Abstract
Retinoic acid (RA) is the active form of vitamin A and is synthesized from retinol by two key enzymes, alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH). As the physiological precursor of RA, retinol impacts female reproductive functions and fertility. The expression of Adh1 and Adh5 as well as Aldh1a1 and Aldh1a7 are significantly increased in the ovaries of mice treated with equine chorionic gonadotropin/FSH. The RA receptor is expressed and localized in granulosa cells and is activated by endogenous RA as indicated by LacZ expression in granulosa cells of RA-responsive transgene-LacZ transgenic mice (RA reporter mice). Coinjection of the ADH inhibitor, 4-methylpyrazole, with equine chorionic gonadotropin significantly decreases the number and developmental competence of oocytes ovulated in response to human chorionic gonadotropin/LH as compared with controls. Injections of RA completely reverse the effects of the inhibitor of ovulation and oocyte development. When mice were fed a retinol-free, vitamin A-deficient diet that significantly reduced the serum levels of retinol, the expression of the LH receptor (Lhcgr) was significantly lower in the ovaries of the vitamin A-deficient mice, and injections of human chorionic gonadotropin failed to induce genes controlling ovulation. These results indicate that ovarian de novo biosynthesis of RA is required for the follicular expression of Lhcgr in granulosa cells and their ability to respond to the ovulatory LH surge.
Multiple intraovarian factors enhance FSH-induced proliferation and differentiation of granulosa cells (1–4). One of these factors is activin, which is expressed in the granulosa cells of growing follicles (3–5) and enhances the FSH regulation of granulosa cell proliferation and estradiol production (6–9). Kipp et al (10) showed that activin decreases granulosa cell expression of Cyp26b1, a gene that encodes an enzyme that degrades retinoic acid (RA). Moreover, the CYP26B1 inhibitor, R115866, enhanced granulosa cell proliferation of secondary follicles in culture. Thus, RA produced in secondary follicles by the reduction of Cyp26b1 expression appears to play an important role(s) in granulosa cells during the early follicular development to the small antral stage.
In serum, the physiological precursor of RA, retinol, is high, whereas RA itself is a minor component of the vitamin A family. Retinol is imported into cells by the transporter stimulated by retinoic acid 6 (STRA6) or by passive transport into cells that directly interact with capillaries (11, 12). Within cells, retinol is converted to retinal by alcohol dehydrogenases (ADH); retinal is then converted to RA by acetaldehyde dehydrogenases (ALDH) (13–16). Our previous study revealed that expression of the Aldh family members, Aldh1a1, Aldh1a2, and Aldh1a7 were increased in the mouse ovary by equine chorionic gonadotropin (eCG) priming (17). Inhibition of ALDH activity significantly suppressed the number of oocytes released in response to an ovulatory dose of human chorionic gonadotropin (hCG) (17). ALDH family members are known to not only induce RA but also to degrade the steroidogenic metabolic byproduct acetaldehyde, which can have toxic effects on cells (18). Injection of an ALDH inhibitor in vivo increased ovarian levels of acetaldehyde in ovary and granulosa cell apoptosis (17). However, our previous study did not resolve the physiological roles of the ALDH family in the production of cellular RA.
RA binds the nuclear receptors, retinoic acid receptor (RAR) or retinoid X receptor (19). The expression of RARs and retinoid X receptors in the rat ovary and the positive effects of RA on granulosa cell proliferation and oocyte maturation in vitro have been reported (20–23), suggesting that de novo synthesis of RA from retinol in antral follicles is required for the induction of granulosa cell differentiation and the acquisition of responsiveness to ovulation stimuli. However, less is known about the metabolic pathways that control RA biosynthesis in the ovary in vivo and the roles of de novo-synthesized RA in follicular development in a molecular level. Therefore, in this study we investigated which members of the ADH and ALDH families were expressed in preovulatory follicles and analyzed the role of RA in vivo using two different approaches: 1) mice were fed a retinol-free, vitamin A-deficient (VAD) diet or 2) mice were injected with a pharmacological ADH inhibitor. The de novo-synthesized RA was monitored using RA-responsible element (RARE) reporter mice harboring a RA-responsive transgene (RARE-Hspa1b-LacZ), which allows the visualization of the distribution of RA signaling by X-galactosidase (X-Gal) staining in the intact ovary.
