Abstract
In homeotherms, injury to the brain, such as a penetrating wound, increases microglial cytokine expression and astroglial aromatase (estrogen synthase). In songbirds, injury-induced synthesis of estrogens is neuroprotective as aromatase inhibition and replacement with estradiol (E2) exacerbates and mitigates the extent of damage, respectively. The influence of induced aromatization on inflammation, however, remains unstudied. We hypothesized that injury-induced aromatization, via E2 synthesis, may affect neuroinflammation after a penetrating brain injury. Using adult zebra finches, we first documented an increase in the transcription of cytokines but not aromatase, 2 hours after the injury. Twenty-four hours after the injury, however, aromatase was dramatically elevated and cytokine expression had returned to baseline, suggesting that aromatization may be involved in the decrease of cytokines and neuroinflammation. In two subsequent experiments, we tested the influence of the inhibition of induced aromatization and aromatase inhibition with concomitant central E2 replacement on the transcription of the cytokines TNF-α, IL-1β, and IL-6, the enzyme cyclooxygenase-2 (cox-2), and its product prostaglandin E2 (PGE2). Administration of fadrozole, an aromatase inhibitor, caused a sustained elevation of IL-1β in females and TNF-α, cox-2, and PGE2 in both sexes. This prolonged neuroinflammation appears to be due to a failure to synthesize E2 locally because intracranial E2 replacement lowered IL-1β in females, TNF-α in males, and cox-2 and PGE2 in both sexes. IL-6 was not affected by injury, aromatase inhibition, or E2 replacement in either sex. These data suggest that E2 synthesis after a penetrating brain injury is a potent and inducible anti-inflammatory signal, with specific modulation of discrete cytokine signaling.
Estrogens like 17β-estradiol (E2) organize and activate the vertebrate brain (1–3) and are potent regulators of many neural processes across vertebrates (4–6). E2 also regulates peripheral physiology with dramatic effects on metabolism, muscle function, and the immune system (7, 8). Indeed, premenopausal women are at a lower risk for several diseases including cardiac disease, osteoporosis, and stroke, compared with age-matched men and postmenopausal women not on hormone replacement therapy, suggesting that E2 may be protective centrally and peripherally (9, 10).
E2 is synthesized in multiple tissues including but not limited to the ovary, adipose, and liver (8). Several vertebrates can also synthesize E2 in the brain, via expression of the enzyme aromatase (estrogen synthase) in neurons at discrete neural loci (11–15). However, in songbirds and mammals, after an injury to the brain, such as a penetrating stab wound, aromatase is induced in reactive astrocytes at the site of damage (16–19). In songbirds, the induction of aromatase in astroglia is particularly dramatic (19–21), with demonstrated neuroprotective effects such as decreases in injury-induced apoptosis (22–24), and increases in cyto- and neurogenesis (25).
Injury to the homeotherm brain also causes neuroinflammation, characterized in part by the rapid synthesis and secretion of proinflammatory cytokines like TNF-α, and interleukins, IL-1β and IL-6 (26–30). The secretion of these cytokines induces cyclooxygenase-2 (cox-2), which synthesizes the prostanoid prostaglandin E2 (PGE2) (31, 32). PGE2 is responsible for classic signs of tissue damage, including but not limited to edema, erythema, and pain (33). PGE2 also can recruit macrophages and neutrophils, activate microglia, and increase inflammatory processes by amplifying proinflammatory cytokine and chemokine secretion (31, 33, 34). Inflammatory signaling can have beneficial and detrimental effects that may differ between the acute and chronic periods after trauma. Specifically, although the inflammatory response can initially promote phagocytosis and repair, the chronic expression of inflammatory signaling can activate toxic cascades and, in the central nervous system (CNS), can promote further neurodegeneration (35–38).
Previous studies have revealed a strong interaction between estrogens and the innate immune system. Specifically, TNF-α and IL-1β increase in women with low circulating E2 due either to natural (39) or surgical menopause (40). Resident macrophages isolated from female mice are more plentiful and express higher levels of toll-like receptors, compared with males (41), perhaps suggesting a higher sensitivity of the female immune system. Indeed, ovariectomized mice have higher neural cytokine expression after peripheral endotoxin treatment relative to sham controls (42), suggesting an anti-inflammatory role for circulating E2.
Whereas interactions between the endocrine and immune system have been studied in songbirds (43–45), surprisingly little is known about how E2 affects the innate immune system. Given the interactions among a penetrating brain injury, cytokine expression and E2-dependent neuroprotection, we hypothesized an influence of injury-induced aromatization on neuroinflammation. Because zebra finches (Taeniopygia guttata) of both sexes demonstrate rapid increases in cytokines (20, 46) and a robust, sustained, and reliable induction of glial aromatase (21, 46) after a penetrating brain injury we hypothesized that E2 synthesis, via aromatization, may affect neuroinflammation in males and females of this species. First, we assessed the time course of cytokine and aromatase induction. Using time points established from this experiment, we tested the effect of aromatase inhibition and E2 replacement on the transcription of the proinflammatory cytokines TNFα, IL-1β, and IL-6. Toward understanding the downstream effects of cytokine expression, in these same subjects, we also assessed the effects of injury-induced aromatization and E2 on the expression of cox-2 and the central levels of PGE2.
Materials and Methods
Adult male and female zebra finches (>100 d after hatch) were obtained from a commercial breeder and housed in same-sex aviaries at The American University, under a 12-hour light, 12-hour dark cycle, with food and water provided ad libitum. The American University Institutional Use and Animal Care Committee approved all animal procedures.
