminished neuronal activity in dehydrated rats, a condition in which we showed HO-1 expression to also be up-regulated (28, 46).
Besides the increased HO-1 expression and CO excitatory actions described above during HF, we also observed, as previously described, a diminished nNOS protein and mRNA expression (31–35) in VP neurons during HF. Importantly, the diminished basal NO availability within the PVN has been shown to contribute to elevated neurohumoral drive in chronic HF (34). Still, the mechanisms leading to diminished NO availability and actions during HF remain unknown.
Although CO and NO actions in the PVN could involve independent mechanisms, a growing body of evidence supports the possibility of a direct cross talk between these 2 gas signaling mechanisms (66, 67). This is supported by the fact that both HO-1 and nNOS enzymes colocalize in a relatively large proportion of PVN VP neurons (28), suggesting that VP neurons have the ability to act as sources of both CO and NO gas molecules. Moreover, this and previous studies support the fact that their expression can be regulated either in similar or opposite directions, in a state-dependent manner. Thus, although both HO-1 and nNOS are up-regulated during dehydration or an osmotic stimulation (28), HO-1 and nNOS are up- and down-regulated, respectively, during HF, as shown in this study.
A direct mechanistic link between CO and NO in the modulation of VP neuronal activity during HF is also supported by our electrophysiological studies. Thus, we found that the decreased in VP firing activity after blockade of endogenous CO production during HF was blunted by previous blockade of NOS activation and consequently NO synthesis. These results indicate that the excitatory effect mediated by endogenous CO (unveiled in our studies as an inhibition of firing after its blockade) is dependent on NOS activity and NO availability. Given that others and we have previously shown NO to be a potent inhibitor of supraoptic nucleus and PVN neuronal activity (20–25), along with the fact that NOS expression and NO bioavailability are diminished in the PVN of HF rats (31–35), we interpret our findings to suggest that the elevated endogenous CO levels in HF contribute to the blunted NO availability and thus, increased VP firing activity in HF. Accordingly, diminishing CO levels by inhibiting HO-1 activity would increase NO availability inhibiting VP neuronal activity (in a NOS-dependent manner).
Previous studies have shown that NO inhibition of VP neuronal activity and sympathoexcitatory outflow from the PVN are mediated via activation of GABAergic synaptic inputs (34, 37, 40, 42). Moreover, a diminished NO availability within the PVN, resulting in a blunted of GABAergic transmission (36–38, 40, 68), has been shown to contribute to elevated neurohumoral drive in HF rats. Thus, our results showing that the excitatory effect mediated by endogenous CO was also occluded by previous GABAA receptor blockade, further supports a direct CO-NO interaction in the regulation of VP neuronal activity during HF. To confirm a link between CO and GABAergic synaptic activity in VP neurons during HF, we directly monitored GABAA-mediated IPSCs in VP neurons. We found that inhibition of HO activity enhanced GABA-mediated IPSCs frequency in VP neurons in HF, but not in sham rats. Thus, based on all these results, it is reasonable to speculate that the increased CO availability in the PVN of HF rats contributes to a blunted NO-GABA signaling which, in turn, increases VP neuronal activity and neurohumoral activation during HF.
Various mechanisms could mediate a functional link between CO and NO in the PVN during HF. For example, previous studies showed that CO can directly bind to NOS, leading to its subsequent inhibition and diminished NO production (69–71). Moreover, NO synthesis is also regulated by intracellular haeme levels. Thus, increased HO activity could directly degrade the haeme located in the active site of NOS, reducing the available haeme amount for new synthesis of NOS (69). Finally, CO and NO both modulate the activity of soluble guanylate cyclase (18, 44, 45, 72) and, in tissues in which both NOS and HO are expressed, HO activity can suppress cGMP levels (45, 73), interfering thus with NOS signaling at targets downstream to NOS, without changing NO bioavailability. Whether any of these mechanisms contribute to the CO-NO interaction during HF described in this study, remains to be determined.
