Abstract
In pregnancies complicated by placental insufficiency and intrauterine growth restriction (IUGR), fetal glucose and oxygen concentrations are reduced, whereas plasma norepinephrine and epinephrine concentrations are elevated throughout the final third of gestation. Here we study the effects of chronic hypoxemia and hypercatecholaminemia on β-cell function in fetal sheep with placental insufficiency-induced IUGR that is produced by maternal hyperthermia. IUGR and control fetuses underwent a sham (intact) or bilateral adrenal demedullation (AD) surgical procedure at 0.65 gestation. As expected, AD-IUGR fetuses had lower norepinephrine concentrations than intact-IUGR fetuses despite being hypoxemic and hypoglycemic. Placental insufficiency reduced fetal weights, but the severity of IUGR was less with AD. Although basal plasma insulin concentrations were lower in intact-IUGR and AD-IUGR fetuses compared with intact-controls, glucose-stimulated insulin concentrations were greater in AD-IUGR fetuses compared with intact-IUGR fetuses. Interestingly, AD-controls had lower glucose- and arginine-stimulated insulin concentrations than intact-controls, but AD-IUGR and AD-control insulin responses were not different. To investigate chronic hypoxemia in the IUGR fetus, arterial oxygen tension was increased to normal levels by increasing the maternal inspired oxygen fraction. Oxygenation of IUGR fetuses enhanced glucose-stimulated insulin concentrations 3.3-fold in intact-IUGR and 1.7-fold in AD-IUGR fetuses but did not lower norepinephrine and epinephrine concentrations. Together these findings show that chronic hypoxemia and hypercatecholaminemia have distinct but complementary roles in the suppression of β-cell responsiveness in IUGR fetuses.
Placental insufficiency restricts the supply of oxygen and nutrients to the fetus and causes intrauterine growth restriction (IUGR) (1, 2). The resulting fetal hypoxemia and hypoglycemia provoke endocrine responses that lower plasma insulin concentrations (3–5). High norepinephrine and epinephrine concentrations are a hallmark of both human and animal IUGR fetuses (6–11). These high concentrations of catecholamines inhibit insulin secretion from pancreatic β-cells and may contribute to very low insulin concentrations in the IUGR fetus (12, 13). In addition, chronic elevation of norepinephrine has been shown to slow fetal growth and induce asymmetric growth of fetal tissues (14, 15).
Our ovine model of placental insufficiency-induced IUGR reproduces many critical pathologies observed in the human IUGR fetus, including hypoglycemia, hypoxemia, and hypoinsulinemia (16, 17). In addition, plasma norepinephrine and epinephrine concentrations are elevated in both the human and sheep IUGR fetus in late gestation (9, 18). We have shown that elevated catecholamines play a major role in suppressing fetal insulin concentrations in the IUGR sheep fetus because an acute pharmacological blockade of adrenergic receptors increased insulin concentrations (19, 20). In fact, the adrenergic blockade exposed a compensatory gain in the insulin secretion response of IUGR fetuses because glucose-stimulated insulin concentrations increased to control levels despite substantially lower β-cell mass. We also observed compensatory insulin secretion responsiveness in sheep fetuses after a continuous 1-week infusion of norepinephrine (21). These findings demonstrate some of the functional adaptations in fetal β-cells in response to chronic hypercatecholaminemia. Other adaptations that we have observed in IUGR and norepinephrine-infused fetal sheep include lower adrenergic sensitivity in adipose tissue and skeletal muscle, a phenotype that persisted in young IUGR lambs (22–24).
The evidence for catecholamine-induced adaptive responses in IUGR fetuses warrants further investigation. Thus, the present work was designed to test the two following hypotheses: 1) chronically elevated catecholamines in the IUGR fetus inhibit glucose-stimulated insulin concentrations and are responsible for compensatory insulin secretion independently of hypoglycemia, hypoxemia, and hypoinsulinemia and 2) maternal oxygen therapy will increase blood oxygen content in the IUGR fetus to mitigate catecholamine concentrations and enhance glucose-stimulated insulin concentrations. To test these hypotheses, control and IUGR sheep fetuses, produced by hyperthermia-induced placental insufficiency, were studied during late gestation after either a bilateral adrenal demedullation (AD) to eliminate adrenal catecholamine release or sham (intact) procedure. In vivo insulin secretion was determined under ambient laboratory conditions in all fetuses and after fetal arterial oxygen tension was increased to normoxemia in IUGR fetuses.
Materials and Methods
Fetal sheep preparations
Animal procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. All procedures were performed at The University of Arizona in facilities approved by the Association for Assessment and Accreditation for Laboratory Animal Care International.
Columbia-Rambouillet cross-bred ewes carrying singleton pregnancies were purchased from Nebeker Ranch. Fetal number was confirmed by ultrasonography prior to treatment assignment. Ewes were assigned by a simple randomization method to one of two treatment groups, control or IUGR, at receipt. Placental insufficiency-induced IUGR fetuses (n = 20) were generated by exposing pregnant ewes to elevated ambient temperatures (40°C for 12 h; 35°C for 12 h; dew point 22°C) from 39 ± 1 day gestational age (dGA) to 93 ± 1 dGA (term 149 dGA) as described previously (19). Control fetuses (n = 17) were from ewes that were maintained at 25°C and pair fed to the average feed intake of IUGR ewes. All ewes were fed Standard-Bread alfalfa pellets (Sacate Pellet Mills) and had ad libitum access to water. A flow diagram of animal use is shown (Figure 1). For a majority of animals used in this experiment, we have previously determined pancreatic endocrine cell mass and reported these findings along with fetal and organ weights in 2015 by Davis et al (15). The overlap by group includes six intact-controls, six AD-controls, six intact-IUGRs, and four AD-IUGR fetuses.
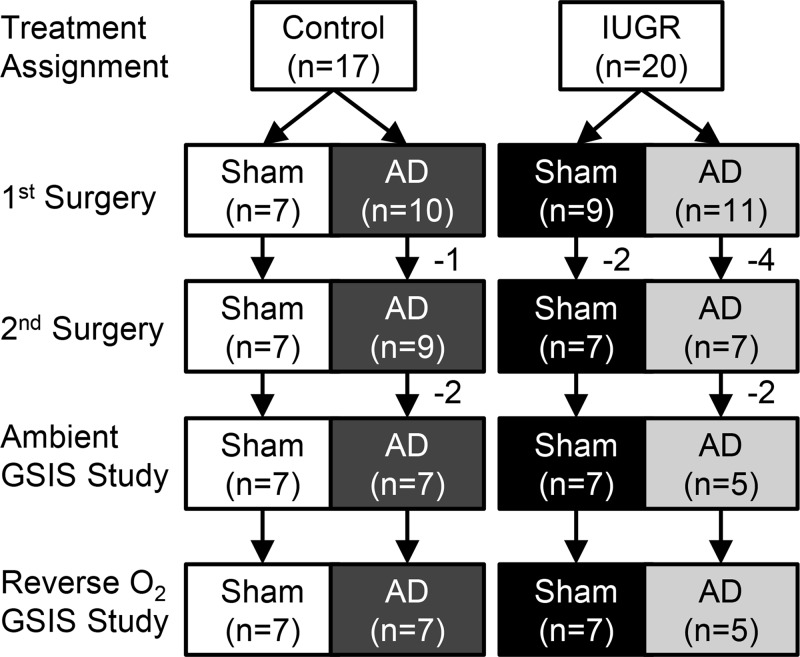
Figure 1.