Surgery and tissue preparation
In all experiments, subjects served as their own controls (within animal design) with experimental treatments administered to contralateral brain hemispheres. Within each experiment (see below), all treatments were counterbalanced between the left and the right telencephalic hemispheres. As previously published (20, 22–25), animals were anesthetized and positioned in a stereotaxic apparatus with the head angled at 45°. The cranium was exposed, a craniotomy was performed, and a 22-gauge Hamilton syringe (Hamilton Co) was targeted toward the entopallial nucleus, 2 mm anterior to the pineal gland, 3 mm lateral to the midline, and 3 mm ventral to the brain surface (see 22, 23). This form of injury is similar to penetrating brain injury used in other laboratories (47–51). Two or 24 hours after the injury, birds were decapitated, and the telencephalon was detached from the rest of the brain. To concentrate the contribution of the injury tract to the tissue processed, the anterior telencephalon approximately 4 mm anterior to the pineal (52) was discarded. The injury site was always in the posterior lobes and, based on previous studies, is estimated to span about 10%–20% of the remaining telencephalic volume (23–25). The posterior telencephalon was separated across the midline, weighed, and then stored at −80°C. Just prior to the procedures described below, samples were homogenized in 1 mL of 0.1M phosphate buffer.
Experiments
Experiment 1
Time course of injury-induced changes in the expression of TNF-α, IL-1β, IL-6, and aromatase mRNA after a neural injury
To determine the time course of cytokine and aromatase induction, 20 adult zebra finches (10 per sex) received a unilateral brain injury as described above. A 5-μL injection of a steroid suspension vehicle (SSV) (22, 25) was administered through the syringe over 60 seconds. Control hemispheres were left uninjured. Birds were either killed at 2 hours (n = 10; five per sex) or 24 hours after the injury (n = 10; five per sex), and the tissue was collected as described above. Tissue was subsequently processed for quantitative PCR (qPCR) to measure the expression of TNF-α, IL-1β, IL-6, and aromatase (see below).
Experiment 2
Expression of TNF-α, IL-1β, IL-6, cox-2, and PGE2 after aromatase inhibition
Ten adult zebra finches (five per sex) received bilateral injuries as described above. Subjects received either 5 μL of a 10-mg/mL solution of the aromatase inhibitor fadrozole suspended in SSV (50 μg of fadrozole per injection) (23) or 5 μL of SSV into contralateral hemispheres. Animals were killed 24 hours after the injury, a time when injury-induced aromatase expression is robust and reliable (23–25). The expression of TNF-α, IL-1β, IL-6, and cox-2 was assessed using qPCR, and neural levels of PGE2 were measured using a validated enzyme immunoassay (EIA) (see below).
Experiment 3
Expression of TNF-α, IL-1β, IL-6, cox-2, and PGE2 after E2 replacement
Ten adult zebra finches (five per sex) received bilateral injuries through which either 5 μL of 10-mg/mL fadrozole suspended in SSV alone or 5 μL of the same solution containing 1 μg of E2 was administered (24). Animals were killed 24 hours after the injury, and qPCR and EIA were used to analyze the expression of TNF-α, IL-1β, IL-6, cox-2, or PGE2 as described below.
Quantitative PCR
Total RNA was isolated from 500 μL of homogenate using the RNeasy mini extraction kit (QIAGEN) according to the manufacturer's instructions. The purity and concentration were analyzed on a ND-1000 spectrophotometer (NanoDrop), and only extracts that exceeded a 260:280 ratio of 1.9 were used.
Primers for aromatase, TNF-α, IL-1β, IL-6, cox-2, and the housekeeping gene, GAPDH (see Table 1) were designed based on the known zebra finch sequence for each gene. Amplicons were run on an agarose gel to confirm the presence of a single band of the expected size (approximately). Because the size of small sequences (∼100bp) is often difficult to determine unequivocally, the bands were sequenced (Genewiz; Beckman Coulter Genomics) and compared with the zebra finch genome. We also performed a serial dilution by varying template concentration. Efficiency was assessed at greater than 100%. Melt curves of the PCR products were evaluated to confirm the specificity of primer annealing.
Table 1.
List of Primers Used for Amplification for qPCR
| Gene Symbol | Accession Number | Forward Primer (5-′–3-′) | Reverse Primer (5-′–3-′) |
|---|---|---|---|
| Aromatase | AH008861.2 | CTCCACCGACAAAAATCCAC | GGGTTTCCAGGACCATCT TT |
| GAPDH | NM_OO1198610.1 | TGCTGCTCAGAACATTATCCC | TTTCCCACAGCCTAGCAGCT |
| TNF-α | XM_002197321.2 | TGTCCCATCTGC ACCACCTTCTTA | ATTCCCTTCCATCTGGCTTCTGT |
| IL-1β | XM_002195564.2 | TTCCGGTGCATCAGAGGCAGTTAT | GCACGAAGCACTTGTGGTCAATGT |
| IL-6 | XM_002191284.2 | CGTCTGCCAGAACAGCATGGAAAT | TATCCTCATTAAAGCCGGCGAGCA |
The expression of target transcripts was analyzed using 96-well optical plates and the SuperScript Sybr Green one-step quantitative RT-PCR kit (Invitrogen). Each sample was amplified in triplicate, and each well (reaction) had 75 ng total RNA in a 15-μL reaction. In each experiment, samples for one target gene were run on a single plate. To permit and assess for plate-to-plate variation (53) an interrun calibrator was used on each plate, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was analyzed using a one-way ANOVA to ensure that GAPDH did not differ between groups (data not shown). A no-template control and a no-reverse transcriptase control were run on each plate to eliminate the possibility of contaminants.
Prostaglandin E2 enzyme immunoassay
A combined ether and solid-phase extraction were performed on 300 μL of homogenate to maximize the recovery of steroids from the zebra finch brain as previously described (54, 55).