Previous studies have shown that changes in the expression and/or function of the local GABAergic, nitrergic and or glutamate circuitry within the PVN, as revealed in these in vitro experiments, translated into changes in sympathetic activity and or blood pressure regulation in HF rats (31, 34, 40, 74–76). Thus, it is reasonable to speculate that the differences we report here in the PVN CO-NO-GABA signaling in HF rats have a functional implication within the context of neurohumoral activation during HF. Nonetheless; future studies are warranted to directly examine the effects of CO manipulations within the PVN at the whole animal level.
We acknowledge that there are several technical limitations associated to semiquantitative immunofluorescence, including variations in fixation, background contamination, signal fading and cross-reactivity of secondary antibodies, among others (see Refs. 77, 78). We have standardized a procedure aimed at minimizing, to the best of our capabilities, these limitations. This includes running all proper control groups (ie, validation of antibody specificity, omission of primary or secondary antibodies), ensuring similar background noise across specimens, and the use of sequential laser scanning along with extensive tests to avoid cross talk of fluorescent signals, critical for colocalization analysis (see more in Materials and Methods). Still, we cannot completely rule out that any of these factors affected our analysis, so these limitations need to still be considered when interpreting our results. Note however, that our interpretations and conclusions are based not a single technique, but rather on the combination of complementary approaches including immunohistochemistry, Western blotting, single-cell RT-PCR, and electrophysiology.
In summary, results from this study provide evidence to support the notion of a functional interaction between enhanced excitatory CO transmission and blunted inhibitory NO-GABA signaling in PVN VP neurons, contributing to exacerbated VP neuronal activity, and thus neurohumoral activation during HF. To what extent changes in HO/CO expression/function are dependent on other critical pathophysiological players in HF, including overactivation of the renin-angiotensin system (79, 80), oxidative stress, and/or cytokine overproduction (32, 61, 81), remains to be determined.
Acknowledgments
We thank Dr Yoichi Ueta for the kind donation of eGFP-VP rat founders.
This work was supported by the National Institutes of Health Grant R01 090948 (to J.E.S.) and by the American Heart Association Grant 12POST12060540 (to W.L.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- CNS
- central nervous system
- CO
- carbon monoxide
- CORM
- tricarbonyldichlororuthenium dimer
- carboxy-PTIO
- 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- CT
- threshold cycle
- DEPC
- diethylpyrocarbonate
- eGFP
- endogenously green-fluorescent protein
- GABA
- γ-aminobutyric acid
- HF
- heart failure
- HO
- haeme-oxygenase
- IPSC
- inhibitory postsynaptic current
- LM
- lateral magnocellular
- LNAME
- NG-nitro-larginine methyl
- MNC
- magnocellular neurosecretory cell
- nNOS
- neuronal NO synathase
- NO
- nitric oxide
- NS
- nonsignificant
- PVN
- paraventricular nucleus
- SnPP
- Tin-protoporphyrin IX
- VP
- vasopressin.
