Abstract
Environmental and occupational exposure to bisphenol A (BPA), a chemical widely used in polycarbonate plastics and epoxy resins, has received much attention in female reproductive health due to its widespread toxic effects. Although BPA has been linked to infertility and recurrent miscarriage in women, the impact of its exposure on uterine function during early pregnancy remains unclear. In this study, we addressed the effect of prolonged exposure to an environmental relevant dose of BPA on embryo implantation and establishment of pregnancy. Our studies revealed that treatment of mice with BPA led to improper endometrial epithelial and stromal functions thus affecting embryo implantation and establishment of pregnancy. Upon further analyses, we found that the expression of progesterone receptor (PGR) and its downstream target gene, HAND2 (heart and neural crest derivatives expressed 2), was markedly suppressed in BPA-exposed uterine tissues. Previous studies have shown that HAND2 controls embryo implantation by repressing fibroblast growth factor and the MAPK signaling pathways and inhibiting epithelial proliferation. Interestingly, we observed that down-regulation of PGR and HAND2 expression in uterine stroma upon BPA exposure was associated with enhanced activation of fibroblast growth factor and MAPK signaling in the epithelium, thus contributing to aberrant proliferation and lack of uterine receptivity. Further, the differentiation of endometrial stromal cells to decidual cells, an event critical for the establishment and maintenance of pregnancy, was severely compromised in response to BPA. In summary, our studies revealed that chronic exposure to BPA impairs PGR-HAND2 pathway and adversely affects implantation and the establishment of pregnancy.
The physiological functions of mammalian uterus are governed by the concerted actions of steroid hormones 17β-estradiol (E2) and progesterone (P4). These hormones act via their cognate receptors to control proliferation and differentiation of the uterine epithelium and make it competent for embryo implantation during early pregnancy. After attachment of the embryo to the luminal epithelium, E2 and P4 regulate proliferation and differentiation of the underlying stromal cells into unique decidual cells in a process termed as decidualization. Paracrine factors derived from decidual cells control many biological processes that are critical for uterine remodeling, maternal immune response, angiogenesis, and early embryonic growth. Thus, proper decidualization is a prerequisite for successful implantation and establishment of pregnancy (1–3).
In the mouse, an experimentally induced delayed implantation model provided the evidence that E2 plays an essential role in embryo implantation and establishment of pregnancy (4, 5). In this model, deprivation of endogenous steroid hormones by ovariectomy during early pregnancy leads to suspension of embryo implantation. Administration of P4 to these animals allows the embryos at the blastocyst stage to remain viable within the uterus but is insufficient to initiate the implantation process. Administration of E2 to these P4-primed pregnant mice allows attachment of the blastocyst to the luminal epithelium within 12–24 hours and promotes differentiation of the underlying stromal cells to decidual cells within 48 hours. Previous studies using this model have shown that the optimal E2 levels for embryo implantation fall in a narrow range of 0.12- to 4-μg/kg body weight. Beyond this range, E2 is either insufficient or detrimental to establishment and maintenance of early pregnancy (4, 6). Hence, it is clear that the biological processes such as uterine epithelial receptivity and stromal cell decidualization are acutely dependent on the steroid hormone signaling pathways that operate in the uterus during early pregnancy. Indeed, a slight perturbation in estrogen receptor (ER)-α (ESR1) or P4 receptor (PGR)-mediated signaling in the uterus leads to the development of various reproductive disorders (1, 2).
Environmental and occupational exposure to endocrine-disrupting chemicals (EDCs) is a major threat to female reproductive health (7, 8). Bisphenol A (BPA) is an environmental toxicant that is commonly found in polycarbonate plastics and epoxy resins and has received much attention due to its widespread use and high risk of chronic exposure in human (9, 10). BPA is detectable in body fluids of humans worldwide, with higher levels present in preschool children, adolescents, and occupational workers (11). Clinically, blood BPA concentrations in women are associated with reproductive disorders, such as endometrial hyperplasia, endometriosis, recurrent miscarriages, and decreased rate of pregnancy in those who seek assisted reproductive technologies (12–14).
BPA is considered a weak estrogen due to its low binding affinity to ERα and ERβ (15–17). Although most current studies address the estrogenic activity of BPA in target tissues at no or low E2 background, there is increasing evidence to suggest that BPA may also exhibit antiestrogenic activities in the presence of E2 (17, 18). BPA also binds the estrogen receptor-related proteins, G protein-coupled receptor 30, or estrogen-related receptor-γ, which are known to stimulate rapid intracellular responses through nongenomic signaling pathways (17, 19). More recent studies also suggest that BPA exposure could lead to a long-term change in expression levels of target genes via epigenetic mechanisms (20–24).
Previous studies have shown that fetal or neonatal female rodents upon prolonged exposures to BPA encounter numerous reproductive disorders later in life, including abnormal puberty, oocyte aneuploidy, as well as a decline in reproductive capacity (25–30). The impact of the BPA exposure on uterine function remains unclear. In this study, we addressed how chronic exposure to low levels of BPA in young female mice affects uterine epithelial receptivity and stromal cell decidualization, 2 critical biological events that are acutely dependent on steroid hormone-dependent signaling during early pregnancy.
Materials and Methods
Reagents
P4 (P8783), E2 (E2758), and BPA (239658) were purchased from Sigma Chemical Co. Antibodies were purchased from BD PharMingen (KI67, 550609), Dako (PGR, A0098), Leica Biosystems (ERα, NCL-L-ER-6F11), R&D Systems (phospho-fibroblast growth factor substrate 2, p-FRS2, AF5126; Leukemia inhibitory factor, LIF, ab-449), Sigma (phospho-extracellular signal-regulated kinases 1/2, p-ERK1/2, M9692), Santa Cruz Biotechnology, Inc (heart and neural crest derivatives expressed 2, HAND2, sc-9409), and Chemicon (decidual prolactin-related protein [PRL8A2/dPRP], Ab 12930), respectively (Supplemental Table 1).