Flow diagram of animals use for experimental procedures. After eligibility was determined, pregnant ewes were assigned at random to an experimental treatment, IUGR or control, at 39 ± 1 day of gestation. Sham or bilateral AD surgical procedures were performed at 98 ± 1 day. One control and one IUGR fetus were lost during the first surgery due to complications. Two Intact-IUGR and three AD-IUGR fetuses died between the first and second surgeries. All fetuses survived the second surgical procedure when catheters were inserted. Two AD-control and two AD-IUGR fetuses were excluded from the analysis due to incomplete AD. The final groups are composed of fetuses that completed both ambient and oxygen reversal GSIS studies.
At 98 ± 1 dGA (0.65 gestation) control and IUGR fetuses were assigned by a simple randomization method to undergo bilateral AD or a sham surgical procedure (intact) as previously described (25). Briefly, the fetal adrenal glands were isolated via retroperitoneal incisions and cauterized axially with an electrocautery needle. The current applied was sufficient to destroy the medulla, but portions of the adrenal cortex remained intact, which was confirmed by immunohistochemistry At 121 ± 1 dGA, each fetus underwent a second surgical procedure to place indwelling arterial and venous catheters for blood sampling and infusion as described previously (9). At this time, a tracheal catheter was also surgically placed in each ewe (25, 26). Animals were allowed to recover for more than 5 days prior to the in vivo experimental procedures. The catheters were flushed daily with heparinized saline solution (100 U/mL heparin in 0.9% NaCl solution; Vedco, Inc).
Ewes and fetuses were euthanized at 134 ± 1 dGA with an iv overdose of sodium pentobarbital (86 mg/kg) and phenytoin sodium (11 mg/kg; Euthasol; Virbac Animal Health). The fetus and placentomes were removed and weighed. Adrenal glands were collected, fixed in 4% paraformaldehyde, and embedded in optimum cutting temperature compound (Sakura Finetek USA) as previously described (25).
Experimental design
In vivo studies were performed on all fetuses to measure basal and glucose-stimulated insulin concentrations on two separate occasions (Figure 1). The first glucose-stimulated insulin secretion (GSIS) study was conducted under ambient laboratory conditions. After a 24- to 48-hour recovery period, a second GSIS study was performed under experimentally induced, steady-state reversal of fetal blood oxygenation conditions. Control fetuses were made hypoxemic by infusing 100% nitrogen gas into the maternal trachea catheter (∼5–10 L/h), and IUGR fetuses were made normoxemic (ie, to match arterial O2 pressure [PaO2] measured in control fetuses under ambient conditions) by infusing 100% oxygen gas. The targeted steady-state PaO2 was maintained for 60 minutes prior to baseline blood sample collections and throughout the duration of the study period. Glucose-potentiated arginine-induced insulin secretion (GPAIS) was measured under ambient conditions only.
Insulin secretion responsiveness
Fetal insulin secretion responsiveness was measured with a square-wave hyperglycemic clamp as described previously (19, 27). Briefly, a continuous transfusion of maternal blood into the fetus (10 mL/h) was initiated 45 minutes prior to baseline sample collection to compensate for repeated sampling throughout the study. Three arterial blood samples were collected at 5-minute intervals under baseline steady-state conditions and averaged to generate basal period means. Steady-state hyperglycemia was induced by an iv dextrose bolus (∼0.6 mmol/kg estimated fetal weight) followed by a constant iv infusion of 33% dextrose solution. Fetal plasma glucose concentration was measured at 5- to 10-minute intervals during the initial 30 minutes, and the dextrose infusion rate was adjusted accordingly to achieve a steady-state fetal plasma glucose concentration of 3 ± 0.5 mmol/L, which produces maximal glucose-stimulated insulin concentrations in near-term fetal sheep (27). Steady-state conditions were confirmed during the sampling period when arterial plasma glucose concentrations varied less than ±6% of the mean and showed no systematic trend with time. Thirty minutes after steady-state hyperglycemia was achieved, three blood samples were collected at 5-minute intervals and averaged to generate hyperglycemic period means.
Maximal fetal insulin secretion responsiveness, an assessment of readily-releasable insulin, was measured by glucose-potentiated arginine-induced insulin secretion, as described previously (28, 29). Fetal blood samples were collected 5, 15, and 30 minutes after an arginine bolus (0.5 mmol/kg estimated fetal weight; Sigma-Aldrich Co), and the area under the curve was calculated in GraphPad Prism 6.01 (GraphPad Software, Inc).
Blood and plasma analysis
Fetal arterial blood (∼1.5 mL) was collected in EDTA-lined syringes (Sigma-Aldrich Co) and divided into two tubes, one of which was mixed to a final concentration of 0.5 mM EDTA and 0.33 mM reduced glutathione for catecholamine measurements. Plasma from both samples was separated by centrifugation (14 000 × g; 2 min). Glucose and lactate concentrations were determined in a small volume of plasma with a YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments), and the remaining plasma was stored at −80°C. Additional fetal blood (∼200 μL) was collected in heparin-lined syringes (Elkins-Sinn, Inc), and blood gases, pH, hematocrit, and oximetry were analyzed with an ABL 720, (temperature corrected to 39.1°C; Radiometer).
Plasma hormone concentrations were determined by an ELISA for insulin (Ovine Insulin ELISA; ALPCO Diagnostics; sensitivity 0.07 ng/mL; intra- and interassay coefficients of variation, 3% and 6%, respectively), cortisol (Oxford Biomedical Research; sensitivity 10 pg/mL; intra- and interassay coefficients of variation, 9% and 12%, respectively), norepinephrine (Labor Diagnostika Nord; sensitivity 25 pg/mL; intra- and interassay coefficients of variation, 6% and 14%, respectively), and epinephrine (Labor Diagnostika Nord; sensitivity 8.3 pg/mL; intra- and interassay coefficients of variation, 11% and 17%, respectively).
Immunohistochemistry (Table 1)
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Bovine P450c17 | Antibovine P450c17 | A. J. Conley, University of California, Davis | Rabbit polyclonal | 500 | |
| Chromogranin A | AntichromograninA (LK2H10 + PHE5) | Abcam, ab715, lot 871225 | Mouse monoclonal | 250 |
Tissue sections (10 μm) of fetal adrenal glands were immunostained with rabbit antibovine P450c17 (1:500; a gift from A. J. Conley, University of California, Davis; [30]) and mouse monoclonal antichromogranin A (LK2H10+PHE5) (1:250; Abcam; ab715, lot 871225) as described previously (25). Immunocomplexes were detected with affinity-purified secondary antibodies conjugated to Alexa Fluor488 or Alexa Fluor594 (Jackson ImmunoResearch Laboratories). Fluorescence images were visualized on a Leica DM5500 microscope system and digitally captured with a Hamamatsu Digital Camera ORCA flash4.0LT.
Statistical analysis
Fetal treatment groups in this study were intact-controls (n = 7), AD-controls (n = 7), intact-IUGR (n = 7), and AD-IUGR (n = 5). The fetal sex ratio (percentage males) was 57% for intact-control, 86% for AD-control, 71% for intact-IUGR, and 80% for AD-IUGR groups. Fetuses with incomplete adrenal demedullation, identified by their catecholamine response to hypoxemia, were excluded. Values are expressed as mean ± SE. After the second surgery, animals were assigned a new identification number that did not correspond with treatment or previous identification to facilitate blinding and eliminate bias in subsequent analyses. Fetal weights and GPAIS area under the curve were analyzed by a one-way ANOVA (four treatment groups) using the general linear means procedure of SAS software (version 9.4; SAS Institute), and differences were determined with a post hoc least significant difference test. Basal and hyperglycemic period means for biochemical, hematological, and hormone values were analyzed by an ANOVA using MIXED procedures with fetus as the random effect. Main effects for GSIS studies in ambient conditions were treatment, period, or their interaction. The main effects for reverse oxygen tension study were treatment, period, and study, and by experimental design the interactions for treatment by period or treatment by study were analyzed. Fetal sex was included as a variable in the GSIS ANOVA but removed from the model because no significant effect was found. Significant differences reported are P ≤ .05.