Materials and Methods
Materials
eCG and hCG were purchased from Asuka Seiyaku. DMEM-F12 medium and penicillin-streptomycin were purchased from Invitrogen, fetal bovine serum from Life Technologies Inc, oligonucleotide poly-(deoxythymidine) from Invitrogen, and avian myeloma virus reverse transcriptase from Promega Corp. Routine chemicals and reagents were obtained from Nacalai Chemical Co or Sigma Chemical Co.
Animals
Immature female (3 wk old) C57BL/6 mice were obtained from Charles River Laboratories Japan. On day 23 of age, female mice were injected ip with 4 IU of eCG to stimulate follicular growth followed 48 hours later with 5 IU hCG to stimulate ovulation and luteinization. Other immature mice used for nutritional experiments were fed a vitamin A-deficient (VAD) diet (Oriental Bio-service Co) for up to 6 weeks. Some of the VAD mice were injected with eCG and/or 2.5 mg/kg body weight of RA (Sigma Chemical Co) followed 48 hours later with 5 IU hCG. To analyze the transcriptional activity of RAR in the ovary, RARE reporter mice (CD1) harboring a RA-responsive transgene (RARE-Hspa1b-LacZ) were obtained from Jackson Laboratory. The RARE reporter mice allow the visualization of the distribution of RA signaling by X-Gal staining (24). Animals were housed under a 12-hour light, 12-hour dark schedule at the Experiment Animal Center at Hiroshima University and provided with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Hiroshima University.
Vaginal smear tests
Vaginal components were collected (in PBS[−]) at the same time each morning and were smeared onto a glass slide and fully air dried. These samples were fixed for methanol (Nacalai Chemical Co). After fixation, these samples were washed with water from back side of the slide glass and stained with Giemsa stain (WAKO). Evaluations of vaginal smear image were observed under a system microscope CX41 (Olympus).
Collection of fertilized eggs and development to the blastocyst stage
Female mice fed the VAD diet or a normal diet for 6 weeks from weaning were injected ip with eCG (5 IU) at the diestrous stage of the estrus cycle. After 48 hours these mice were treated with hCG (5 IU). At 16 hours after the hCG administration, each female mouse was mated with an adult male mouse, and mating was confirmed by the presence of the vaginal plug the following morning. Fertilized/unfertilized eggs or fragmented eggs were collected from oviducts by perfusion using 0.4% (wt/vol) PVP (Sigma Chemical Co) supplemented by DMEM-F12 containing penicillin and streptomycin (Invitrogen) after 8 hours from mating. The fertilized eggs were cultured in a development medium (KSOM + amino acid; Millipore) for 3 days. At that time the number of embryos developed to the blastocyst stage was scored.
The treatment with 4-methylpyrazole (4MP)
Some of the immature mice (3 wk old) were injected ip with eCG (4 IU) to induce follicular development using the routine ovulatory regimen. During this period, 8 mg/kg body weight of 4MP (Sigma Chemical Co) were injected two times every 24 hours. After 48 hours, some of the treated mice were killed to collect the samples. Other mice were treated with hCG (5 IU) to induce ovulation. To overcome the inhibitory effects of 4MP, we injected RA (2.5 mg/kg body weight) or 17β-estradiol (10 mg/kg body weight; Sigma) into eCG-primed immature mice treated with 4MP or vehicle.
To identify the effects of 4MP on the ovulation process, immature mice were treated with eCG alone to induce follicular development. After 48 hours, the mice were further treated with hCG and 4MP. Sixteen hour after hCG injection, the ovulated cumulus-oocyte complexes (COCs) and oocytes were collected from oviducts and counted.
The detection of retinol level by HPLC-mass spectrometry
Female mice were fed a VAD diet or a normal diet for 6 weeks from weaning. Tail blood was collected on every week. Blood samples were centrifuged at 10 000 × g for 1 minute. The supernatant was stored at −80°C until use. After 6 weeks, the mice were killed. Liver samples and ovaries were isolated from these mice and stored at −80°C until use.
Retinol extraction from the liver and ovaries of control and VAD mice was performed based on the method described by Kane et al (25). Specifically, mouse tissues were homogenized in H2O (125 mg/500 μL) and then 1 mL of 0.025 M KOH/ethanol was added and gradually mixed. Ten microliters of hexane were added, and the samples were vortexed for 15 minutes. After centrifugation at 750 × g for 3 minutes to facilitate phase separation, the hexane layer (8 mL) was transferred to separate tube. The hexane layer was evaporated by vacuum extraction for 12 hours using a centrifugal concentrator (VC-96N; TAITEC) under light-blocking conditions. Samples were suspended in 500 μL of methanol and filtrated by a 0.45-μm cosmonice filter S (Nacalai Chemical Co).