References
- 1. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. [DOI] [PubMed] [Google Scholar]
- 2. Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. [DOI] [PubMed] [Google Scholar]
- 3. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. [DOI] [PubMed] [Google Scholar]
- 4. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B [DOI] [PubMed] [Google Scholar]
- 5. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. [DOI] [PubMed] [Google Scholar]
- 6. Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol. 1993;265:R923–R928. [DOI] [PubMed] [Google Scholar]
- 7. Riegger GA, Liebau G, Bauer E, Kochsiek K. Vasopressin and renin in high output heart failure of rats: hemodynamic effects of elevated plasma hormone levels. J Cardiovasc Pharmacol. 1985;7:1–5. [DOI] [PubMed] [Google Scholar]
- 8. Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol. 1998;275:H2140–H2146. [DOI] [PubMed] [Google Scholar]
- 9. Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J. 1981;102:509–514. [DOI] [PubMed] [Google Scholar]
- 10. Hodsman GP, Kohzuki M, Howes LG, Sumithran E, Tsunoda K, Johnston CI. Neurohumoral responses to chronic myocardial infarction in rats. Circulation. 1988;78:376–381. [DOI] [PubMed] [Google Scholar]
- 11. Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730. [DOI] [PubMed] [Google Scholar]
- 12. Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. [DOI] [PubMed] [Google Scholar]
- 13. Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation. 1990;82:1724–1729. [DOI] [PubMed] [Google Scholar]
- 14. Goldsmith SR, Francis GS, Cowley AW, Jr, Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. [DOI] [PubMed] [Google Scholar]
- 15. Rouleau JL, Packer M, Moyé L, et al. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol. 1994;24:583–591. [DOI] [PubMed] [Google Scholar]
- 16. Bourque CW, Oliet SH, Kirkpatrick K, Richard D, Fisher TE. Extrinsic and intrinsic modulatory mechanisms involved in regulating the electrical activity of supraoptic neurons. Ann NY Acad Sci. 1993;689:512–519. [DOI] [PubMed] [Google Scholar]
- 17. Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. [DOI] [PubMed] [Google Scholar]
- 18. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 19. van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. [DOI] [PubMed] [Google Scholar]
- 20. Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol. 1997;499(pt 3):733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol. 2007;581:221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol. 1995;484(pt 2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park JB, Skalska S, Stern JE. Characterization of a novel tonic γ-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. [DOI] [PubMed] [Google Scholar]
- 24. Randle JC, Renaud LP. Actions of γ-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol. 1987;387:629–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol. 2004;84:197–215. [DOI] [PubMed] [Google Scholar]
- 26. Kadekaro M. Nitric oxide modulation of the hypothalamo-neurohypophyseal system. Braz J Med Biol Res. 2004;37:441–450. [DOI] [PubMed] [Google Scholar]
- 27. Reis WL, Giusti-Paiva A, Ventura RR, et al. Central nitric oxide blocks vasopressin, oxytocin and atrial natriuretic peptide release and antidiuretic and natriuretic responses induced by central angiotensin II in conscious rats. Exp Physiol. 2007;92:903–911. [DOI] [PubMed] [Google Scholar]
- 28. Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Carbon monoxide and nitric oxide interactions in magnocellular neurosecretory neurones during water deprivation. J Neuroendocrinol. 2015;27:111–122. [DOI] [PubMed] [Google Scholar]
- 29. Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1815–R1822. [DOI] [PubMed] [Google Scholar]
- 30. Stern JE, Zhang W. Cellular sources, targets and actions of constitutive nitric oxide in the magnocellular neurosecretory system of the rat. J Physiol. 2005;562:725–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension. 2011;58:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res. 1996;734:109–115. [PubMed] [Google Scholar]
- 34. Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. [DOI] [PubMed] [Google Scholar]
- 35. Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. [DOI] [PubMed] [Google Scholar]
- 36. Han F, Lu YM, Hasegawa H, et al. Inhibition of dystrophin breakdown and endothelial nitric-oxide synthase uncoupling accounts for cytoprotection by 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazo lylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) in left ventricular hypertrophied Mice. J Pharmacol Exp Ther. 2010;332:421–428. [DOI] [PubMed] [Google Scholar]
- 37. Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. [DOI] [PubMed] [Google Scholar]
- 38. Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol. 2011;106:1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci. 2013;33:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1006–R1015. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience. 2003;118:585–601. [DOI] [PubMed] [Google Scholar]