Results
Immunofluorescent staining of fetal adrenal glands
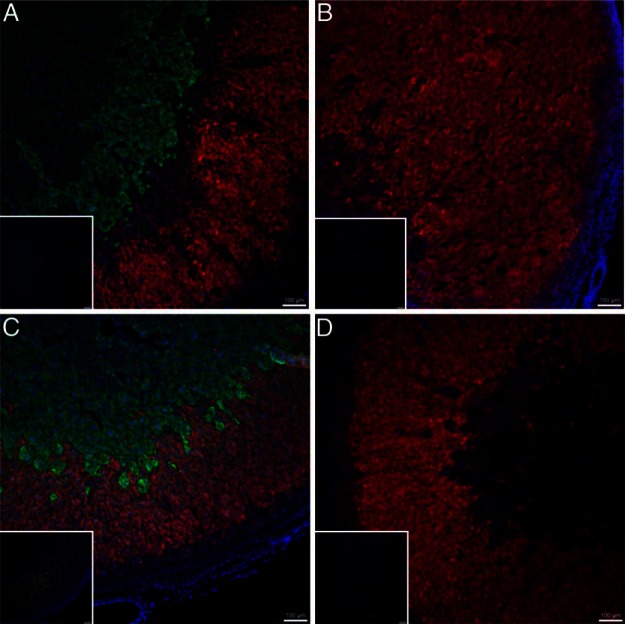
Immunostaining of the fetal adrenals identified successful depletion of the adrenal medulla but not cortex in AD fetuses (Figure 2). Adrenal gland in intact-IUGR fetuses have similar areas of adrenal cortex and medulla compared with intact-controls.
Figure 2.
Immunofluorescent staining of fetal adrenal glands. Fetal adrenal glands were immunostained with rabbit antibovine P450c17 (Alexa Fluor594; red), mouse antihuman chromogranin A (Alexa Fluor488; green), and 4′,6-diamidino-2-phenylindole (blue) to identify glucocorticoid-producing steroidogenic cells, chromaffin cells, and nuclear DNA, respectively. Overlays of representative photomicrographs are shown for intact-control (A), AD-control (B), intact-IUGR (C), and AD-IUGR fetuses (D). Negative controls are depicted in the insert for each adrenal gland. The bar in the lower right corner is 100 μm
Fetal growth restriction in IUGR fetuses
Fetal and placental weights have been reported previously for a portion of the animals used in this study (15). Similar to the previous report, the average placental weights for these treatments showed that intact-IUGR fetuses (110.0 ± 11.9 g) and AD-IUGR fetuses (160.5 ± 27.4 g) were lighter (P < .05) than intact-controls (345.9 ± 18.4 g) and AD-controls (280.4 ± 13.0 g). AD-control placentas weighed less than intact-controls, but AD-IUGR placentas tended to be heavier (P = .06) than intact-IUGR placentas. Intact-IUGR fetuses were smaller than intact-controls (1.4 ± 0.2 kg vs 3.4 ± 0.1 kg, P < .05), and AD-IUGR fetuses weighed less than AD-controls (2.2 ± 0.4 kg vs 3.2 ± 0.2 kg, P < .05). Surgical AD had no effect on fetal weight in controls, but AD-IUGR fetuses were heavier than intact-IUGR fetuses.
Adrenal demedullation increases GSIS in IUGR fetuses
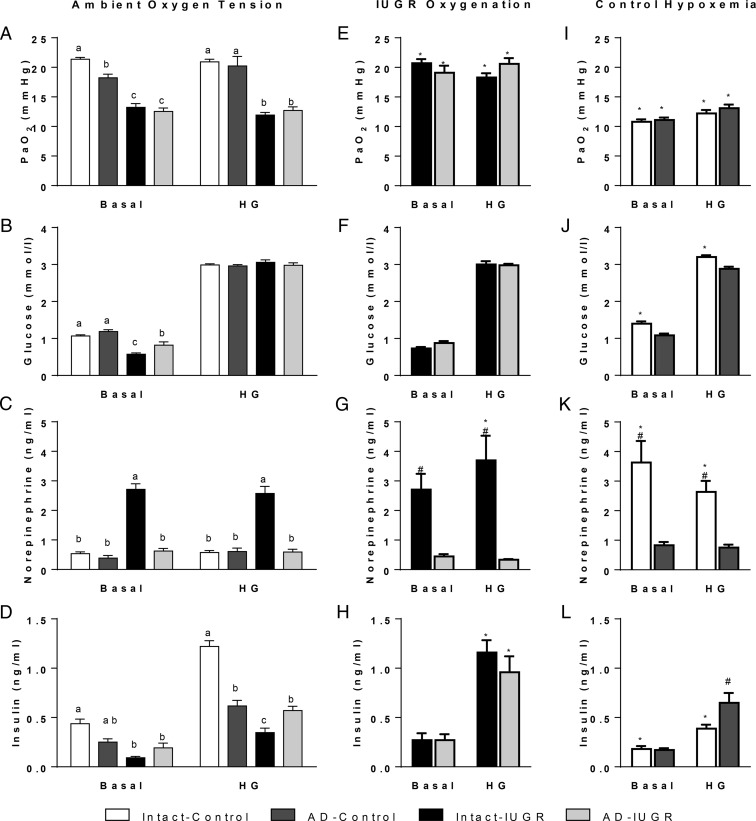
Under ambient laboratory conditions, PaO2 was lower in intact-IUGR and AD-IUGR fetuses than their respective controls during both basal and hyperglycemic periods but similar between IUGR groups (Figure 3A). During the basal period, PaO2 was 15% lower in AD-controls than intact-controls but was not different between the two during the hyperglycemic period. Arterial O2 content followed the same pattern as PaO2 (Table 2).
Figure 3.
GSIS studies during ambient and reversed fetal oxygenation. Mean values for basal and hyperglycemic (HG) steady-state periods are presented for blood PaO2 and plasma concentrations of glucose, norepinephrine, and insulin. GSIS studies were performed in intact-control (n = 7), AD-control (n = 7), intact-IUGR (n = 7), and AD-IUGR fetuses (n = 5). Under ambient laboratory conditions (panels A–D), significant treatment differences (P < .05) between means within the period are represented by different letters. The maternal inspired O2 fraction was increased to oxygenate IUGR fetuses during a second GSIS study (panels E–H). In control fetuses, the maternal inspired O2 fraction was decrease to produce fetal hypoxemia (panels I–L). The oxygen conditions for treatment groups are indicated above the representative column of panels. Comparisons were made between reversed and ambient studies as well as within the reversed oxygen tension study. *, Significant differences (P < .05) between GSIS studies; #, significant treatment difference within the GSIS study.
Table 2.