BPA exposure protocol
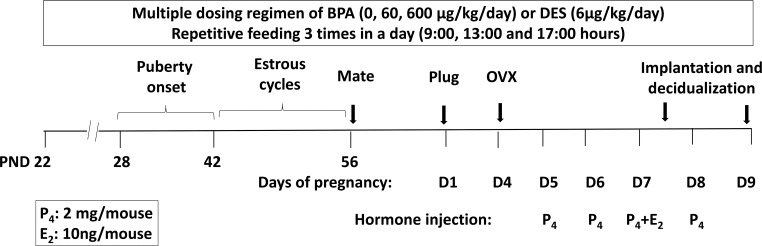
All experiments involving animals were conducted in accordance with National Institutes of Health standards for the use and care of animals. The animal protocols were approved by the University of Illinois Institutional Animal Care and Use Committee. CD-1 female mice were purchased from Charles River. Mice were kept in a standard light-controlled animal room (12-h day light) at 23°C–25°C in polypropylene cages and provided with Teklad Rodent Diet 2016 (Harlan) diet and reverse-osmosis-treated water in glass bottles, ad libitum, to minimize exposure to estrogenic chemicals from food, water, and caging as suggested previously (16). Two batches of animals at postnatal day (PND)21 were ordered separately to minimize interbatch variability. Animals were randomly separated into 4 groups and orally exposed to 0, 60, and 600 μg/kg · d of BPA (designated as BPA-0, BPA-60, BPA-600, n = 8–10/experimental group), or 6 μg/kg of diethylstilbestrol (DES) (designated as DES-6, n = 3) in 3 feedings daily starting at PND22. The drugs were administered in tocopherol-stripped corn oil in 3 equal feedings (9 am, 1 pm, and 5 pm) by placing a micropipette with the dosing solution at one side of the mouth for 5 weeks (Figure 1). Exposure volumes were adjusted daily based on body weight. Vaginal opening was monitored daily by visual examination of the vulva. Upon vaginal opening, vaginal smears were taken and examined to record the ages of pubertal onset and duration of each stage in the estrous cycles. The 60 μg/kg · d dose is relevant to the level of exposure in occupational workers and is close to the dosage of BPA that is considered safe for human consumption, 50 μg/kg · d (11).
Figure 1.
Chronic BPA exposure protocol and the delayed implantation mouse model. Prepubertal CD-1 female mice at PND22 were exposed to 60 or 600 μg/kg · d of BPA (n = 8–10), 6 μg/kg · d of DES (n = 3), or vehicle control in 3 split feedings until tissue collection. Vaginal opening and smears were examined daily from PND42 to PND56 to record the age of pubertal onset (first estrus) and the duration of each stage in the estrous cycle. At PND56, female mice were paired with fertile untreated males. The presence of copulatory plug was designated as day 1 of pregnancy. The pregnant female mice were subjected to ovariectomy (OVX) on the morning of day 4 before embryo implantation, and then supplemented with 2 mg/mouse of P4 daily. Implantation was initiated by a single E2 injection (10 ng/mouse). Uterine tissues were collected at 12 and 48 hours after E2 administration to examine uterine receptivity to embryo implantation and stromal cell decidualization, respectively.
Delayed implantation mouse model
Adult female mice at PND56 were paired with fertile male mice to induce pregnancy. The presence of a vaginal semen plug was considered day 1 of pregnancy. Blastocysts before implantation were retrieved by flushing uterine horns with Hank's Balanced Salt Solution on day 4 of pregnancy and counted. The number and morphology of blastocysts were assessed under a dissecting microscope. Delayed implantation was performed as described previously (4). Briefly, embryo implantation was temporarily suspended in pregnant female mice by deprivation of ovarian steroid hormones (via ovariectomy) before embryo attachment on day 4 of pregnancy. Pregnancy was then maintained by daily injection of P4 (2 mg/mouse) from days 5 to 7. To resume embryo implantation, a single dose of E2 (10 ng/mouse) was administered to the pregnant female mice. Uterine tissues were harvested 12 or 48 hours after E2 injection to examine epithelial receptivity or stromal cell decidualization, respectively.
To visualize the sites of implanted embryos, Chicago blue dye solution was injected iv as described previously (4). The blue bands, which arise along the uterine horns due to increased endometrial vascular permeability at the sites of blastocyst apposition, were counted. The uterine tissues were then subjected to longitudinal sectioning and histological evaluation after hematoxylin and eosin staining. The maximal widths of implantation chambers were determined using the measurement tools in Adobe Photoshop CS6 software.
RNA isolation and quantitative real-time PCR (qPCR) analysis
Uterine tissue collection, RNA purification, cDNA synthesis, and qPCR were performed as described previously (31). Primer sequences corresponding to specific target genes are listed in Supplemental Table 2. 36B4, encoding an acidic ribosomal phosphoprotein, or cytokeratin 18 (Krt18) were used as an internal control. The mean ΔCt was calculated from individual ΔCt values obtained from a minimum of 3 replicates. ΔΔCt was calculated as the difference between the mean ΔCt values of the experimental and control samples. The fold change of gene expression in each sample relative to a control was computed as 2−ΔΔCt. The relative gene expression level was expressed as the average fold change ± SEM from at least 3 independent experiments.
Immunohistochemistry (IHC)
IHC analysis was performed as described previously (31). Briefly, sections of paraffin-embedded uterine tissue were deparaffinized and rehydrated in xylene and a series of graded ethanol. After antigen retrieval with citrate buffer (pH 6.0), uterine sections were incubated with a primary antibody against ERα (ESR1), PGR, LIF, PRL8A2/PRP, p-FRS2, p-ERK1/2, KI67, or HAND2, respectively. Immunostaining was performed using the horseradish peroxidase-labeled Avidin-Biotin system (Vector Laboratories) and AEC (3-amino-9-ethylcarbazole) solution. Cells were briefly counterstained with hematoxylin, mounted, and examined under microscope. Red deposits indicate the sites of immunostaining. The slides were analyzed using ImageJ software (ImageJ 1.49; NIH) as described previously (32). The number of positive cells and the staining intensities were quantified by comparing the average number of positively stained cells with the total number of cells in the demarcated areas from 3 independent samples.