- 42. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2332–H2341. [DOI] [PubMed] [Google Scholar]
- 43. Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maines MD. Carbon monoxide: an emerging regulator of cGMP in the brain. Mol Cell Neurosci. 1993;4:389–397. [DOI] [PubMed] [Google Scholar]
- 45. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. [DOI] [PubMed] [Google Scholar]
- 46. Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Enhanced expression of heme oxygenase-1 and carbon monoxide excitatory effects in oxytocin and vasopressin neurones during water deprivation. J Neuroendocrinol. 2012;24:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol. 2000;46:573–585. [PubMed] [Google Scholar]
- 48. Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci USA. 1991;88:5364–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ewing JF, Maines MD. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512–1519. [DOI] [PubMed] [Google Scholar]
- 50. Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. [DOI] [PubMed] [Google Scholar]
- 51. Ueta Y, Fujihara H, Serino R, et al. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146:406–413. [DOI] [PubMed] [Google Scholar]
- 52. Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–R1745. [DOI] [PubMed] [Google Scholar]
- 53. Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates. Compact 3rd ed San Diego, CA: Academic Press; 1997. [Google Scholar]
- 54. Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. [DOI] [PubMed] [Google Scholar]
- 55. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. [DOI] [PubMed] [Google Scholar]
- 56. Rorato R, Reis WL, de Carvalho Borges B, Antunes-Rodrigues J, Elias LL. Cannabinoid CB(1) receptor restrains accentuated activity of hypothalamic corticotropin-releasing factor and brainstem tyrosine hydroxylase neurons in endotoxemia-induced hypophagia in rats. Neuropharmacology. 2012;63:154–160. [DOI] [PubMed] [Google Scholar]
- 57. Sonner PM, Lee S, Ryu PD, Lee SY, Stern JE. Imbalanced K+ and Ca2+ subthreshold interactions contribute to increased hypothalamic presympathetic neuronal excitability in hypertensive rats. J Physiol. 2011;589:667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gomes DA, Giusti-Paiva A, Ventura RR, Elias LL, Cunha FQ, Antunes-Rodrigues J. Carbon monoxide and nitric oxide modulate hyperosmolality-induced oxytocin secretion by the hypothalamus in vitro. Biosci Rep. 2010;30:351–357. [DOI] [PubMed] [Google Scholar]
- 59. Vincent SR, Das S, Maines MD. Brain heme oxygenase isoenzymes and nitric oxide synthase are co-localized in select neurons. Neuroscience. 1994;63:223–231. [DOI] [PubMed] [Google Scholar]
- 60. Aizawa T, Ishizaka N, Taguchi J, et al. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension. 2000;35:800–806. [DOI] [PubMed] [Google Scholar]
- 61. Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guggilam A, Haque M, Kerut EK, et al. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–H609. [DOI] [PubMed] [Google Scholar]
- 63. Kang YM, Ma Y, Zheng JP, et al. Brain nuclear factor-κ B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu XJ, Suo YP, Qi J, et al. Interaction between AT1 receptor and NF-κB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol. 2013;13:381–390. [DOI] [PubMed] [Google Scholar]
- 66. Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic Res. 1999;31:459–475. [DOI] [PubMed] [Google Scholar]
- 67. Hartsfield CL. Cross talk between carbon monoxide and nitric oxide. Antioxid Redox Signal. 2002;4:301–307. [DOI] [PubMed] [Google Scholar]
- 68. Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004;554:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. [DOI] [PubMed] [Google Scholar]
- 70. McMillan K, Bredt DS, Hirsch DJ, Snyder SH, Clark JE, Masters BS. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci USA. 1992;89:11141–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627–6631. [DOI] [PubMed] [Google Scholar]
- 72. Snyder SH, Bredt DS. Biological roles of nitric oxide. Sci Am. 1992;266:68–71, 74–67. [DOI] [PubMed] [Google Scholar]
- 73. Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. [DOI] [PubMed] [Google Scholar]
- 74. Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. [DOI] [PubMed] [Google Scholar]
- 75. Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H527–H537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zinchuk V, Wu Y, Grossenbacher-Zinchuk O. Bridging the gap between qualitative and quantitative colocalization results in fluorescence microscopy studies. Sci Rep. 2013;3:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Teerlink JR. Neurohumoral mechanisms in heart failure: a central role for the renin-angiotensin system. J Cardiovasc Pharmacol. 1996;27(suppl 2):S1–S8. [DOI] [PubMed] [Google Scholar]
- 80. Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond). 2014;126:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koba S, Hisatome I, Watanabe T. Central command dysfunction in rats with heart failure is mediated by brain oxidative stress and normalized by exercise training. J Physiol. 2014;592:3917–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]