Arterial Values in Ambient, Oxygenated, and Hypoxemic GSIS Studies
| Study Period | Ambient Oxygen Tension |
Oxygenation |
Hypoxemia |
|||||
|---|---|---|---|---|---|---|---|---|
| Intact-Control (n = 7) | AD-Control (n = 7) | Intact-IUGR (n = 7) | AD-IUGR (n = 5) | Intact-IUGR (n = 7) | AD-IUGR (n = 5) | Intact-Control (n = 7) | AD-Control (n = 7) | |
| Basal | ||||||||
| O2 content, mmol/L | 3.8 ± 0.1a | 3.0 ± 0.1b | 2.1 ± 0.2c | 1.8 ± 0.2c | 3.5 ± 0.2d | 3.4 ± 0.3d | 1.3 ± 0.1d | 1.2 ± 0.1d |
| PaCO2, mm Hg | 47.4 ± 0.5a | 51.6 ± 0.6b | 51.3 ± 1.3b | 54.4 ± 1.1b | 49.9 ± 0.9 | 54.1 ± 1.3 | 40.9 ± 0.7d | 46.4 ± 1.3d,e |
| pH | 7.30 ± 0.01a,b | 7.30 ± 0.01a,b | 7.32 ± 0.01a | 7.28 ± 0.01b | 7.35 ± 0.01d,e | 7.29 ± 0.01 | 7.28 ± 0.01 | 7.28 ± 0.01 |
| Hematocrit, % | 33.8 ± 0.5a | 35.7 ± 1.3a,b | 42.5 ± 1.5c | 40.9 ± 1.9b,c | 38.5 ± 1.7d | 37.5 ± 2.0d | 36.1 ± 0.4d | 37.6 ± 0.9d |
| Bicarbonate, mmol/L | 21.2 ± 0.3 | 22.1 ± 0.3 | 22.2 ± 0.4 | 21.3 ± 0.3 | 24.5 ± 0.4d,e | 22.1 ± 0.3d | 17.5 ± 0.7d | 18.6 ± 0.5d |
| Lactate, mmol/L | 1.62 ± 0.09a | 2.68 ± 0.18a,b | 2.84 ± 0.19a,b | 3.84 ± 0.50b | 4.30 ± 0.83d | 4.42 ± 0.91 | 8.15 ± 0.96d | 8.20 ± 0.83d |
| Hyperglycemic | ||||||||
| O2 content, mmol/L | 3.5 ± 0.1a,f | 2.9 ± 0.1b,f | 1.3 ± 0.1c,f | 1.6 ± 0.1c,f | 2.6 ± 0.2d | 3.0 ± 0.3d | 1.2 ± 0.1d | 1.2 ± 0.1d |
| PaCO2, mm Hg | 48.5 ± 0.6a | 52.4 ± 0.9a,b | 54.5 ± 0.8b,f | 56.5 ± 1.2b,f | 54.5 ± 1.2 | 56.4 ± 1.2 | 40.0 ± 0.6d | 48.8 ± 1.2d,e |
| pH | 7.29 ± 0.01a,f | 7.29 ± 0.01a,f | 7.26 ± 0.01a,b,f | 7.25 ± 0.01b,f | 7.31 ± 0.01d,e | 7.27 ± 0.01 | 7.16 ± 0.02d | 7.16 ± 0.03d |
| Hematocrit, % | 33.4 ± 0.5a | 35.5 ± 1.2a,b | 40.9 ± 1.4b | 40.2 ± 1.8b | 38.2 ± 1.6d | 36.9 ± 2.0d | 35.0 ± 0.4d | 35.7 ± 0.9e |
| Bicarbonate, mmol/L | 20.6 ± 0.3f | 21.5 ± 0.2f | 19.9 ± 0.4f | 20.0 ± 0.3f | 23.1 ± 0.4d | 21.7 ± 0.4d | 13.3 ± 0.8d | 14.9 ± 0.9d |
| Lactate, mmol/L | 2.18 ± 0.09a,f | 3.05 ± 0.15a,f | 5.70 ± 0.34b,f | 5.23 ± 0.57b,f | 4.38 ± 0.62d | 4.30 ± 0.76 | 13.36 ± 1.39d | 12.44 ± 1.28d |
Letter superscripts denote differences (P ≤ .05) within the period of the ambient oxygen tension study.
Differences (P ≤ .05) within the period between GSIS studies (oxygenation or hypoxemia) vs ambient oxygen tension data.
Treatment differences (P ≤ .05) within steady-state periods for the reverse oxygen tension GSIS studies.
Differences (P ≤ .05) between steady state periods within treatment group for the ambient GSIS study.
Glucose concentrations during the basal period were lower in intact-IUGR and AD-IUGR fetuses compared with intact-controls (Figure 3B). Basal glucose concentrations in AD-IUGR fetuses were greater than intact-IUGR fetuses but still less than all control groups. Hyperglycemic glucose concentrations increased (P < .05) from basal, as expected, and were not different between treatments.
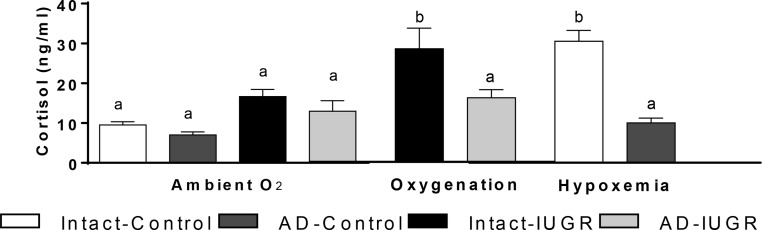
Norepinephrine concentrations were approximately 5-fold greater in intact-IUGR fetuses compared with intact-control fetuses in both periods (Figure 3C). Adrenal demedullation at 98 dGA prevented high norepinephrine concentrations in AD-IUGR fetuses at 134 dGA. In fact, AD-IUGR norepinephrine concentrations were not different from controls. Epinephrine concentrations were also higher (P < .05) in intact-IUGR fetuses (178.1 ± 27.6 pg/mL) than in intact-controls, AD-controls, or AD-IUGR fetuses, which were below the detection limit. Basal cortisol concentrations were not different between treatment groups (Figure 4).
Figure 4.
Fetal cortisol concentrations in the basal period. Plasma cortisol concentrations were measured in intact-control (n = 7), AD-control (n = 7), intact-IUGR (n = 7), and AD-IUGR (n = 5) fetuses. Basal steady-state cortisol concentrations (mean ± SE) are presented for the ambient study and oxygenated (IUGR) or hypoxemic (control) studies. Different superscripts denote significant difference (P < .05).
Basal insulin concentrations were 79% lower in intact-IUGR fetuses and 56% lower in AD-IUGR fetuses compared with intact-controls, with AD-control fetuses being intermediate (Figure 3D). Basal insulin concentrations were not different between IUGR treatments. In all treatments, insulin concentrations increased (P < .001) in response to hyperglycemia. Glucose-stimulated insulin concentrations were 72% lower in intact-IUGR fetuses compared with intact-controls. In AD-controls glucose-stimulated insulin concentrations were approximately 45% lower than in intact-controls but were not different from AD-IUGR fetuses. Glucose-stimulated insulin concentrations were 61% greater in AD-IUGR than intact-IUGR fetuses.
Arterial PaCO2, pH, hematocrit, bicarbonate, and lactate values are presented (Table 1). Compared with intact-controls, PaCO2 was elevated in all other treatments. The pH was lower in AD-IUGR fetuses compared with intact-IUGR at basal. Hyperglycemia lowered blood pH and bicarbonate levels but increased lactate concentrations in all treatments. The hematocrit was elevated in intact-IUGR and AD-IUGR fetuses compared with intact-controls, with AD-controls being intermediate at basal and hyperglycemic periods.