Statistical analysis
All of the numerical values were obtained from at least 3 independent samples and were analyzed by one-way ANOVA followed by Dunnett's post hoc test when comparisons were made between a control group and more than 1 experimental group (GraphPad Prism 5.0; GraphPad Software, Inc). Data are expressed as mean ± SEM. Statistical significance is defined as P < .05.
Results
Exposure to BPA affects the onset of puberty and estrous cyclicity in female mice
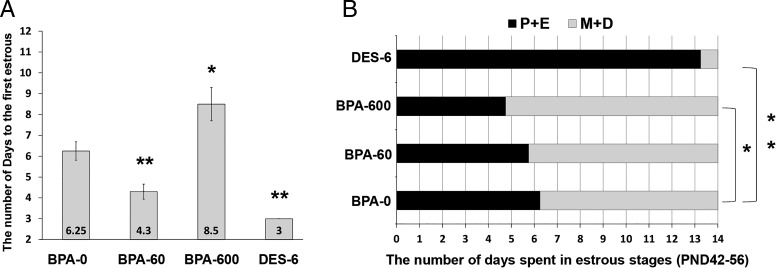
Humans are chronically exposed to BPA primarily through oral intake during multiple times a day (9). In order to recapitulate the BPA exposure situation in human population, particularly in teenagers, we designed a multiple dosing regimen for BPA in young female mice. In this experimental paradigm, mice were exposed to BPA or DES during pubertal development starting from PND22. The chemicals were administered daily in 3 equal feedings (Figure 1). Vaginal opening and smears were examined daily from PND28 to PND56 to record the age of first estrus and the onset of puberty. We also determined the number of days the mice spent at each stage of the estrous cycle (33). No apparent difference in the age with regard to the timing of vaginal opening was observed in mice with or without exposure to either BPA or DES (Supplemental Figure 1). However, the days to the first estrus were markedly different between control group and those exposed to DES or BPA. Although BPA-60 or DES-6 advanced the onset of estrous cyclicity, BPA-600 delayed it considerably (Figure 2A). We continued to monitor the estrous cycles of control and BPA- or DES-exposed females up until 8 weeks of age. For each mouse, the number of days spent at proestrus plus estrus or metaestrus plus diestrus was recorded separately. Compared with the vehicle-treated controls, the DES-treated group spent significantly more number of days at proestrus and estrus, whereas BPA-600 group spent considerably less number of days at these stages of the cycle (Figure 2B). These results indicate that repetitive exposure to BPA in young female mice was able to affect the timing of pubertal onset and estrous cyclicity later in life.
Figure 2.
Chronic exposure to BPA adversely affects the onset of puberty and duration of stages during the estrous cycles. Prepubertal CD-1 female mice at PND22 were exposed to 0, 60, or 600 μg/kg · d of BPA (n = 8–10) and 6 μg/kg · d of DES (n = 3) as depicted in Figure 1. Vaginal smears were examined daily. A, Pubertal onset (first estrus). The values represent the number of days from the time of exposure to the appearance of the cornified vaginal epithelial cells. B, Estrous cycles. The values represent the average number of days the mice spent from PND42 to PND56 at proestrous and estrous (P+E) or metestrous and diestrous (M+D) stages of the estrous cycles. Numerical data were analyzed by one-way ANOVA followed by Dunnett's post hoc analysis and expressed as mean ± SEM. Asterisks indicate significant difference from the control group; *, P < .05; **, P < .01.
BPA exposure affects embryo implantation and formation of the decidua during early pregnancy
We next investigated the effect of BPA on uterine function during early pregnancy. We were particularly interested in determining whether BPA exerts any adverse effect on embryo implantation and establishment of pregnancy. We first considered the possibility that BPA exposure may affect ovarian function and steroidogenesis (34, 35), which indirectly can influence uterine function during early gestation. To circumvent this problem, we employed the delayed implantation mouse model. In this model, as described previously, ovarian function is bypassed and embryo implantation is controlled by administration of exogenous E2 and P4 (Figure 1). Experimental and control female mice at 8 weeks (PND56) were mated with fertile males. Pregnant females were subjected to delayed implantation on day 4 of pregnancy. Embryo attachment and decidual response were then evaluated in these mice by administration of exogenous E2. This mouse model of implantation provides a physiologically relevant system to study the effect of an environmental endocrine disruptor with estrogenic or antiestrogenic activities on uterine function during early pregnancy.
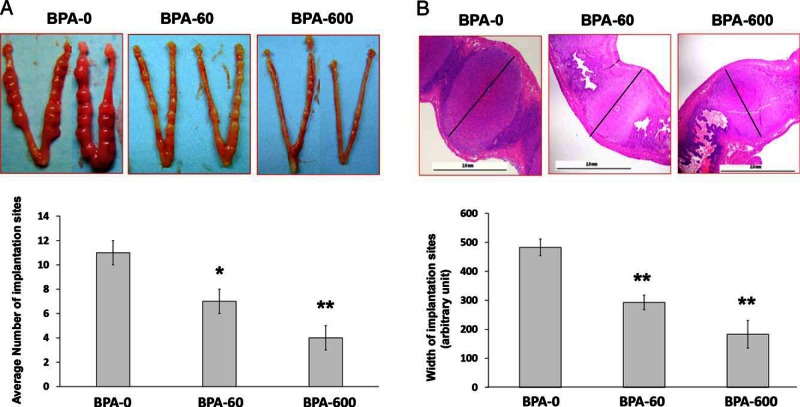
As expected, in pregnant females that were exposed to DES, the uteri were hypertrophic with no signs of embryo implantation (data not shown). The control mice dosed with vehicle displayed well-formed implantation sites within 48 hours after E2 administration (Figure 3A, panel BPA-0). However, the females that were exposed to a chronic BPA regimen, exhibited a dose-dependent decline in the number of implantation sites (Figure 3A). Histological analyses revealed that BPA-exposed uteri exhibited a marked impairment in decidualization as indicated by the reduction in the size of the implantation chambers (Figure 3B). Moreover in the BPA-600 group, 4 out of 12 mice had limited decidual cells surrounding the attached embryo and more than 50% pregnant females showed intrauterine hemorrhage (Supplemental Figure 2). We also recovered the embryos from uteri of mice with or without BPA exposure on day 4 of pregnancy, before implantation. No significant difference was found in the number of the embryos recovered from control or BPA-exposed uteri indicating normal ovulation and fertilization in response to BPA treatment (Supplemental Figure 3). Taken together, these results indicate that chronic exposure to BPA results in an intrauterine environment that is unfavorable for embryo implantation and stromal cell decidualization during early pregnancy.