Ether extraction
Homogenate was extracted with 2 mL of diethyl ether (Sigma-Aldrich), vortexed on a low setting for 30 seconds, and centrifuged at 2100 rpm for 5 minutes at 4°C. After the centrifugation, the mixture was placed in a MeOH/dry ice bath to freeze the aqueous phase, and the organic phase was poured into a clean glass tube (Kimble Chase). This was repeated for a total of three times. After the third extraction, samples were dried down under a stream of air until fully evaporated. Then approximately 50 μL of 1:1 mixture of MeOH/CH2Cl2 was dripped down the side of the tubes and evaporated under air. Fully dried down samples were stored at −80°C until solid-phase extraction.
Solid-phase extraction
Ether-extracted samples (see above) were resuspended in 300 μL of 1.0 M acetate buffer according to the manufacturer's instructions (Arbor Assays). Solid-phase extraction was performed using a Visiprep SPE vacuum manifold (Supelco) and 1 mL per 500 mg C18 cartridges (Agilent). Columns were conditioned under vacuum pressure by adding 500 μL ethanol, followed by 750 μL of ultrapure water. Cartridges were not allowed to dry. Samples were then applied to an SPE cartridge and eluted into a waste container and then were washed with 250 μL of ultrapure water. At this time, the waste bin was replaced with the final collection glass tubes (Kimble Chase), and 300 μL of ethyl acetate containing 1% methanol was applied to the column and was eluted by gravity. Samples were then stored at −80°C.
PGE2 enzyme immunoassay
PGE2 was measured using a commercial kit developed by Cayman Chemical. Cayman Chemical reports a high specificity for PGE2 and low cross-reactivity (<0.01%) with similar prostaglandins or prostaglandin metabolites and a detection range from 7.8 to 1000 pg/mL. On the day of the assay, samples were dried down under air stream, according to the manufacturer's instructions (Arbor Assays). Samples were then resuspended in 300 μL EIA buffer (Cayman Chemical) and assayed at a 1:5 dilution in triplicate according to the manufacturer's instructions.
PGE2 immunoassay validation
To validate the specificity of the assay in the zebra finch, a serial dilution was first performed on zebra finch brain samples. Three dilutions (1:1, 1:3, 1:5 dilution) were chosen and the measured concentrations decreased by expected proportion. Second, the accuracy of the kit was assessed by spiking brain tissue samples with known amounts of PGE2. Before ether and solid-phase extraction, spiked samples were also run to estimate recovery. Two samples were spiked with radioinert PGE2 to 125 pg/mL. There was a high correlation between expected and observed values, and the recovery rate was assessed at 87%. Finally, as part of a different study and as an in vivo assay validation, injections of a cox-2 inhibitor significantly decreased brain levels of PGE2 compared with injected controls (P = .04) (56).
Statistical analyses
Quantitative PCR Data
The delta threshold cycle number (δCt) method was used for quantification using the detection threshold (Ct) for each target gene less the Ct for the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All statistical analyses were performed on δCt, and are presented as means ± SEM in both the text of the results and in the figures (see Figures 1, 3, and 6). Some data are shown as fold change + SEM using the formula 2-δ(δCT), and the normalization of the control condition δCt value is set to 1 (20, 46, 57). In this case, each individual experimental data point is expressed as a function of the control condition mean, resulting in a distribution (with variability). Whereas this permits an intuitive appreciation of the difference in expression between treatment and control hemispheres, the reader is reminded that it obscures any baseline differences across control conditions and groups (see Figures 2, 4, and 7).
Figure 1.
Levels of TNF-α, IL-1β, and aromatase mRNA relative to GAPDH (δCt values ± SEM) in adult male and female zebra finches from experiment 1. In both males and females, TNF-α (A) and IL-1β mRNA (B) is increased 2 hours after the injury but not at 24 hours after the injury. At 24 hours after the injury, females have higher TNF-α expression relative to males (A). At 2 hours, males have increased IL-1β mRNA relative to injured females (B). Aromatase mRNA is increased at 24 hours but not at 2 hours after the injury (C). *, P < .05. Note: y-axes do not begin at zero.
Figure 3.
Levels of TNF-α, IL-1β, and cox-2 mRNA relative to GAPDH (δCt values ± SEM) in adult male and female zebra finches from experiment 2. Only significant gene targets are shown. Fadrozole (FAD) administration increased TNF-α mRNA in both males and females; however, males have more TNF-α, regardless of treatment (A). Fadrozole treatment after the injury increased IL-1β mRNA only in females (B). Fadrozole administration increased cox-2 mRNA in both males and females; however, females have higher cox-2 levels, regardless of treatment (C). *, P < .05. Note: y-axes do not begin at zero.
Figure 6.
Levels of TNF-α, IL-1β, and cox-2 mRNA relative to GAPDH (δCt values ± SEM) in adult male and female zebra finches from experiment 3. Only significant gene targets are shown. E2 replacement decreased TNF-α in males (A) and IL-1β in females (B), and cox-2 in both sexes (C). In addition, males have higher overall levels of TNF-α (A) and cox-2 (C). Females had higher levels of and IL-1β, regardless of treatment (B). *, P < .05. Note: y-axes do not begin at zero. FAD, fadrozole.
Figure 2.
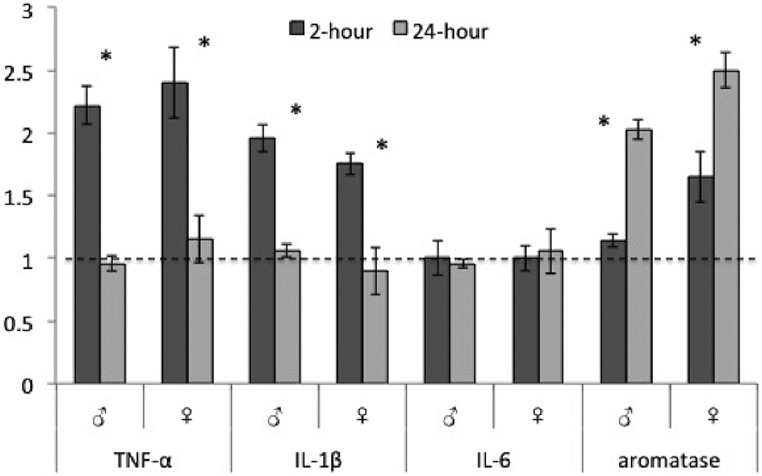
Levels of cytokines and aromatase mRNA relative to GAPDH (fold change values ± SEM) in adult male and female zebra finches. TNF-α and IL-1β mRNA are up-regulated at 2 hours after the injury in both males and females; however, all cytokines are at control levels 24 hours after the injury. At this time point, aromatase transcript is increased in both males and females. Dashed line represents uninjured controls (controls set to 1 for fold change calculation). *, P < .05.