Serum samples (50 μL each) were mixed with 600 μL methanol. Then these samples were allowed to stand at room temperature. After 15 minutes, these samples were centrifuged at 10 000 × g for 5 minutes, and then 500 μL of supernatants filtrated by a 0.45-μm cosmonice filter S were collected to another tube.
Retinol levels in each tissue and the serum sample were determined by ACQUITY ultraperformance liquid chromotography BEH reverse-phase C18 2.1 × 50 mm 1.7 μm column (Waters) using ultraperformance liquid chromotography and mass spectrometry ACQUITY SQD systems (Waters). The injection volume was 5 μL. A gradient curve was generated at 0.4 mL/min: 0–10 minutes, 100% A to 100% B. Mobile-phase A consisted of methanol/H2O/formic acid (75:25:0.1, vol/vol/vol); mobile-phase B consisted of methanol/H2O/formic acid (100:0:0.1, vol/vol/vol). The detection was performed at 325 nm. These data were analyzed with MassLynx4.1 software (Waters). The instrumental coefficient of variance was obtained by repeat measurements of standard samples (6.41%). Standard curves are linear between 0.01 and 1 nmol/mL. Each recovery rate of retinol in each tissue and serum sample was 73.33% in the tissue sample and 91.10% in the serum sample.
Quantitative determination of estrogen levels in serum
The level of serum estrogen during follicular development was determined using a rodent estradiol ELISA test kit (Endocrine Technologies, Inc) according to the manufacturer's instructions. Serum samples (50 μL each) were analyzed using a microplate reader to determine the amount of substrate converted at 450 nm.
RNA extraction and RT-PCR
Total RNA was obtained from mouse ovaries or granulosa cells using the RNAeasy mini kit (QIAGEN Sciences) according to the manufacturer's instruction. One hundred nanograms of total RNA were reverse transcribed using 500 ng polydeoxythymidine (Amersham Pharmacia Biotech) and 0.25 U avian myeloblastosis virus-reverse transcriptase (Promega Corp) at 42°C for 75 minutes and 95°C for 5 minutes. RT-PCR analyses were performed with KOD FX Neo (TOYOBO Life Science) according to the manufacturer's instructions. Specific primers pairs used in the RT-PCRs are shown in Supplemental Table 1; cDNA products were resolved on 2% (wt/vol) agarose gels.
Real-time quantitative PCR assay
cDNA and primers were added to 15 μL total reaction volume of the Power SYBR Green PCR master mix (Applied Biosystems). PCRs were then performed using the StepOne real-time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 minutes at 95°C followed by 45 cycles each of 15 seconds at 95°C and 1 minute at 60°C–64°C. Specific primers pairs were selected and analyzed as indicated in Supplemental Table 1. L19 was used as a control for reaction efficiency and variations in concentrations of mRNA in the original reverse transcription reaction.
Western blot analyses
Protein samples were obtained by homogenizing whole ovaries and by lysing granulosa cells in the lysate buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] sodium deoxycholate, 1 mM EDTA, and 0.1% [wt/vol] sodium dodecyl sulfate) containing complete protease inhibitors (Roche Diagnostics GmbH) and then diluted with 2× sodium dodecyl sulfate sample buffer. Proteins (10 μg protein) were separated by SDS-PAGE (10%) and transferred to polyvinyl difluoride membranes (GE Bioscience). Membranes were blocked in Tris-buffered saline and Tween 20 (10 mM Tris, pH 7.5; 150 mM NaCl; and 0.05% Tween 20) containing 5% nonfat Carnation instant milk (Nestle Co). Blots were incubated with primary antibody (1:500 of anti-STRA6 antibody; Abcam; 1:1000 of anti-ALDH1A1 antibody; Abcam; 1:1000 of anti-ADH1 antibody; Gene Tex; 1:1000 of anti-ADH5 antibody; ProteinTech; 1:500 of anti-RARγ antibody; Signalway Antibody; 1:10 000 of anti-βactin antibody; Sigma Chemical Co) overnight at 4°C. After washing in Tris-buffered saline and Tween 20, enhanced chemiluminescence detection was performed by using the enhanced chemiluminescence system according to the manufacture's specifications (GE Bioscience) and appropriate exposure of the blots to Fuji X-ray film (Fujifilm).