Figure 3.
Chronic exposure to BPA adversely affects embryo implantation and stromal cell decidualization during early pregnancy. Prepubertal female mice at PND22 were exposed to 0, 60, or 600 μg/kg · d of BPA (n = 8–10), and then paired with fertile males at PND56 to induce pregnancy. Pregnant females were subjected to delayed implantation as described above and assessed for embryo implantation at 48 hours after E2 injection. A, Embryo implantation. Upper panel shows the representative images of uteri collected from each treated group. Lower panel shows the average number of implantation sites from each experimental group. B, Histological examination of implantation chambers. Serial longitudinal sections of uterine horns of BPA-exposed pregnant mice were examined by H&E staining. The maximal width of each implantation site (black bars) was determined using measurement tools in Adobe Photoshop CS3 software. The representative images from each BPA-treated group are shown. Numerical data were analyzed by one-way ANOVA followed by Dunnett's post hoc analysis and expressed as mean ± SEM. Asterisks indicate significant difference from the control group (BPA-0); *, P < .05; **, P < .01.
BPA affects ERα- and PGR-dependent molecular pathways to influence epithelial receptivity during embryo implantation
To gain insights into the mechanism by which chronic BPA exposure disrupts uterine function during early pregnancy, we first examined the expression of ERα (Esr1) and PGR in vehicle- or BPA-exposed uterine samples collected 12 hours after E2 administration. This time frame corresponds to the time of blastocyst attachment to the receptive luminal epithelium in mice. We focused our studies on the BPA-60 group because the exposure of BPA at this level (60 μg/kg · d) is close to the reference safe dose (50 μg/kg · d) for daily consumption in human (11). IHC and qPCR analyses showed that there was no appreciable difference in ERα expression in the uterine tissues that were dosed with or without BPA (Figures 4, A, upper panels, and B, upper panel). However, we observed a marked decline in the level of PGR in uteri of mice that have been exposed to BPA. Although PGR expression in the epithelium was low but comparable in both control and BPA-exposed uterine tissues, the expression of PGR in the stroma was significantly reduced in uteri of mice that have been exposed to BPA (Figure 4, A, middle panels, and B, upper panel). Consistent with these observations we found that the targets of PGR in epithelial cells, such as Ihh, Alox15, and Irg1 (36), were expressed at comparable levels in vehicle- or BPA-treated uterine samples. In contrast, the stromal targets of PGR including Hand2 and Hoxa10 (31, 37) were markedly reduced in the BPA-exposed uteri (Figures 4, A, lower panels, and B, upper panel).
Figure 4.
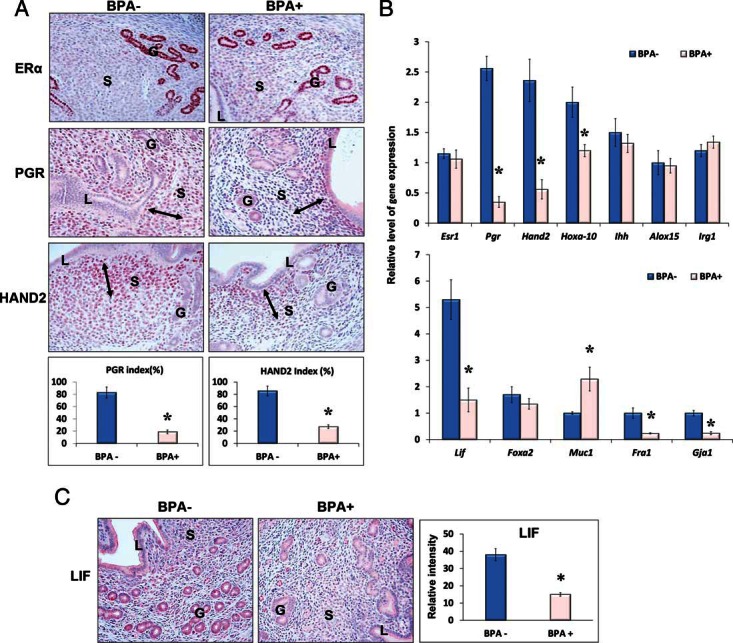
Chronic exposure to BPA impairs ERα- and PGR-mediated signaling at the time of embryo implantation. Prepubertal female mice were exposed to 60 μg/kg · d of BPA (BPA+) or vehicle control (BPA−) and subjected to the delayed implantation mouse model as described above. Uterine tissues were collected at 12 hours after E2 administration. A, Uterine sections were subjected to IHC analysis using antibodies against ERα, PGR, and HAND2. B, Total RNA was purified from BPA-exposed and unexposed uterine tissues and subjected to qPCR using primers specific for Esr1, Pgr, Hand2, Hoxa10, Ihh, Alox15, and Irg1 (upper panel) and Lif, Foxa2, Muc1, Fra1, and Gja1 (lower panel). 36b4 or Krt18 was used as internal controls. C, Uterine sections were subjected to IHC analysis using an antibody against LIF. Representative images in each treatment group are shown. The percentages of the immunostaining positive cells for PGR and HAND2 in the indicated areas (left and right arrows) and the relative intensities of LIF staining were analyzed by ImageJ software. The values represent mean ± SEM of 3 independent samples (n = 3). L, G, and S denote luminal epithelium, glandular epithelium, and stroma, respectively. Asterisks indicate significant difference from the control group; *, P < .01.