Figure 4.
Levels of cytokine mRNA relative to GAPDH (fold change values ± SEM) after injury with fadrozole administration in adult male and female zebra finches. Fadrozole administration resulted in sustained elevation of TNF-α and cox-2 in both males and females compared with injured controls, with males having a more pronounced increase of TNF-α. Aromatase inhibition also resulted in a sustained elevation of IL-1β but is evident only in females. Dashed line represents injured controls (controls set to 1 for fold change calculation). *, P < .05.
Figure 7.
Levels of cytokine mRNA relative to GAPDH (fold change values ± SEM) after an injury with fadrozole administration and central E2 replacement in adult male and female zebra finches. Injury with central E2 replacement rescued sustained TNF-α elevation after fadrozole in males and sustained IL-1β in females. It also rescued sustained cox-2 elevation in both sexes. Dashed line represents injured aromatase inhibited fadrozole controls (controls set to 1 for fold change calculation). *, P < .05.
δCt-values for TNF-α, IL-1β, IL-6, and aromatase were analyzed separately using a three-way ANOVA with treatment, time, and sex (experiment 1) and a two-way ANOVA with treatment and sex (experiments 2 and 3) as the main variables. In all experiments, treatment was coded as a within-subject variable. The source of significant main effects was queried using Tukey-Kramer post hoc analysis, and significant interactions were assessed with Fisher least significant differences pairwise comparisons.
PGE2 enzyme immunoassay
To account for brain weight, data obtained in picograms per milliliter were converted to picograms per milligram based on the wet weight of samples collected at the time the animals were killed. A two-way ANOVA was run with variables of treatment and sex, and treatment was coded as a within-subject variable, and post hoc analysis was conducted as described above.
Results
Experiment 1: Time course of injury-induced changes in the expression of TNF-α, IL-1β, IL-6, and aromatase mRNA after neural injury
Tumor necrosis factor-α
Statistical analysis revealed the main effects of treatment, time, and sex. Overall, TNF-α expression was higher in the injured relative to the uninjured lobe (F [1, 8] = 38.51, P < .01), at 2 hours relative to 24 hours after the injury (time [F [1, 8] = 70.30], P = .01), and in females relative to males (F [1, 8] = 8.715, P = .01). We also found a significant interaction between treatment × time (F [1, 8] = 36.167, P < .01). This interaction reflects a higher expression in the injured relative to uninjured lobes at 2 (4.95 ± 0.26 vs 6.67 ± 0.19, respectively; P < .01) but not at 24 hours after the injury (7.71 ± 0.24 vs 7.68 ± 0.24, respectively; P = .88). There was also a significant interaction of sex × time (F [1, 8] = 8.76, P < .01). This interaction reflects similar levels of expression across sexes at 2 hours (5.81 ± 0.32 vs 5.82 ± 0.40, P = .96; males vs females) but a lower expression in the male relative to the female brain at 24 hours (8.27 ± 0.28 vs 7.16 ± 0.19, P = .01; males vs females). There were no other significant interactions (treatment × sex: F [1, 8] = 1.34, P = .23; treatment × sex × time: F [1, 8] = 0.064, P = .80). Means ± SEM of δCt (in which a lower number equals a higher expression) are shown in Figure 1A. Fold change data are depicted in Figure 2.
Interleukin-1β
In a largely similar pattern, IL-1β transcription varied systematically over treatment (F [1, 8] = 12.45, P < .01) and time (F [1, 8] = 16.49, P < .01) but not across sex (F [1, 8] = 1.01, P = .33). We also found a significant interaction of treatment × time (F [1, 8] = 16.23, P < .01). As above, this interaction reflects a higher expression in the injured relative to uninjured lobe at 2 hours (7.61 ± 0.31 vs 8.59 ± 0.35, respectively; P < .01) but not at 24 hours (9.19 ± 0.72 vs 9.05 ± 0.21, respectively; P = .81) after the injury. There also was a significant interaction of sex × time (F [1, 8] = 24.24, P < .01). This interaction reflects a higher expression in males relative to females at 2 hours after the injury (7.3 ± 0.29 vs 8.39 ± 0.28, P < .01; males vs females) but not 24 hours after the damage (9.56 ± 0.11 vs 9.6 ± 0.19, P = .64; males vs females). No other significant interactions were found (treatment × sex: (F [1, 8] = 0.51, P = .48; treatment × sex × time: (F [1, 8] = 0.12, P = .91). Means ± SEM of delta]Ct (in which the lower number equals a higher expression) are shown in Figure 1B and fold change data are depicted in Figure 2.
IL-6
In contrast to the other cytokines investigated, no significant main effects or interactions on IL-6 expression were detected (treatment: F [1, 8] = 1.008, P = .33; time: F [1, 8] = 0.24, P = .64; sex: F [1, 8] = 0.009, P = .93) or interactions (treatment × time: F [1, 8] = 1.28, P = .28; treatment × sex: F [1, 8] = 0.033, P = .89; sex × time: F [1, 8] = 0.30, P = .59; treatment × sex × time (F [1, 8] = 0.57, P = .46; see Figure 2).