Glucose-potentiated arginine-induced insulin secretion was observed in all treatment groups (Figure 5). After arginine administration, insulin concentrations were greater in intact-controls than in all other treatments, which were not different from one another. Area under the curve for GPAIS showed that intact-control fetuses (60.9 ± 4.7 μg/min · L) had a greater (P < .01) maximum insulin response than AD-controls (33.6 ± 5.5 μg/min · L), intact-IUGR fetuses (27.1 ± 6.1 μg/min · L), and AD-IUGR fetuses (22.2 ± 2.1 μg/min · L).
Figure 5.
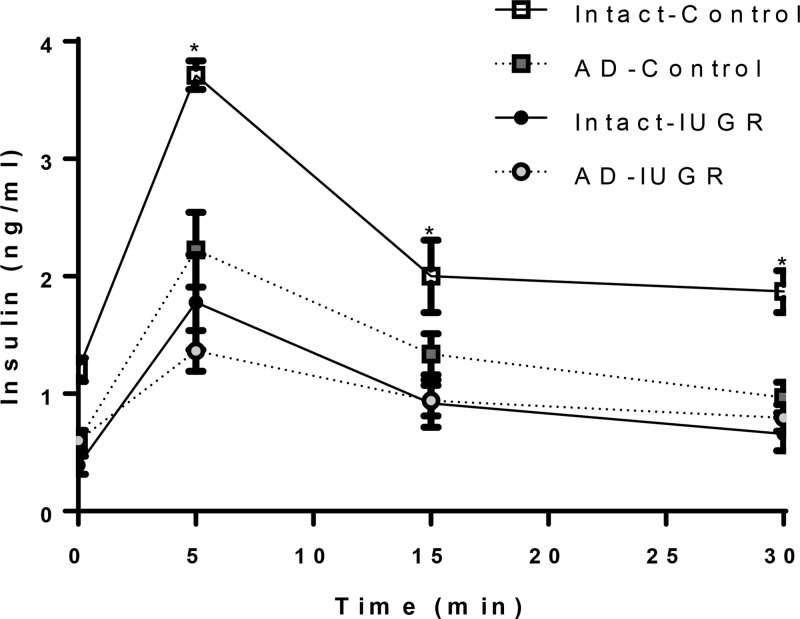
Glucose potentiated arginine-stimulated insulin secretion. Arginine was administered after the collection of the hyperglycemic samples. Plasma insulin concentrations (mean ± SE) are present for the GPAIS period. The sampling times after the arginine bolus at time 0 are presented on the x-axis. Symbols identifying the four treatment groups are described in the legend for seven intact-control, seven AD-control, seven intact-IUGR, and five AD-IUGR fetuses. The asterisks denote significant difference (P < .05) between treatment groups.
Oxygenation increases GSIS in IUGR fetuses
In IUGR treatments maternal insufflation of 100% oxygen increased fetal PaO2 to levels observed in intact-control fetuses (Figure 3E). Similarly, blood O2 content increased in IUGR fetuses (Table 1). PaO2 and O2 content were not different between oxygenated study periods or treatments.
Basal and hyperglycemic glucose concentrations were not different between ambient (Figure 3B) and oxygenated GSIS studies (Figure 3F). Glucose concentrations also were not different between intact-IUGR and AD-IUGR fetuses during the basal or hyperglycemic period of the oxygenated GSIS study.
Norepinephrine concentrations remained greater in intact-IUGR fetuses compared with AD-IUGR fetuses in both periods of the oxygenated GSIS study (Figure 3G). In intact-IUGR fetuses, norepinephrine concentrations were increased in the oxygenated-hyperglycemic period compared with the ambient-hyperglycemic period. AD-IUGR fetal norepinephrine concentrations were not different between studies for either period. Epinephrine concentrations remained high (P < .05) in intact-IUGR fetuses (177.0 ± 41.0 pg/mL) compared with AD-IUGR fetuses, which remained undetectable. Basal cortisol concentrations increased in intact-IUGR fetuses, but not AD-IUGR fetuses, compared with the ambient study (Figure 4).
Glucose-stimulated insulin concentrations were 3.3-fold greater (P < .05) in intact-IUGR fetuses and 1.7-fold greater (P < .05) in AD-IUGR fetuses during the oxygenated-hyperglycemic period compared with their own ambient-hyperglycemic period (Figure 3H). Basal insulin concentrations were not different between studies. No differences were found between IUGR treatments within the oxygenated GSIS study periods.
Oxygenation increased blood pH in intact-IUGR fetuses during both periods but did not affect pH in AD-IUGR fetuses (Table 2). During oxygenation, basal and hyperglycemic bicarbonate concentrations increased in intact-IUGR and AD-IUGR fetuses. Oxygenation lowered the hematocrit in both IUGR treatments. In intact-IUGR fetuses, basal lactate concentrations increased with oxygenation but were lower with oxygenation during the hyperglycemic period. Lactate concentrations were not different between studies in AD-IUGR fetuses.
Hypoxemia-induced norepinephrine inhibits GSIS in control fetuses
Maternal hypoxemia lowered blood PaO2 in control fetuses to the levels observed in IUGR fetuses at ambient conditions (Figure 3I). Blood O2 content was also decrease in control fetuses (Table 2).
In intact-controls, hypoxemia increased basal and hyperglycemic glucose concentrations by 31% and 7%, respectively, compared with the ambient study (Figure 3J). Glucose concentrations were not different between hypoxemic and ambient GSIS studies for AD-control fetuses or between intact-controls and AD-controls in the hypoxemic GSIS study periods.
Hypoxemia increased norepinephrine concentrations in intact-control fetuses by 6.8-fold during the basal period and by 4.6-fold during the hyperglycemic period (Figure 3K). Norepinephrine concentrations were unaffected by hypoxemia in AD-controls. Epinephrine concentrations were increased (P < .05) in intact-control fetuses (168.8 ± 74.5 pg/mL) compared with AD-controls, which remained undetectable. Basal cortisol concentrations were increased in intact-controls compared with the ambient study but were unchanged in AD-control fetuses (Figure 4).
In intact-control fetuses, hypoxemia decreased basal and hyperglycemic insulin concentrations, whereas AD-control insulin concentrations were unaffected (Figure 3L). Glucose-stimulated insulin concentrations were 1.7-fold greater in AD-controls than intact-controls during hypoxemia.
Hypoxemia decreased PaCO2, but intact-control fetuses had lower PaCO2 levels compared with AD-controls (Table 2). Blood pH decreased with hypoxemia and hyperglycemia. Bicarbonate concentrations also decreased during hypoxemia. Hypoxemia increased the hematocrit in intact-control fetuses during both periods. In AD-control fetuses, the hematocrit increased during the basal-hypoxemic period but decreased during the hyperglycemic-hypoxic period, which was not different from the ambient hyperglycemic mean. Lactate concentrations were increased with hypoxemia and further increases with hyperglycemia but not different between control treatments.