Immunofluorescence staining
Intact ovaries were collected from immature mice treated with or without eCG were fixed in 4% paraformaldehyde (Nacalai Chemical Co) for 24 hours at 4°C. Subsequently, these tissues were washed with PBS and were embedded in paraffin wax (Therrmo Fisher Scientific Inc) after having been dehydrated. Paraffin-embedded tissue sections (5 μm) were deparaffinized. After washing with PBS, the sections were treated with 10 mM citric acid buffer (pH 6.0) (Nacalai Chemical Co) for antigen activation for 15 minutes in boiling water. Then the sections were washed three times for 5 minutes with PBS and blocked by 5% (vol/vol) BSA (Nacalai Chemical Co). The sections were incubated with a primary antibody (1:100 of anti-STRA6 antibody, anti-ALDH1A1 antibody, anti-ADH1 antibody, anti-ADH5 antibody, anti-RARγ antibody, anti-CYP26B1 antibody [Proteintech Group, Inc]) in 5% (vol/vol) BSA (Nacalai Chemical Co) overnight at 4°C. After washing by 0.3% (vol/vol) Triton X-100 in PBS(−), the ovaries were visualized with Cy3-conjugated goat antirabbit IgG (1:100; Sigma Chemical Co) and 4′,6′-diamino-2-phenylindole (Vector Laboratories Inc). Digital images were captured using Keyence BZ-9000 microscope (Keyence Co).
Hematoxylin-eosin staining
Intact ovaries were fixed, embedded, and sectioned as above. After deparaffinization and washing with PBS, tissues were stained with eosin (Sakura-Finetek Japan) and hematoxylin (Sakura-Finetek Japan) to visualize the cytoplasm and nuclei, respectively. Tissues were observed under light microscope.
X-Gal staining
For whole-mount staining, fresh ovaries were collected from RARE-lacZ transgenic mice treated with or without eCG. Collected ovaries were fixed at room temperature for 1 hour in 0.5% (vol/vol) of glutalaldehyde, 1.25 mM EGTA, and 0.4 ml 2 mM of MgCl2. Tissues were rinsed with wash solution (2 mM MgCl2, 0.01% [vol/vol] deoxycholate, and 0.02% [vol/vol] Nonidet P-40). X-Gal staining was performed with a stain solution containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6/3H2O, and 1 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal substrate; Sigma Chemical Co) in wash solution and incubated at 37°C for 24 hours. The tissues were postfixed in 2% (wt/vol) of paraformaldehyde at 4°C for 24 hours and transferred to optimum cutting temperature compound (Sakura-Finetek Japan) on dry ice. The optimum cutting temperature-embedded tissues were stored at −80°C. Tissues were sectioned at 10 μm and were observed under a light microscope.
Quantitative β-galactosidase activity assay
Ovaries or granulosa cells were lysed with cell extract buffer (20 mM sodium phosphate solution, pH 7.2; 50 mM 2-mercaptoethanol solution; and 1 mM MgCl2). After ultrasonication, extracts were centrifuged at 15 000 rpm at 4°C for 10 minutes; protein concentrations were determined in each supernatant. Ten microliters of 30 mg/mL chlorophenolred-β-D-galactopyranoside (CPRG)/double-distilled H2O were added to each 200-μg cell extract and incubated at 37°C for 12 hours. The reaction was stopped by adding 100 μL of 1 M sodium carbonate. β-Galactosidase activity was measured using a microplate reader to determine the amount of substrate converted at 595 nm every hour.
Three-dimensional ovarian follicle culture
Ovaries isolated from immature mice were cut and opened by forceps to form a sheet under the microscope. After washing with PBS, the ovarian sheet was placed into 0.5% (wt/vol) sodium alginate (WAKO)/PBS (Ca2+, Mg2+ free) and then dropped into 50 mM CaCl2 (Nacalai Chemical Co)/saline to cross-link the alginate. The piece of ovary was rinsed and then cultured for 48 hours in the medium (DMEM-F12 containing penicillin and streptomycin) containing 500 ng/mL ovine FSH (National Institute of Diabetes and Digestive and Kidney Diseases, Torrance, CA) and 10 ng/mL T (Sigma Chemical Co) in the presence or absence of 1% (vol/vol) serum. These ovaries were further treated with the ADH inhibitor, 4MP. Retinol was also added at the concentration described elsewhere (see Figure 7A.
Figure 7.