Aromatase
Three-way ANOVA revealed significant effects of treatment (F [1, 8] = 39.238, P < .01) and time (F [1, 8] = 381.09, P < .01) but not sex (F [1, 8] = 0.57, P = .46). We also detected a significant interaction of treatment × time (F [1, 8] = 15.35, P < .01) but no other interactions (treatment × sex: F [1, 8] = 0.21, P = .89; sex × time: F [1, 8] = 0.59, P = .81; treatment × sex × time: (F [1, 8] = 1.91, P = .19). The interaction of treatment × time is driven by higher aromatase in injured lobes at 24 hours but not at 2 hours after the injury (7.16 ± 0.14 vs 10.81 ± 0.08, P < .01; 24 hours vs 2 h). Means ± SEM of δCt (in which a lower number equals a higher expression), and fold change data are depicted in Figures 1C and 2, respectively.
Experiment 2: Expression of TNF-α, IL-1β, IL-6, cox-2, and PGE2 after aromatase inhibition
Tumor necrosis factor-α
At 24 hours after the injury, a time when aromatase, but not cytokines, is elevated (experiment 1), we found significant effects of aromatase inhibition (F [1, 8] = 29.72, P = .01) and sex (F [1, 8] = 73.83, P < .01) but no interaction (treatment × sex: F [1, 8] = 2.847, P = .13). Fadrozole administration increased TNF-α mRNA expression 24 hours after injury compared with control SSV treatment (4.71 ± 0.18 vs 5.45 ± 0.168), independent of sex. Overall, males had higher TNF-α than females, regardless of treatment (4.56 ± 0.13 vs 5.61 ± 0.15; males vs females). Means ± SEM of δCt and fold changes are presented in Figures 3A and 4, respectively.
Interleukin-1β
Two-way ANOVA indicated a main effect of treatment (treatment (F [1, 8] = 24.52, P < .01), sex (F [1, 8] = 7.71, P < .01), and their interaction (F [1, 8] = 27.45, P < .01). IL-1β expression was higher in injured lobes treated with fadrozole compared with vehicle, independent of sex (8.04 ± 0.32 vs 8.63 ± 0.12). Furthermore, females had a higher IL-1β expression than males independent of treatment (7.69 ± 0.21 vs 8.98 ± 0.11). The treatment × sex interaction reflects higher IL-1β expression in the lobe treated with fadrozole compared with SSV in females (7.05 ± 0.13 vs 8.34 ± 0.13; P = .01; fadrozole vs SSV) but not in males (9.03 ± 0.20 vs 8.93 ± 0.13, P = .74; fadrozole vs SSV). Means ± SEM of δCt and fold changes are presented in Figures 3B and 4, respectively.
Interleukin-6
As in experiment 1, we failed to detect any differences in IL-6 expression. Two-way ANOVA indicated no significant effects (treatment: F [1, 8] = 0.69, P = .43; sex: F [1, 8] = 0.59, P = .46) or interactions (treatment × sex: F [1, 8] = 2.09, P = .19; see Figure 4).
Cyclooxygenase-2
We found significant effects of treatment (F [1, 8] = 17.0, P < .01) and sex (F [1, 8] = 27.5, P < .01) on cox-2 with no interaction of treatment × sex (F [1, 8] = 0.83, P = .83). Cox-2 expression was higher in lobes treated with fadrozole compared with SSV (11.49 ± 0.42 vs 12.76 ± 0.45) independent of sex. Overall, females had higher cox-2 expression compared with males independent of treatment (10.95 ± 0.31 vs 12.30 ± 12.51). Means ± SEM of δCt and fold changes are presented in Figures 3C and 4, respectively.
Prostaglandin E2
Inhibition of aromatase increased PGE2 levels, with a main effect of treatment (F [1, 8] = 33.8, P < .01) and its interaction with sex (F [1, 8] = 9.30 P < .01) but not sex alone (F [1, 8] = 1.09 P = .32). PGE2 levels were higher in lobes treated with fadrozole compared with SSV (27.53 ± 3.47 vs 7.59 ± 1.16, respectively), independent of sex. The interaction of treatment × sex is due to lower PGE2 expression after an injury with SSV in females compared with males (3.97 ± 0.48 vs 11.21 ± 0.34 pg/mg, P < .01; females vs males) but higher expression in females compared with males after an injury with fadrozole (34.38 ± 6.17 vs 20.69 ± 1.95, P = .05; females vs males). Data, presented in picograms per milligram, are shown in Figure 5.
Figure 5.
Levels of PGE2 after injury with fadrozole (FAD) administration in adult male and female zebra finches (A) or after injury with fadrozole administration and central E2 replacement (B) in adult male and female zebra finches. Fadrozole administration resulted in increased levels of PGE2 in both male and females compared with injured controls. Injury with central E2 replacement rescued PGE2 levels in females, and there was a trend for males. *, P < .05.
Experiment 3: Expression of TNF-α, IL-1β, IL-6, cox-2, and PGE2 after E2 replacement
Tumor necrosis factor-α
Replacement with E2 did not affect TNF-α expression (F [1, 8] = 4.148, P = .76), but significant effects of sex (F [1, 8] = 18.82, P < .01) and its interaction with treatment were found (F [1, 8] = 7.87, P = .02). Overall, TNF-α expression was higher in males relative to females (4.63 ± 0.24 vs 6.16 ± 0.42, respectively), independent of treatment. The interaction of treatment and sex reflects that E2 replacement compared with just aromatase inhibition lowered TNF-α expression in males (4.99 ± 0.29 vs 4.01 ± 0.33; P = .02; E2 vs fadrozole) but had no such effect in females (6.11 ± 0.27 vs 6.22 ± 0.11; P = .63; E2 vs fadrozole). Means ± SEM of δCt and fold changes are presented in Figures 6A and 7, respectively.