Discussion
This study shows that the prevention of high-plasma catecholamines in the IUGR fetus augments glucose-stimulated insulin concentrations. These findings demonstrate a predominant role for elevated catecholamines in IUGR pathology that is independent of hypoxemia, hypoglycemia, and other fetal conditions. Moreover, adaptations to chronic adrenergic stimulation such as compensatory gain in β-cell responsiveness were not present in IUGR fetuses that had undergone adrenal demedullation. Experimental results for our second hypothesis demonstrate that increasing the maternal inspired O2 fraction increases blood O2 content in IUGR fetuses. However, acute oxygenation in intact-IUGR fetuses to normal levels did not lower plasma catecholamine concentrations but enhanced glucose-stimulated insulin concentrations by more than 3-fold. Moreover, fetal oxygenation improved maximal glucose-stimulated insulin concentrations in AD-IUGR fetuses. These findings indicate that chronic fetal hypoxemia impairs pancreatic β-cell responsiveness and demonstrate that oxygen tension exerts effects independent of catecholamines, which is not the case for control fetuses with acute hypoxemia. Together our results indicate that prevention of chronic hypercatecholaminemia and normalization of oxygen tension can each independently potentiate glucose-stimulated insulin secretion in IUGR fetuses, and thus, chronic hypoxemia and hypercatecholaminemia likely play distinct but complementary roles in the suppression of glucose-stimulated insulin secretion in IUGR fetuses.
Growing evidence indicates that inhibition of insulin secretion is mediated by several different regulatory factors and cellular mechanisms (31). In this study, IUGR reduced basal insulin concentrations by 79%, which is consistent with our previous findings (9, 19). Catecholamines suppress insulin secretion, and we expected adrenal demedullation of IUGR fetuses to increase basal insulin concentrations in concert with lower catecholamine concentrations. However, we found that intact-IUGR and AD-IUGR fetuses had similar basal insulin concentrations despite substantially higher concentrations of catecholamines in the former. The lack of correlation between basal insulin and catecholamine concentrations demonstrates that low basal insulin results from hypoglycemia and hypoxemia, which were comparable between intact and AD-IUGR fetuses and have been shown to directly impair β-cell function (9, 32, 33).
In contrast to basal insulin concentrations, adrenal demedullation augmented glucose-stimulated insulin concentrations in IUGR fetuses but blunted it in control fetuses. Adrenal demedullation did not improve maximal readily releasable insulin secretion in IUGR fetuses and, in fact, reduced insulin secretion in control fetuses by approximately 50%. This is consistent with expectations that nonadrenergic factors influence insulin secretion in IUGR fetuses and provides a basis for the adrenal medulla in regulating insulin stimulus-secretion coupling in the healthy fetus. We have previously observed similar insulin secretion deficits in normal sheep fetuses after adrenal demedullation at 0.82 gestation (25), which suggests that suppression of β-cell function in this study is not caused by limitations in β-cell development or maturation. An alternative explanation is that adrenal chromaffin cells may offer developing β-cells protection against temporary bouts of hypoglycemia and hypoxemia that are known to occur during pregnancy because β-cells have been shown to be especially susceptible to such stressors (32, 34, 35).
Previous work in fetuses with chronically high catecholamines elucidated programming adaptations in adrenergic responsive tissues (22–24). Our studies and others demonstrated acute pharmacological adrenergic receptor blockade enhanced the capacity for glucose-stimulated insulin secretion in IUGR fetuses (19, 20). This β-cell compensatory response to chronic catecholamine exposure was similar to phenomena previously reported in humans and animals after chronic adrenergic stimulation (21, 36–38). Our findings show that preventing chronic hypercatecholaminemia eliminates the impetus for developing compensatory β-cells responsiveness. Future work will more clearly determine whether these adaptive responses in adrenergic signaling continue their role during the transition to postnatal life.
The acute return of blood oxygen levels to normal restored glucose-stimulated insulin concentrations in IUGR fetuses to normal as well. It unexpectedly did so without decreasing catecholamines in intact-IUGR fetuses. Also, oxygenation potentiated glucose-stimulated insulin secretion in AD-IUGR fetuses, which lacked any catecholamine influence. In normal fetuses, monoamine oxidase and catechol-O-methyltransferase enzymes are active in the liver and kidneys near term, yet placental clearance still accounts for approximately 70% of fetal catecholamine disappearance (39, 40). In previous studies using fetal sheep with healthy placentas, iatrogenic catecholamine infusion or hypoxemia-induced acute elevations in fetal catecholamine concentrations promptly returned to resting values within an hour of removing the stimulus or source of exogenous catecholamines (41, 42). However, our model of IUGR produces substantial reductions in placental mass and functional capacity (43–45), making it reasonable to postulate that placental catecholamine clearance is also impaired. In addition, we previously found that plasma norepinephrine concentrations remained elevated in normal fetal sheep 4 hours after terminating a week-long norepinephrine infusion (21), which indicates that chronic elevation of catecholamines may also stimulate increases in their own synthesis and secretion.
The ability of acute oxygenation to improve glucose-stimulated insulin secretion in IUGR fetuses and more specifically in AD-IUGR fetuses shows that chronic hypoxemia exerts an independent role in limiting insulin secretion. Although normal fetuses are naturally hypoxemic relative to the maternal blood oxygen tension, increasing PaO2 in normal fetuses did not augment basal or stimulated insulin concentrations, indicating that oxygen is not limiting under normal conditions (12, 16). However, hyperglycemia increases oxygen consumption, which can lower intracellular oxygen tension and activate hypoxia inducible factor 1α (34, 46), and we show here that the 50% reduction in PaO2 observed in our IUGR fetuses limits insulin secretion in response to glucose, which may explain the intolerance to prolonged euglycemic correction previously observed in IUGR fetuses (47). Fetal hypoxemia in IUGR may also have a more direct influence on β-cells because perifusion experiments on rat and canine islets show that second-phase insulin secretion has a graded response to intermediate PaO2 levels and insulin secretion is inhibited by 50% at 27 mm Hg (48). In a previous study, pancreatic islet vascularity in the near-term IUGR fetus was similar to controls (49). This indicates inadequate oxygen delivery to islets is likely due to arterial hypoxemia caused by placental insufficiency. However, adaptation to chronic hypoxemia appears to be an important factor considering acute hypoxemia of AD-control fetuses was unaffected, which is demonstrated here and in our previous study (25). Interestingly, reoxygenating islets after acute intermediate hypoxia elicited a transient spike in insulin secretion even though these IUGR fetuses have reduced β-cell mass (15, 48). Enhanced insulin secretion with oxygen in the IUGR fetus also appears to be transient because when the duration of oxygenation was extended to six hours in two intact-IUGR fetuses, the augmentation was lost. Additional work is required to define mechanisms for the pronounced rebound and its potential loss in insulin secretion in IUGR fetuses.
In this study we also observed fluctuations in the hematocrit in response to the fetal oxygen status. Although mechanisms responsible for these differences were not directly investigated, we hypothesize that chronic hypoxemia in the IUGR fetus stimulate erythropoiesis (50, 51). However, differences in hematocrit have not been reported previously for this model (19, 52). Acute reversal in PaO2 produced reciprocal responses in IUGR and control fetal hematocrit that were not dependent on catecholamines. The acute alterations may represent vasomotor responses or atrial natriuretic peptide concentrations that affect plasma volume (53–55). Nonetheless, the hematocrit is within an acceptable range demonstrated to support maximal oxygen delivery to fetal organs and tissues (56).