Although ERα expression was not affected upon BPA exposure, we considered the possibility that BPA interacts with ERα to modulate its transcriptional activity. We, therefore, monitored the effect of BPA on factors that are regulated by ERα and play a critical role in epithelial receptivity and stromal cell differentiation during early pregnancy. Targets of ERα in the luminal epithelium (Muc1), the glandular epithelium (Lif), as well as the stroma cells (Fra1 and Gja1) were selected for the analyses (38–40). Interestingly, we observed that compared with the vehicle-treated controls, Muc1 expression was significantly up-regulated, whereas expressions of Lif, Fra-1, and Gja1 were markedly down-regulated in BPA-exposed uteri (Figures 4, B, lower panel, and C). In contrast the glandular factor Foxa2, which is not a target of ERα (41), was expressed at a comparable level in control and BPA-treated uteri (Figure 4B, lower panel). Collectively these results indicate that BPA interferes with ERα- and PGR-mediated signaling pathways in the uterus during early pregnancy.
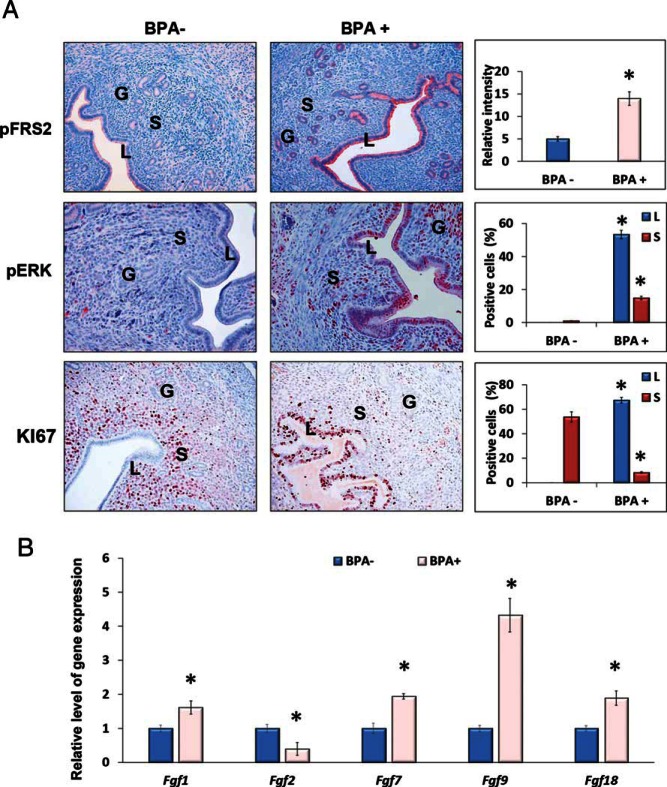
In many species including mice, the receptive state is marked by a cessation in epithelial cell proliferation before implantation. Our recent studies have shown that this cessation of epithelial proliferation is mediated by a basic helix-loop-helix transcription factor Hand2, which is expressed downstream of stromal PGR (31). In the receptive phase, Hand2 expressed in the stroma suppresses the production of fibroblast growth factors (FGFs) to inhibit cell proliferation. In mouse uteri lacking Hand2, continued induction of FGFs in the stroma activates FGF receptor (FGFR) signaling in the epithelium to promote cell proliferation and impair implantation (31). Because expression of PGR and Hand2 in the uterine stroma was affected by BPA exposure (Figure 4), it was of interest to determine the effect of this EDC on FGFR signaling during implantation. The activation of the FGFR signaling pathway in control and BPA-exposed uterine tissues at the time of implantation was monitored by examining the tyrosine phosphorylation status of FRS2, an adapter protein that links activated FGR receptors to downstream signaling pathways (42). As expected, only low levels of phospho-FRS2 were observed in the uterine epithelia of control mice at the time of implantation. In contrast, a marked increase in the level of phospho-FRS2 was seen in the epithelia of BPA-exposed uteri, indicating elevated FGF signaling in response to this EDC (Figure 5A, upper panels).
Figure 5.
Chronic BPA enhances FGFR-ERK1/2-mediated MAPK signaling and mitotic activity in uterine epithelial cells. Female mice exposed to 60 μg/kg · d of BPA (BPA+) or vehicle control (BPA−) were subjected to the delayed-implantation mouse protocol as described above. Uterine tissues were collected at 12 hours after E2 administration (n = 3). A, IHC analysis using antibodies against p-FRS2, p-ERK1/2, and KI67. Representative images in each treatment group are shown. B, qPCR analysis using primers specific for Fgf1, Fgf2, Fgf7, Fgf9, and Fgf18 are shown. 36b4 was used as internal control. The percentages of the immunostaining positive cells for p-ERK1/2 and KI67 in the luminal epithelium and the underling stroma and the relative intensities for pFRS2 staining were analyzed by ImageJ software. The values represent mean ± SEM of 3 independent samples (n = 3). Asterisks indicate significant difference from the control group; *, P < .01. L, G, and S denote luminal epithelium, glandular epithelium, and stroma, respectively.
We next investigated whether the ERK1/2 pathway, which is known to be activated downstream of FGFR (43), is stimulated in the epithelia of BPA-exposed uteri. As shown in Figure 5A, middle panels, a dramatic increase in the level of p-ERK1/2 was seen in the uterine epithelia of BPA-exposed mice at the time of implantation. Consistent with this observation we found an enhanced proliferation of uterine epithelial cells, as indicated by KI67 staining, in response to BPA exposure. The control uterine sections, as expected, exhibited enhanced stromal proliferation but minimal p-ERK1/2 expression and epithelial proliferation at the time of implantation (Figure 5A, lower panels). We also determined the gene expression levels of FGF family members in the uterine stroma in response to BPA exposure. As shown in Figure 5B, Fgf2 expression is down-regulated, whereas Fgf1, Fgf7, Fgf9, and Fgf18 levels are markedly elevated in BPA-exposed uteri compared with the vehicle-treated controls. These results indicate that down-regulation of Hand2 in the uterine stroma in response to BPA results in persistent activation of FGFR-ERK1/2 pathway and enhanced cell proliferation in the epithelium, making the uterus nonreceptive for implantation.