Interleukin-1β
IL-1β expression showed no effect of E2 replacement (F [1, 8] = 4.30, P = .07) but an effect of sex (F [1,8] = 20.09, P < .01) and its interaction with treatment (F [1, 8] = 6.12, P = .03). Overall, females had higher IL-1β expression compared with males independent of treatment (8.07 ± 0.18 vs 9.84 ± 0.17). The interaction of treatment × sex is driven by females (8.46 ± 0.08 vs 7.59 ± 0.26, P = .03; E2 vs fadrozole) but not males (9.98 ± 0.29 vs 9.81 ± 0.22, P = .78; E2 vs fadrozole), showing decreases in IL-1β mRNA after an injury with E2 replacement, compared with fadrozole-injured birds. Means ± SEM of δCt and fold changes are presented in Figures 6B and 7, respectively.
Interleukin-6
Once again, no significant effects on IL-6 expression were found (treatment: F [1, 8] = 0.94, P = .36; sex: F [1, 8] = 2.65, P = .14; treatment × sex: F [1, 8] = 0.27, P = .13; see Figure 7).
Cyclooxygenase-2
Replacement with E2 after an injury affected cox-2 expression with significant effects of treatment (F [1, 8] = 52.8, P < .01), sex (F [1, 8] = 77.5, P < .01), and a significant interaction of treatment × sex (F [1, 8] = 25.2, P < .01). Both sexes showed decreases in cox-2 levels after an injury with E2 treatment compared with fadrozole alone (11.75 ± 0.26 vs 10.63 ± 0.43, respectively). In addition, males had higher cox-2 expression compared with females independent of treatment (10.27 ± 0.31 vs 11.11 ± 0.20). The interaction of these variables reflects a marginally stronger decrease following E2 replacement compared with fadrozole in males (11.07 vs 10.26, respectively; P = .05; E2 vs fadrozole) relative to that seen in females (12.42 ± 0.3 vs 11.9 ± 0.17, respectively, P = .07; E2 vs fadrozole). Means ± SEM of δCt and fold changes are presented in Figures 6C and 7, respectively.
Prostaglandin E2
In partial agreement with the data above, PGE2 levels were affected by E2 replacement (F [1, 8] = 29.5 P < .01) and sex (F [1, 8] = 6.58 P < .03) but not by the interaction of these variables (F [1, 8] = 3.78 P < .08). PGE2 levels were lower in lobes receiving E2 replacement compared with just fadrozole (8.80 ± 1.27 vs 21.79 ± 2.28, respectively) independent of sex. Additionally, independent of treatment, females had higher levels of PGE2 compared with males (18.81 ± 3.45 vs 11.77 ± 2.12). Data, presented in picograms per milligram, are shown in Figure 5.
Discussion
In adult zebra finches of both sexes, we found that postinjury elevation of TNF-α and IL-1β was low when aromatase was elevated (experiment 1), remained elevated upon pharmacological inhibition of aromatase activity (experiment 2), and did so in an E2-dependent manner (experiment 3). In addition, inhibition of induced aromatase sustained the expression of, whereas replacement with E2 appeared to lower, cox-2 and PGE2 expression, respectively. Most effects were apparent in both sexes except that E2 replacement decreased PGE2 levels in females, but its effect in males was not significant (P = .09). These data strongly support the idea that E2, synthesized in response to brain damage, is a potent anti-inflammatory signal that may prevent the CNS from the effects of chronic neuroinflammation.
In the zebra finch, there is extensive documentation of injury and inflammation-dependent aromatase expression in cells of astrocytic morphology (18, 19, 23) and those that coexpress the astrocytic marker vimentin (20–22, 24). Importantly, these changes in astrocytic aromatase expression are in excellent agreement with penetrating brain injury and inflammation-induced increases in aromatase transcription (20, 21). Thus, we have good reason to believe that the observed changes in aromatase transcription and the manipulation of its activity in the current set of studies reflect the injury-dependent expression of this enzyme in reactive astrocytes around the site of damage that has been reported over a decade (20, 21, 46). However, because the present studies rely on homogenates from telencephalic regions that express abundant neuronal aromatase, we cannot exclude this source of E2 as a contributor to the effects described. Further studies that restrict the samples to just the injured entopallium (where aromatase is exclusively glial) are necessary to unequivocally test the specific effects of astrocytic aromatase to neuroinflammation.
Similarly, whereas measurement of cytokine expression in the current study was limited to transcript, we hypothesize that these cytokines are indeed translated into protein and secreted. Administration of fadrozole increased, and replacement with E2 lowered, the expression of cox-2 and PGE2, respectively. There is excellent agreement between the expression of cytokines and that of cox-2 in the present studies. Given these similarities and because cox-2 expression and activity represents a point farther downstream in signaling, (31–33), it is very likely that the changes in cytokine transcription measured in the current studies do result from the activity of multiple elements of the inflammatory signaling cascade. Indeed, the measured changes in PGE2 protein are strong evidence of robust cytokine secretion and activation of the inflammatory cascade including the expression and activity of cox-2. Taken together, the data strongly suggest that injury-induced E2 synthesis is a potent modulator of neuroinflammation associated with brain damage from a penetrating brain injury in the finch brain.
There is substantial evidence supporting a role for circulating E2 as an anti-inflammatory in vertebrates. Indices of inflammation are higher in postmenopausal women and ovariectomized mice compared with premenopausal, age-matched controls and intact animals, respectively. Specifically, expression and secretion of TNF-α, IL-1β, and IL-6 are higher at times of low circulating E2 relative to controls, as is the expression of their cognate receptors (39). The present data extend these findings to the brain by demonstrating a role for injury-induced aromatization within the CNS, one that involves a potent inhibition of multiple components of the inflammatory cascade within neural tissue.