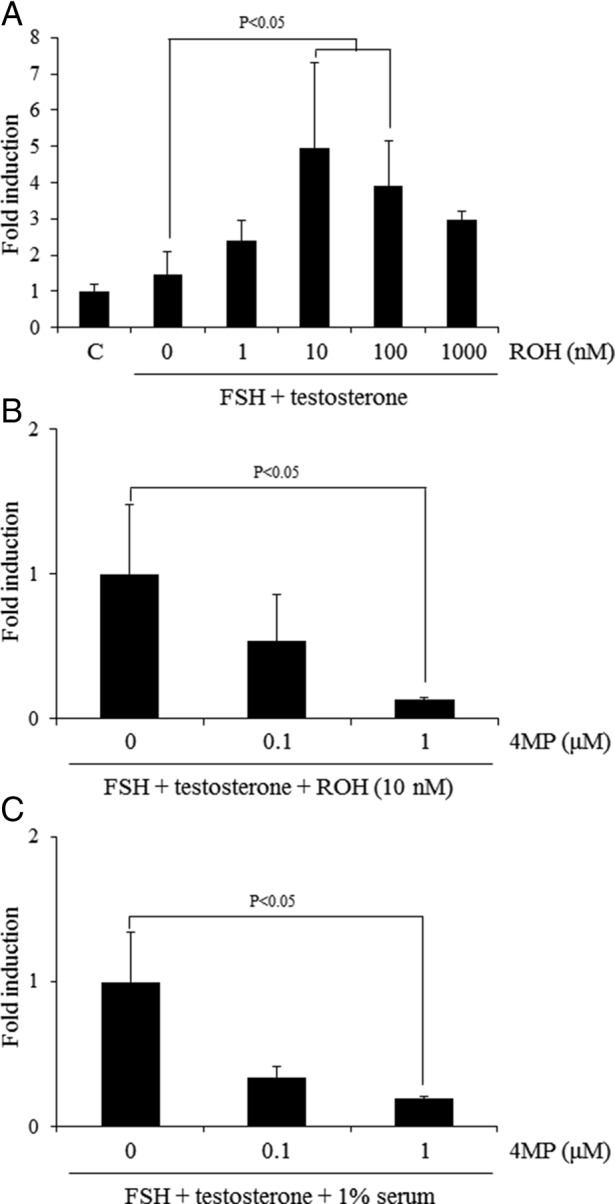
The effect of activation of the RA synthesis from retinol in the ovary on the Lhcgr expression. A, The effects of additional retinol (ROH) to FSH+T-containing medium on the expression of Lhcgr when the ovary was cultured in the absence of serum. For reference, the control (C) value was set as 1, and the data are presented as fold induction. The addition of 10 or 100 nM of retinol (ROH) to FSH and T-containing medium significantly increased the expression level of Lhcgr as compared with those in the ovaries cultured for 48 hours with FSH and T (P < .05). Values are mean ± SEM of three replicates. B, The conversion from retinol to RA is required for the induction of Lhcgr expression in vitro under serum-free condition. For reference, FSH+T+ROH (10 nM) value was set as 1, and the data are presented as fold induction. **, The treatment with 1 μM 4MP significantly decreased the expression level of Lhcgr as compared with that in ovary cultured with FSH and T with 10 nM of retinol (ROH) (P < .05). Values are mean ± SEM of three replicates. C, Ovaries of immature day 21 mice were embedded in alginate gels and then cultured for 48 hours without any hormones. The conversion from retinol to RA is required for the induction of Lhcgr expression in vitro under 1% serum condition. For reference, FSH+T value was set as 1, and the data are presented as fold induction. ***, The treatment with 1 μM 4MP significantly decreased the expression level of Lhcgr as compared with that in ovary cultured with FSH and T (P < .05). Values are mean ± SEM of three replicates.
Statistics
Statistical analyses of data from three or four replicates for comparison were carried out by either a Student's t test or a one-way ANOVA followed by Duncan's multiple-range test (Statview; Abacus Concepts, Inc). In some of the VAD mice studies, the data obtained from three or four mice were carried out by a two-way ANOVA.