In response to stress, like hypoxemia, cortisol is secreted from the adrenal gland and has been shown to slow fetal growth, alter carbohydrate metabolism, and promote maturation of fetal tissues (57–59). In this study, cortisol concentrations were not different between the four treatment groups in ambient conditions, which is consistent with previous reports for this model of placental insufficiency-induced IUGR (60, 61) but not all reports (62, 63). Comparison between previous, independent IUGR reports indicate that cortisol concentrations associate positively with lactate concentrations, reflecting higher concentrations with greater severity of placental insufficiency (60). Therefore, intact-IUGR fetuses may perceive acute oxygenation as a stress because cortisol concentrations increased, which are also associated with higher lactate concentrations in this study and elevated norepinephrine concentrations during hyperglycemia in intact-IUGR fetuses. Although the presence of the fetal adrenal cortex was confirmed with P450c17 immunostaining, glucocorticoid synthesis in hypoxemic AD-control and oxygenated AD-IUGR fetuses was blunted compared with intact fetuses. We postulate that early gestational ablation of the adrenal medulla impaired the functional development of the adrenal cortex or hypothalamic-pituitary-adrenal axis because adrenal demedullation at later ages did not disrupt the cortisol response to hypoxemia (64).
In the intact-IUGR fetus, oxygenation increased basal lactate concentrations but lowered lactate concentrations during the hyperglycemic period. Plasma lactate concentrations are dependent on placental uptake, fetal production from glucose and nonglucose sources, and fetal consumption (65). We and others have proposed that a new equilibrium between lactate production and use is reached to support hepatic glucose production in IUGR fetuses (60, 66). IUGR fetuses develop a limited capacity for glucose oxidation, despite normal glucose use rates (60, 62). This limitation was associated with increased liver expression of pyruvate dehydrogenase kinase 4 and lactate dehydrogenase A to support intrahepatic lactate production for gluconeogenesis (62). Although not measured here, discrepancies between lactate production and use are apparent during oxygenation of intact-IUGR fetuses because plasma lactate concentrations increase at basal but did not increase further with hyperglycemia, unlike the ambient study. We have shown previously that although hyperglycemia improves rates of glucose use and oxidation, it also increases lactate concentrations (60). This suggests that in IUGR oxygenation alters the equilibrium of fetal lactate production and use during hypoglycemic conditions but allows rates of glucose oxidation to increase with hyperglycemia. The mechanic explanation for these findings remains unknown.
In conclusion, our findings show that chronic fetal hypercatecholaminemia and hypoxemia independently contribute to reduced fetal growth and impaired insulin secretion in the IUGR fetus. Acutely increasing arterial oxygen tension in the IUGR fetus substantially augmented glucose-stimulated insulin secretion, regardless of whether fetal adrenal medullae were intact or ablated, which shows that fetal hypoxemia influences regulation of insulin stimulus-secretion function, even without adrenergic intercession. This study shows that, contrary to our original hypothesis, adrenergic activity may not overshadow the efficacy and safety of single-nutrient replacement therapies. However, based on detrimental outcomes of previous attempts at nutrient supplementation (47, 67–70), we postulate that the first successful therapeutic intervention to improve fetal and neonatal outcomes in the context of placental insufficiency-induced IUGR will likely require the combination of nutrient supplementation (eg, glucose and amino acids), oxygen delivery, and endocrine modulation. These studies indicate that nutrient replacement strategies must take into account the modulation of the fetal endocrine profile and identify elevated plasma catecholamines as a critical factor that warrants further investigation in IUGR fetuses.
Acknowledgments
We thank Mandie M. Dunham for technical assistance.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by National Institutes of Health Grant R01 DK084842 (S.W.L., principal investigator). A.R.M. and L.E.C. were supported by Grant T32 HL7249 (J. Burt, principal investigator). D.T.Y. was supported by Award 2012-67012-19855 (D.T.Y., principal investigator) from the National Institute of Food and Agriculture, US Department of Agriculture. X.C. was supported by the Chongqing Science and Technology Commission, Chongqing, China (Grant NO.CSTC2014JCYJA80036) and Southwest University (Grant NO.20140090).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AD
- adrenal demedullation
- dGA
- days gestational age
- GPAIS
- glucose-potentiated arginine-induced insulin secretion
- GSIS
- glucose-stimulated insulin secretion
- IUGR
- intrauterine growth restriction
- PaO2
- arterial O2 pressure.
References
- 1. Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. [DOI] [PubMed] [Google Scholar]
- 2. Pardi G, Cetin I, Marconi AM, Lanfranchi A, et al. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–696. [DOI] [PubMed] [Google Scholar]
- 3. Economides DL, Proudler A, Nicolaides KH. Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;160:1091–1094. [DOI] [PubMed] [Google Scholar]
- 4. Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. [DOI] [PubMed] [Google Scholar]
- 5. Hubinont C, Nicolini U, Fisk NM, Tannirandorn Y, Rodeck CH. Endocrine pancreatic function in growth-retarded fetuses. Obstet Gynecol. 1991;77:541–544. [PubMed] [Google Scholar]
- 6. Hiraoka T, Kudo T, Kishimoto Y. Catecholamines in experimentally growth-retarded rat fetus. Asia Oceania J Obstet Gynaecol. 1991;17:341–348. [DOI] [PubMed] [Google Scholar]
- 7. Jones CT, Robinson JS. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. J Dev Physiol. 1983;5:77–87. [PubMed] [Google Scholar]
- 8. Lagercrantz H, Sjoquist B, Bremme K, Lunell NO, Somell C. Catecholamine metabolites in amniotic fluid as indicators of intrauterine stress. Am J Obstet Gynecol. 1980;136:1067–1070. [DOI] [PubMed] [Google Scholar]
- 9. Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–1497. [DOI] [PubMed] [Google Scholar]
- 10. Okamura K, Watanabe T, Tanigawara S, et al. Catecholamine levels and their correlation to blood gases in umbilical venous blood obtained by cordocentesis. Fetal Diagn Ther. 1990;5:147–152. [DOI] [PubMed] [Google Scholar]
- 11. Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev. 1990;23:9–13. [DOI] [PubMed] [Google Scholar]
- 12. Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2179–R2188. [DOI] [PubMed] [Google Scholar]
- 13. Sperling MA, Christensen RA, Ganguli S, Anand R. Adrenergic modulation of pancreatic hormone secretion in utero: studies in fetal sheep. Pediatr Res. 1980;14:203–208. [DOI] [PubMed] [Google Scholar]
- 14. Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol. 1998;274:R1536–R1545. [DOI] [PubMed] [Google Scholar]
- 15. Davis MA, Macko AR, Steyn LV, Anderson MJ, Limesand SW. Fetal adrenal demedullation lowers circulating norepinephrine and attenuates growth restriction but not reduction of endocrine cell mass in an ovine model of intrauterine growth restriction. Nutrients. 2015;7:500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies. Placenta. 2002;23(suppl A):S119–S129. [DOI] [PubMed] [Google Scholar]