Interestingly, although similar in general, we found some intriguing differences in the anti-inflammatory effects of E2 across sexes. When aromatase is inhibited, females have a prolonged elevation of TNF-α and IL-1β, whereas only TNF-α remains high in males (Figure 4). In partial agreement, E2 administration lowers TNF-α in males and IL-1β in females but not vice versa (Figure 7). Previous studies have hypothesized that cytokines may serve different biological functions in men and women (58), and the rate of injury-induced aromatase expression differs between sexes in songbirds (21). Thus, it is likely that E2 manipulation may affect inflammation in a sexually differentiated manner. Experiments varying severities of injury and time points of injury need to be explored to increase confidence in this interpretation. Furthermore, no experiments in the current study detected differences in IL-6 in either sex. Although we did not see changes in IL-6, previous studies have shown that IL-6 protein peaks 4 hours after penetrating brain injury (20). It is possible that IL-6 would have been affected by injury or treatment manipulation at a different time point. Alternatively, there may be specific, highly regulated mechanisms promoting E2's anti-inflammatory properties in the zebra finch brain.
It is difficult, however, to extract overall patterns regarding sex differences, response to injury or E2-dependent signaling, from these studies because only one experimental group is shared across experiments. Specifically, although the injured lobe 24 hours after the damage and the SSV lobe are similar between experiments 1 and 2, it is the fadrozole group that is similar between experiments 2 and 3. In general, however, interactions between injury-induced aromatization and inflammation appear to demonstrate strong effects on TNF-α expression in the male brain but stronger effects on IL-1β expression in the female brain. First, 24 hours after the injury, TNF-α appeared to return to lower levels in the male brain relative to the female brain (experiment 1), and the effect of E2 replacement affected TNF-α only in males (experiment 3). In contrast, the female brain responded to aromatase inhibition with higher levels of IL-1β expression compared with males (experiment 2); and IL-1β, but not TNF-α, was affected by E2 replacement (experiment 3).
Because these studies did not contain any measures of unmanipulated birds, it is difficult to know whether these differences are due to baseline differences in cytokine expression or differences in responsivity of the inflammatory cascade to injury and/or aromatization. The latter hypothesis is supported by a report of a more rapid induction of aromatase in the female brain after an injury (21), suggesting that E2-dependent effects may be temporally distinct between sexes. Potential differences in the temporal patterns of response to injury and inflammation may prove even more important in terms of the interaction between PGE2 and aromatase. In rodents, aromatase is sensitive to PGE2 both developmentally and in adulthood (59, 60), perhaps via a distinct binding site on the aromatase promoter (61). This interaction may prove key in the dynamics of injury-induced aromatase expression, the feedback effects of locally synthesized E2, and their modulation by PGE2. Current work in our laboratory is testing these hypotheses (56).
Nevertheless, in both sexes, aromatization and consequent E2 synthesis affects signaling cascades downstream of cytokine expression, as evidenced by changes in the expression of cox-2 and central PGE2. Thus, independent of the particular cytokine(s) affected, the present data suggest that the inflammatory cascade is potently affected by aromatization in both sexes. Current studies in our laboratory focus on the mechanisms whereby E2 may affect microglial cytokine synthesis, and furthermore, how these cytokines may impact inflammatory cascades in neurons and other cells within the CNS.
Whereas E2 appears to be a potent, inducible anti-inflammatory after an injury, the mechanism by which it decreases cytokine activity is unknown. Microglia, the producers of cytokines after injury, contain classical estrogen receptors (ER)-α and ER-β and also the nonclassical receptor G protein-coupled receptor-30. These receptors are up-regulated after neural damage in mammals (62, 63), which may help E2 reduce cytokine production and therefore limit neural damage. However, whether passerine microglia express ERs is unclear and requires further study.
In summary, these experiments suggest that induced E2 synthesis via astrocytic aromatization is a potent anti-inflammatory in the brain. E2 synthesis may impact inflammatory expression and secretion after injury to the brain, thereby reducing the deleterious effects of chronic neuroinflammation. We believe this is the first demonstration of induced steroid synthesis acting as an anti-inflammatory in the vertebrate brain.
Acknowledgments
This work was supported by National Institutes of Health Grants 042767 and 080585.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CNS
- central nervous system
- cox-2
- cyclooxygenase-2
- Ct
- detection threshold
- δCt
- δ-threshold cycle number
- E2
- 17β-estradiol
- EIA
- enzyme immunoassay
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PGE2
- prostaglandin E2
- qPCR
- quantitative PCR
- SSV
- steroid suspension vehicle.