Uterine stromal cell differentiation is impaired in response to BPA exposure
After attachment of the embryo to the uterine epithelium, P4 functioning via PGR also promotes the proliferation and differentiation of the underlying stromal cells into decidual cells that maintain an environment conducive to the growth and development of the implanting embryo (2, 3, 44). We, therefore, investigated the expression of PGR in BPA-exposed and unexposed uterine tissues during the decidual phase of pregnancy (Figure 6A). Uterine sections were collected from these mice at 48 hours after E2 stimulation, which overlaps with the decidual phase of pregnancy. IHC analysis revealed that BPA exposure led to aberrant expression of PGR in the endometrial stroma during decidualization. In the control unexposed uterine sections, as expected, PGR was localized to the stromal cells outside the primary decidual zone (Figure 6A, A and a). The primary decidual zone includes a few layers of cells in the immediate vicinity of the implanted embryo. In the BPA-exposed uterine samples, the cells in the primary decidual zone expressed PGR, whereas the cells outside this zone were devoid of PGR expression (Figure 6A, B and b).
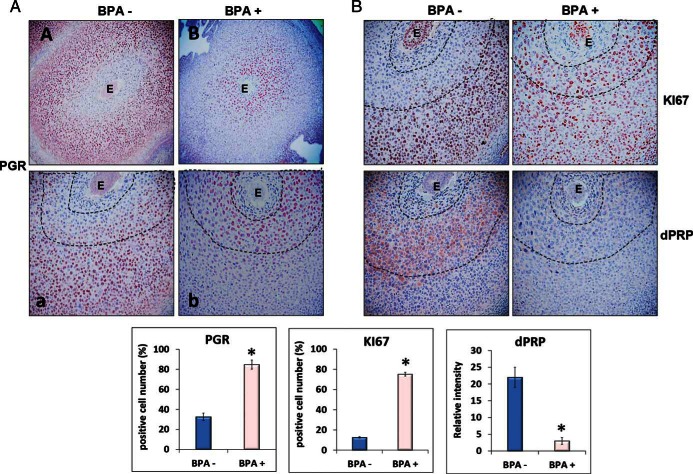
Figure 6.
Chronic BPA affects uterine stromal cell proliferation and differentiation during decidualization. Female mice exposed to 60 μg/kg · d of BPA (BPA+) or vehicle control (BPA−) were subjected to the delayed-implantation mouse protocol as indicated in Figure 1. Uterine sections were collected at 48 hours after E2 administration and subjected to IHC analysis using antibodies against PGR (A), KI67 or dPRP (B), respectively. Panels a and b in A are magnified images of A and B, respectively. Representative images in each treatment group are shown. The percentages of the immunostaining positive cells for PGR and KI67 in the indicated areas and the relative intensities of dPRP staining (within dash lines) were analyzed by ImageJ software. The values represent mean ± SEM of 3 independent samples (n = 3). E, embryo.
Aberrant PGR expression in uterine stroma upon BPA exposure raised the possibility that stromal proliferation and differentiation might be affected in response to this EDC during early pregnancy. IHC analysis was performed using antibodies against KI67 and PRL8A2/dPRP, to assess stromal cell proliferation and differentiation, respectively (45). As expected, in control uterine tissues, the stromal cells in close vicinity of the embryos were largely devoid of any KI67 staining indicating that these cells have exited the cell cycle and entered the differentiation program (Figure 6B, upper left panel). Indeed, these cells expressed PRL8A2/dPRP, a differentiation marker of stromal cells (Figure 6B, lower left panel). In contrast, the uterine sections from BPA-exposed mice exhibited wide spread staining of KI67 in the stromal cells that are near the embryo (Figure 6B, upper left panel). These cells remained proliferative and did not undergo differentiation as indicated by the lack of expression of PRL8A2/dPRP (Figure 6B, lower right panel). These results indicate that BPA exposure affects the differentiation of uterine stromal cells to decidual cells that is critical for the establishment of pregnancy.
Discussion
It is generally believed that humans face the risk of chronic exposure to BPA, albeit at low levels, through consumption of contaminated food and beverages multiple times a day (9, 10). This exposure situation, however, is not properly reflected in many animal studies where BPA is administered either orally or by sc injection in a single daily dose. These studies suggested that the toxic effects of BPA exposure is of minimal concern because a high dose of BPA is needed to obtain the lowest-observed-adverse-effect-level in rodent uteri (11). This is most likely due to the short life-span of BPA in vivo after ingestion or iv injection (46–48). To mimic the human exposure situation, we utilized a multiple dosing protocol for BPA exposure in prepubertal female mice, in which the daily dose of BPA was split into 3 equal feedings. We reasoned that there would be greater health risks under this exposure paradigm because of the persistent bioavailability of BPA and its long-lasting biological effects in target tissues (46, 49). Indeed, reproductive abnormalities were found with regard to the pubertal onset, duration of estrous cycles, and uterine functions during early pregnancy, as a result of ingestion of 60 μg/kg · d of BPA. This dose is far below the lowest-observed-adverse-effect-level established previously (50 mg/kg · d) and close to the reference safe dose for human consumption (50 μg/kg · d). We note, with interest, that the levels of biologically active, unconjugated BPA measured in human serum falls in a range of 0.5–10 ng/mL, with an average level of approximately 2 ng/mL (9, 10). Previous pharmacokinetic studies of BPA in adult CD1 female mice have shown a linear relationship between the serum levels and an oral dose of BPA ranging from 2 to 100 000 μg/kg (48). After oral administration of 400-μg/kg BPA, the unconjugated serum BPA levels were reported to be maximal at 1 hour (3.28 ng/mL), which then declined rapidly by 4 hours. The average area under the curve (AUC)0–24 was 0.7 ng/mL (48). Because there is a lack of evidence for bioaccumulation of BPA after repeated dosing, we estimate that oral exposure to BPA at 200-μg/kg feeding (3 times in a day with 4-h interval) would correspond to serum BPA level of 1.64 ng/mL at 1 hour after each ingestion. The average AUC0–24 after ingestion of 3 doses of 200-μg/kg BPA in a day would correspond to 1.05 ng/mL.