- 17. Wallace JM, Regnault TR, Limesand SW, Hay WW, Jr, Anthony RV. Investigating the causes of low birth weight in contrasting ovine paradigms. J Physiol. 2005;565:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW., Jr Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab. 2013;304:E516–E523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–E778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macko AR, Yates DT, Chen X, et al. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J Dev Origins Health Dis. 2013;4(5):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am J Physiol Endocrinol Metab. 2014;306:E58–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen X, Fahy AL, Green AS, Anderson MJ, Rhoads RP, Limesand SW. β2-Adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J Physiol. 2010;588:3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yates DT, Macko AR, Nearing M, Chen X, Rhoads RP, Limesand SW. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J Pregnancy. 2012;2012:631038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yates DT, Clarke DS, Macko AR, et al. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J Physiol. 2014;592:3113–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yates DT, Macko AR, Chen X, et al. Hypoxemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinemia in fetal sheep. J Physiol. 2012;590:5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. Am J Physiol. 1993;264:E741–E747. [DOI] [PubMed] [Google Scholar]
- 27. Green AS, Macko AR, Rozance PJ, et al. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab. 2011;300:E817–E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Green AS, Chen X, Macko AR, et al. Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets. J Endocrinol. 2012;212:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gresores A, Anderson S, Hood D, Zerbe GO, Hay WW., Jr Separate and joint effects of arginine and glucose on ovine fetal insulin secretion. Am J Physiol. 1997;272:E68–E73. [DOI] [PubMed] [Google Scholar]
- 30. Peterson JK, Moran F, Conley AJ, Bird IM. Zonal expression of endothelial nitric oxide synthase in sheep and rhesus adrenal cortex. Endocrinology. 2001;142:5351–5363. [DOI] [PubMed] [Google Scholar]
- 31. McDermott AM, Sharp GW. Inhibition of insulin secretion: a fail-safe system. Cell Signal. 1993;5:229–234. [DOI] [PubMed] [Google Scholar]
- 32. Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol. 2003;547(1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cantley J, Grey ST, Maxwell PH, Withers DJ. The hypoxia response pathway and β-cell function. Diabetes Obes Metab. 2010;12(suppl 2):159–67. [DOI] [PubMed] [Google Scholar]
- 34. Sato Y, Endo H, Okuyama H, et al. Cellular hypoxia of pancreatic β-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem. 2011;286:12524–12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng X, Zheng X, Wang X, et al. Acute hypoxia induces apoptosis of pancreatic β-cell by activation of the unfolded protein response and upregulation of CHOP. Cell Death Dis. 2012;3:e322.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grodsky GM, Ma YH, Edwards RH. Chronic sympathetic innervation of islets in transgenic mice results in differential desensitization of α-adrenergic inhibition of insulin secretion. Adv Exp Med Biol. 1997;426:129–138. [DOI] [PubMed] [Google Scholar]
- 37. Rousseau-Migneron S, Nadeau A, LeBlanc J. Effect of adrenaline on insulin secretion in rats treated chronically with adrenaline. Can J Physiol Pharmacol. 1976;54:870–875. [DOI] [PubMed] [Google Scholar]
- 38. Robertson RP, Halter JB, Porte DJ. A role for α-adrenergic receptors in abnormal insulin secretion in diabetes mellitus. J Clin Invest. 1976;57:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bzoskie L, Yen J, Tseng YT, Blount L, Kashiwai K, Padbury JF. Human placental norepinephrine transporter mRNA: expression and correlation with fetal condition at birth. Placenta. 1997;18:205–210. [DOI] [PubMed] [Google Scholar]
- 40. Bzoskie L, Blount L, Kashiwai K, Tseng YT, Hay WW, Jr, Padbury JF. Placental norepinephrine clearance: in vivo measurement and physiological role. Am J Physiol. 1995;269:E145–E149. [DOI] [PubMed] [Google Scholar]
- 41. Jones CT, Ritchie JW. The cardiovascular effects of circulating catecholamines in fetal sheep. J Physiol. 1978;285:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bocking AD, White SE, Kent S, et al. Effect of prolonged catecholamine infusion on heart rate, blood pressure, breathing, and growth in fetal sheep. Can J Physiol Pharmacol. 1995;73:1750–1758. [DOI] [PubMed] [Google Scholar]
- 43. Limesand SW, Regnault TR, Hay WW., Jr Characterization of glucose transporter 8 (GLUT8) in the ovine placenta of normal and growth restricted fetuses. Placenta. 2004;25:70–77. [DOI] [PubMed] [Google Scholar]
- 44. Regnault TR, de Vrijer B, Galan HL, et al. The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J Physiol. 2003;550:641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287(6):E1114–E1124. [DOI] [PubMed] [Google Scholar]
- 46. Bensellam M, Duvillie B, Rybachuk G, et al. Glucose-induced O(2) consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic β-cells. PLoS One. 2012;7:e29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res. 2009;65:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. [DOI] [PubMed] [Google Scholar]
- 49. Rozance PJ, Anderson M, Martinez M, et al. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol. 1989;256:R1348–R1354. [DOI] [PubMed] [Google Scholar]
- 51. Widness JA, Teramo KA, Clemons GK, et al. Temporal response of immunoreactive erythropoietin to acute hypoxemia in fetal sheep. Pediatr Res. 1986;20:15–19. [DOI] [PubMed] [Google Scholar]
- 52. Regnault TR, de VB, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta. 2007;28(7):714–723. [DOI] [PubMed] [Google Scholar]
- 53. Adamson SL, Morrow RJ, Bull SB, Langille BL. Vasomotor responses of the umbilical circulation in fetal sheep. Am J Physiol. 1989;256:R1056–R1062. [DOI] [PubMed] [Google Scholar]
- 54. Cheung CY, Brace RA. Fetal hypoxia elevates plasma atrial natriuretic factor concentration. Am J Obstet Gynecol. 1988;159:1263–1268. [DOI] [PubMed] [Google Scholar]
- 55. Brace RA, Cheung CY. Cardiovascular and fluid responses to atrial natriuretic factor in sheep fetus. Am J Physiol. 1987;253:R561–R567. [DOI] [PubMed] [Google Scholar]
- 56. Fumia FD, Edelstone DI, Holzman IR. Blood flow and oxygen delivery to fetal organs as functions of fetal hematocrit. Am J Obstet Gynecol. 1984;150:274–282. [DOI] [PubMed] [Google Scholar]
- 57. Fowden AL, Forhead AJ. Adrenal glands are essential for activation of glucogenesis during undernutrition in fetal sheep near term. Am J Physiol Endocrinol Metab. 2011;300:E94–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endocrinol. 2002;174:27–36. [DOI] [PubMed] [Google Scholar]
- 59. Gardner DS, Fletcher AJ, Bloomfield MR, Fowden AL, Giussani DA. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol. 2002;540:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2007;293:E1716–E1725. [DOI] [PubMed] [Google Scholar]
- 61. Gadhia MM, Maliszewski AM, O'Meara MC, et al. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab. 2013;304:E352–E362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW, Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2015;309:R920–R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jones CT, Roebuck MM, Walker DW, Lagercrantz H, Johnston BM. Cardiovascular, metabolic and endocrine effects of chemical sympathectomy and of adrenal demedullation in fetal sheep. J Dev Physiol. 1987;9:347–367. [PubMed] [Google Scholar]
- 65. Sparks JW, Hay WW, Jr, Bonds D, Meschia G, Battaglia FC. Simultaneous measurements of lactate turnover rate and umbilical lactate uptake in the fetal lamb. J Clin Invest. 1982;70:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thorn SR, Brown LD, Rozance PJ, Hay WW, Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes. 2012;62:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed ). 2011;3:428–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lavezzi JR, Thorn SR, O'Meara MC, et al. Increased fetal insulin concentrations for one week fail to improve insulin secretion or beta-cell mass in fetal sheep with chronically reduced glucose supply. Am J Physiol Regul Integr Comp Physiol. 2013;304:R50–R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Limesand SW, Rozance PJ, Brown LD, Hay WW., Jr Effects of chronic hypoglycemia and euglycemic correction on lysine metabolism in fetal sheep. Am J Physiol Endocrinol Metab. 2009;296:E879–E887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Say L, Gulmezoglu AM, Hofmeyr GJ. Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst Rev. 2003;1:CD000148. [DOI] [PubMed] [Google Scholar]