References
- 1. Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208(4450):1380–1383. [DOI] [PubMed] [Google Scholar]
- 2. MacLusky N, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. [DOI] [PubMed] [Google Scholar]
- 3. Schumacher M, Balthazart J. Sexual dimorphism in the hypothalamic metabolism of testosterone in the Japanese quail (Coturnix coturnix japonica). Prog Brain Res. 1984;61:53–61. [DOI] [PubMed] [Google Scholar]
- 4. Forlano PM, Sisneros JA, Rohmann KN, Bass AH. Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish. Front Neuroendocrinol. 2015;37:129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Remage-Healey L. Frank Beach Award Winner: steroids as neuromodulators of brain circuits and behavior. Horm Behav. 2014;66(3):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. [DOI] [PubMed] [Google Scholar]
- 7. Boon WC, Chow JDY, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181(08):209–232. [DOI] [PubMed] [Google Scholar]
- 8. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86(3):225–230. [DOI] [PubMed] [Google Scholar]
- 9. Brown CM, Suzuki S, Jelks KAB, Wise PM. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27(3):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830–833. [DOI] [PubMed] [Google Scholar]
- 11. Charlier TD, Po KWL, Newman AEM, Shah AH, Saldanha CJ, Soma KK. 17β-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlier TD, Newman AEM, Heimovics SA, Po KWL, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23(8):742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27(3):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32(4):532–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlinger BA, Remage-Healey L. Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol. 2013;24(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318–329. [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Segura LMM, Wozniak A, Azcoitia I, Rodriguez JRR, Hutchison REE, Hutchison JBB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89(2):567–578. [DOI] [PubMed] [Google Scholar]
- 18. Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata). J Neuroendocrinol. 2001;13(4):317–323. [DOI] [PubMed] [Google Scholar]
- 19. Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475(2):261–269. [DOI] [PubMed] [Google Scholar]
- 20. Duncan K, Saldanha CJ. Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J Neuroinflammation. 2011;8(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saldanha CJ, Burstein SR, Duncan K. Induced synthesis of oestrogens by glia in the songbird brain: EBSCOhost. J Neuroendocrinol. 2013;25(11):1032–1038. [DOI] [PubMed] [Google Scholar]
- 22. Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata). J Neurobiol. 2005;64(2):192–201. [DOI] [PubMed] [Google Scholar]
- 23. Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16(8):676–683. [DOI] [PubMed] [Google Scholar]
- 24. Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. [DOI] [PubMed] [Google Scholar]
- 25. Walters BJ, Alexiades NG, Saldanha CJ. Intracerebral estrogen provision increases cytogenesis and neurogenesis in the injured zebra finch brain. Dev Neurobiol. 2011;71(2):170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albertsson A-M, Bi D, Duan L, et al. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J Neuroinflammation. 2014;11(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anthony DC, Couch Y. The systemic response to CNS injury. Exp Neurol. 2014;258:105–111. [DOI] [PubMed] [Google Scholar]
- 28. Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelso ML, Gendelman HE. Bridge between neuroimmunity and traumatic brain injury. Curr Pharm Des. 2014;20(26):4284–4298. [PMC free article] [PubMed] [Google Scholar]
- 30. Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen C. COX-2's new role in inflammation. Nat Chem Biol. 2010;6(6):401–402. [DOI] [PubMed] [Google Scholar]
- 32. Davidson J, Abul H, Milton A, Rotondo D. Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflügers Arch Eur J Physiol. 2001;442(4):526–533. [DOI] [PubMed] [Google Scholar]
- 33. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar RG, Boles JA, Wagner AK. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 months postinjury. J Head Trauma Rehabil. 2015;30(6):369–381. [DOI] [PubMed] [Google Scholar]
- 36. Marciano PG, Eberwine JH, Ragupathi R, Saatman KE, Meaney DF, McIntosh TK. Expression profiling following traumatic brain injury: a review. Neurochem Res. 2002;27(10):1147–1155. [DOI] [PubMed] [Google Scholar]
- 37. Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258C: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shetty AK, Mishra V, Kodali M, Hattiangady B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci. 2014;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;21(1):90–119. [DOI] [PubMed] [Google Scholar]
- 40. Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA. 1991;88(12):5134–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118(22):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors α and β. Endocrinology. 2010;151(10):4916–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Owen-Ashley NT, Hasselquist D, Wingfield J. Androgens and the immunocompetence handicap hypothesis: Unraveling direct and indirect pathways of immunosuppression in song sparrows. Am Nat. 2004;164(4):490–505. [DOI] [PubMed] [Google Scholar]
- 44. Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii). Horm Behav. 2006;49(1):15–29. [DOI] [PubMed] [Google Scholar]
- 45. Lopes PC, Wingfield JC, Bentley GE. Lipopolysaccharide injection induces rapid decrease of hypothalamic GnRH mRNA and peptide, but does not affect GnIH in zebra finches. Horm Behav. 2012;62(2):173–179. [DOI] [PubMed] [Google Scholar]
- 46. Duncan KA, Walters BJ, Saldanha CJ. Traumatized and inflamed—but resilient: glial aromatization and the avian brain. Horm Behav. 2013;63(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghirnikar R, Lee Y, Li J, Eng L. Chemokine inhibition in rat stab wound brain injury using antisense oligodeoxynucleotides. Neurosci Lett. 1998;247(1):21–24. [DOI] [PubMed] [Google Scholar]
- 48. Barreto G, Santos-Galindo M, Diz-Chaves Y, et al. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Endocrinology. 2009;150(11):5010–5015. [DOI] [PubMed] [Google Scholar]
- 49. Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25(10):3039–3046. [DOI] [PubMed] [Google Scholar]
- 50. Garcıá-Estrada J, Luquıń S, Fernández AM, Garcia-segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci. 1999;17(2):145–151. [DOI] [PubMed] [Google Scholar]
- 51. Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628(1–2):271–278. [DOI] [PubMed] [Google Scholar]
- 52. Nixdorf-Bergweiler BE, Bischof HJ. A stereotaxic atlas of the brain of the zebra finch, Taeniopygia guttata: with special emphasis on telencephalic visual and song system nuclei in transverse and sagittal sections [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2007. Available from: http://www.ncbi.nlm.nih.gov/books/NBK2348/. [Google Scholar]
- 53. Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21(4):1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pedersen A, Saldanha CJ. Pre-treatment with a cyclooxygenase 2 (cox-2) inhibitor mitigates the injury-induced up-regulation of aromatase expression in the adult zebra finch brain. Poster presented at: Society for Neuroscience 2015, Abstract 431.16, at the 44th annual meeting for The Society for Neuroscience; 2015 Oct 17–21; Chicago, IL. [Google Scholar]
- 57. Mirzatoni A, Spence RD, Naranjo KC, Saldanha CJ, Schlinger Ba. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J Neurotrauma. 2010;27(10):1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lynch EA, Dinarello CA, Cannon JG. Gender differences in IL-1α, IL-1β, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J Immunol. 1994;153(1):300–306. [PubMed] [Google Scholar]
- 59. Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7(6):643–650. [DOI] [PubMed] [Google Scholar]
- 60. Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012;35(8):1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81(1–2):55–70. [DOI] [PubMed] [Google Scholar]
- 62. Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J Neuroendocrinol. 2012;24(1):183–190. [DOI] [PubMed] [Google Scholar]
- 63. Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol. 2015;146:3–14. [DOI] [PubMed] [Google Scholar]