It has been reported previously that BPA exposures in prenatal or neonatal female rodents cause aberrant pubertal onset and irregular estrous cycles later in life (50). We also observed that chronic exposures to low levels of BPA affect the timing to pubertal onset in a nonmonotonic dose-dependent manner. Although low-level of BPA (60 μg/kg · d) advanced the onset of the first estrus, a marked delay on the timing of first estrus was observed at the high-level of BPA (600 μg/kg · d). In uterine tissue BPA exposure at both low and high levels affected implantation and establishment of pregnancy. It is possible that BPA at different exposure levels affect the physiological functions of diverse target tissues through distinct mechanisms.
Interestingly, our study revealed that chronic exposure to BPA affected ERα-mediated signaling in the uterus during early pregnancy. LIF, a critical factor for uterine receptivity and regulated by ERα in glandular epithelial cells, was markedly down-regulated upon BPA exposure. Our studies further revealed that Muc1, a target of ERα in the luminal epithelium as well as stromal targets of ERα including Fra-1, Gja1, and PGR are aberrantly expressed in the uterine tissues upon exposure to BPA. The expression of HAND2, a downstream target of PGR in the stroma, is also markedly suppressed in the uterine tissues in response to BPA. We have previously reported that HAND2 is a key mediator of P4/PGR signaling in uterine stromal cells and plays a critical role in inhibition of ERα-dependent epithelial proliferation and mucin 1 (MUC1) expression. Down-regulation of MUC1 from luminal epithelium and cessation of epithelial proliferation are essential for acquisition of uterine receptivity during embryo implantation. In the absence of Hand2, uterine stroma produces a number of FGF family members, which in turn result in persistent activation of FGFR-ERK1/2-mediated MAPK pathway and sustained proliferation of luminal epithelial cells, causing implantation failure (31).
The precise mechanism by which BPA affects PGR/HAND2 expression remains unclear. It is possible that BPA interferes with ERα-dependent gene expression in the uterine stroma including PGR during early pregnancy. Interestingly, recent studies have shown that chronic BPA attenuates PGR expression in the endometrium of nonhuman primates and rodents, as well as in the cultured human endometrial stromal cells (18, 28, 51). A recent study has also shown that pretreatment of E2 reduces the bioavailability of BPA in the uterus after ingestion of 50 μg/kg of BPA by female rodents (49). Collectively, these studies seem to indicate that uterine ERα-PGR signaling is vulnerable to exposure of environmental EDCs with either estrogenic or antiestrogenic activity. We are, however, aware of the fact that BPA may adversely affect blastocyst function impairing its implantation potential. We note with interest that the development and transport of preimplantation embryos was affected in female rats fed with high-butterfat diet mixed with BPA (52). The impact of BPA exposure on the activation of blastocysts at the time of implantation is currently under investigation in the laboratory.
The attachment of the blastocyst to the uterine epithelium is followed by proliferation and differentiation of the subjacent stromal cells into decidual cells, which support the growth and development of the implanted embryos. Studies using genetically engineered mouse models have clearly established that P4 functioning via PGR is the primary driver of this differentiation process (2, 44). In recent years, chromatin immunoprecipitation-sequencing in combination with microarray-based gene expression profiling analyses have revealed that PGR also collaborates with other transcription factors, including CCAAT/enhancer binding protein beta (cEBP/β) and signal transducer and transcription activator 3 (STAT3), to regulate the expression of many target genes that function in concert to properly control uterine stromal cell decidualization (53, 54). Because exposure to BPA leads to aberrant expression PGR in the endometrial stroma, it is likely that BPA interferes with the PGR-dependent pathways and controls the decidualization program during early pregnancy. Indeed, our study revealed that uterine stromal cells exhibited a marked impairment in proliferation and differentiation upon BPA exposure. The mechanism by which BPA affects PGR expression in the uterus during decidualization remains unknown.
There is increasing evidence to suggest that chronic BPA exposure could alter gene expression in target tissues through epigenetic mechanisms, including DNA methylation in “CG” islands of target gene promoters (20, 21, 55). Although the effect of BPA on DNA methylation of PGR has not been reported, a recent study has shown that methylation of Stat3 and Fkbp5 is affected in the liver of BPA-exposed mice (56, 57). Furthermore, studies from the Taylor laboratory have shown that DNA methylation of Hoxa10, a known target of PGR, is affected in response to BPA exposure (23). Interestingly, we noted that the expression of DNA (cytosine-5-)-methyltransferase 3 beta, Dnmt3b, a member of the methyltransferases responsible for establishing methylation patterns (58), is markedly induced in uterine stromal cells in response to chronic low-level BPA exposure (data not shown). Hence, it is possible that BPA exposure affects promoter DNA methylation of PGR and PGR-targets by epigenetic mechanism. These studies are currently under investigation in the laboratory.
In summary, our studies revealed that chronic exposure to low levels of BPA during pubertal development in female mice adversely affects uterine function later in life. We have further shown that BPA exposure affects ERα- and PGR-dependent signaling, impairing epithelial receptivity and stromal cell decidualization during early gestation. Deciphering the underlying mechanism of aberrant steroid receptor-dependent signaling in the uterus in response to BPA exposure will provide insights into the BPA-associated female reproductive disorders, such as infertility, recurrent miscarriages, endometriosis, endometrial hyperplasia, and even endometrial cancers.
Acknowledgments
This research was supported by National Institutes of Health Grants R21 ES024198 (to Q.L.), U54 HD 055787 (to M.K.B. and I.C.B.), P01 ES022848 and EPA RD-83459301 (to J.A.F.), and T32ES007326 (to J.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- DES
- diethylstilbestrol
- E2
- 17β-estradiol
- EDC
- endocrine-disrupting chemical
- ER
- estrogen receptor
- ERK
- extracellular signal-regulated kinase
- FGF
- fibroblast growth factor
- FGFR
- FGF receptor
- FRS2
- fibroblast growth factor substrate 2
- HAND2
- heart and neural crest derivatives expressed 2
- IHC
- immunohistochemistry
- KI67
- antigen identified by monoclonal antibody Ki67
- LIF
- leukemia inhibitory factor
- P4
- progesterone
- PND
- postnatal day
- PGR
- P4 receptor
- PRL8A2/dPRP
- decidual prolactin-related protein
- qPCR
- quantitative real-time PCR.
References
- 1. Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol. 2014;28:1408–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasquez YM, DeMayo FJ. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol. 2013;24:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003;100:2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Cheon YP, Kannan A, Shanker S, Bagchi IC, Bagchi MK. A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor γ regulates implantation in mice. J Biol Chem. 2004;279:11570–11581. [DOI] [PubMed] [Google Scholar]
- 6. Milligan SR, Cohen PE, Finn CA. The minimum requirements for oestradiol to induce uterine sensitivity for implantation and decidualization in mice. Hum Reprod. 1995;10:1502–1506. [DOI] [PubMed] [Google Scholar]
- 7. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15:631–650. [DOI] [PubMed] [Google Scholar]
- 9. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24:139–177. [DOI] [PubMed] [Google Scholar]
- 10. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. [DOI] [PubMed] [Google Scholar]
- 12. Ehrlich S, Williams PL, Missmer SA, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. 2011;95:1816–1819. [DOI] [PubMed] [Google Scholar]
- 14. Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20:2325–2329. [DOI] [PubMed] [Google Scholar]
- 15. Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. [DOI] [PubMed] [Google Scholar]
- 18. Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wetherill YB, Akingbemi BT, Kanno J, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. [DOI] [PubMed] [Google Scholar]
- 20. Anderson OS, Nahar MS, Faulk C, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol a and phthalates. Int J Mol Sci. 2012;13:10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caserta D, Di Segni N, Mallozzi M, et al. Bisphenol A and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol. 2014;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cabaton NJ, Wadia PR, Rubin BS, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bosquiazzo VL, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG. Effects of neonatal exposure to bisphenol A on steroid regulation of vascular endothelial growth factor expression and endothelial cell proliferation in the adult rat uterus. Biol Reprod. 2010;82:86–95. [DOI] [PubMed] [Google Scholar]
- 27. Varayoud J, Ramos JG, Bosquiazzo VL, Muñoz-de-Toro M, Luque EH. Developmental exposure to Bisphenol a impairs the uterine response to ovarian steroids in the adult. Endocrinology. 2008;149:5848–5860. [DOI] [PubMed] [Google Scholar]
- 28. Varayoud J, Ramos JG, Bosquiazzo VL, Lower M, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology. 2011;152:1101–1111. [DOI] [PubMed] [Google Scholar]
- 29. Vigezzi L, Bosquiazzo VL, Kass L, Ramos JG, Muñoz-de-Toro M, Luque EH. Developmental exposure to bisphenol a alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reprod Toxicol. 2015;52:83–92. [DOI] [PubMed] [Google Scholar]
- 30. Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod. 2012;87:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuhrich DG, Lessey BA, Savaris RF. Comparison of HSCORE assessment of endometrial β3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol. 2013;35:210–216. [PMC free article] [PubMed] [Google Scholar]
- 33. Iguchi T, Uchima FD, Ostrander PL, Bern HA. Growth of normal mouse vaginal epithelial cells in and on collagen gels. Proc Natl Acad Sci USA. 1983;80:3743–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee SG, Kim JY, Chung JY, et al. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ Health Perspect. 2013;121:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheon YP, Li Q, Xu X, DeMayo FJ, Bagchi IC, Bagchi MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–2871. [DOI] [PubMed] [Google Scholar]
- 37. Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. [DOI] [PubMed] [Google Scholar]
- 38. Das A, Li Q, Laws MJ, Kaya H, Bagchi MK, Bagchi IC. Estrogen-induced expression of Fos-related antigen 1 (FRA-1) regulates uterine stromal differentiation and remodeling. J Biol Chem. 2012;287:19622–19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laws MJ, Taylor RN, Sidell N, et al. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- 41. Jeong JW, Kwak I, Lee KY, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. [DOI] [PubMed] [Google Scholar]
- 44. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Q, Kannan A, Wang W, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. [DOI] [PubMed] [Google Scholar]
- 46. Doerge DR, Twaddle NC, Woodling KA, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicol Appl Pharmacol. 2010;248:1–11. [DOI] [PubMed] [Google Scholar]
- 47. Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–165. [DOI] [PubMed] [Google Scholar]
- 48. Taylor JA, Vom Saal FS, Welshons WV, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect. 2011;119:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pollock T, deCatanzaro D. Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses. Reprod Toxicol. 2014;49:145–154. [DOI] [PubMed] [Google Scholar]
- 50. Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. [DOI] [PubMed] [Google Scholar]
- 51. Mannelli C, Szóstek AZ, Lukasik K, et al. Bisphenol A modulates receptivity and secretory function of human decidual cells: an in vitro study. Reproduction. 2015;150:115–125. [DOI] [PubMed] [Google Scholar]
- 52. Martinez AM, Cheong A, Ying J, et al. Effects of high-butterfat diet on embryo implantation in female rats exposed to bisphenol A. Biol Reprod. 2015;93:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mantena SR, Kannan A, Cheon YP, et al. C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA. 2006;103:1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–567. [DOI] [PubMed] [Google Scholar]
- 56. Weinhouse C, Bergin IL, Harris C, Dolinoy DC. Stat3 is a candidate epigenetic biomarker of perinatal bisphenol A exposure associated with murine hepatic tumors with implications for human health. Epigenetics. 2015;10:1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kitraki E, Nalvarte I, Alavian-Ghavanini A, Rüegg J. Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response. Mol Cell Endocrinol. 2015;417:191–199. [DOI] [PubMed] [Google Scholar]
- 58. Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]