Results
Expression of retinol-retinoic acid metabolic pathway genes in ovaries and granulosa cells of eCG-stimulated mice
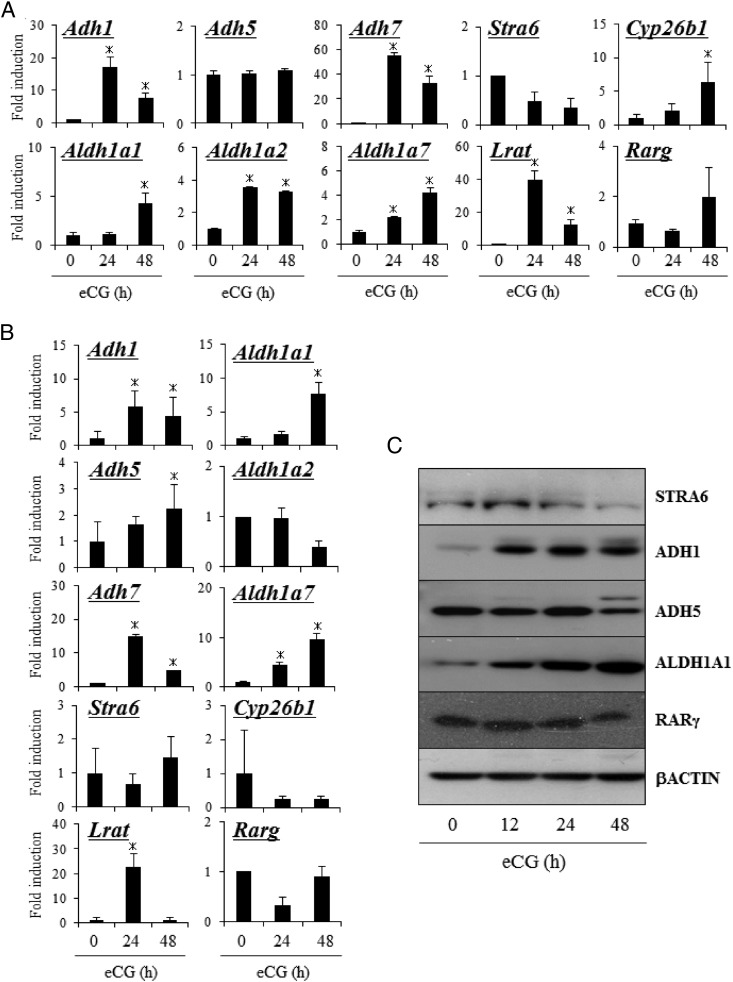
Using primer sets that recognize each of the ADH and ALDH1 family members, mRNA expression for three members of the ADH family (Adh1, Adh5, and Adh7) and three members of the ALDH1 family (Aldh1a1, Aldh1a2, and Aldh1a7) were detected by RT-PCR in eCG-stimulated mouse ovaries (Supplemental Figure 2). To determine the kinetic changes in the expression of these genes during follicular development, immature mice were treated with eCG to stimulate preovulatory follicle development. Using whole-ovary samples, the expression levels of Adh1 and Adh7, Aldh1a1, Aldh1a2, and Aldh1a7 as well as genes involved in RA metabolism (Lrat and Cyp26b1) were significantly increased, whereas Adh5, Stra6, and Rarg did not change in response to eCG (Figure 1A). When granulosa cells were analyzed, Adh5 was induced by eCG treatment concomitantly with the induction of Adh1 and Adh7, Aldh1a1, and Aldh1a7, and Lrat (Figure 1B). Aldh1a2, Stra6, Cyp26b1, and Rarg were not induced (Figure 1B).
Figure 1.
The expression of genes related to RA synthesis in ovary of mice treated with or without eCG. A, Kinetic changes in the expression of Stra6, Adh1,5,7, Aldh1a1, Aldh1a2, Aldh1a7, Stra6, Lrat, Cyp26b1, and Rarg mRNA in ovaries of mice treated with or without eCG. For reference, the eCG 0 hour value in ovary is set as 1, and the data are presented as fold induction. Values are mean ± SEM of three replicates. *, Significant differences are observed between ovaries from control compared with those of eCG-primed mice (eCG 0 h) (P < .05). B, Kinetic changes in the expression of Stra6, Adh1, Aldh5, Aldh7, Aldh1a1, Aldh1a2, Aldh1a7, Stra6, Lrat, Cyp26b1, and Rarg mRNA in granulosa cells (GCs) of mice treated with or without eCG. For reference, the eCG 0 hour value in GCs is set as 1, and the data are presented as fold induction. Values are mean ± SEM of three replicates. *, Significant differences are observed between GCs from control mice compared with those of eCG-primed mice (eCG 0 h) (P < .05). C, The expression of STRA6, ADH1, ADH5, ALDH1A1, RARγ, and β-actin protein in whole-ovary samples detected by Western blot analysis.
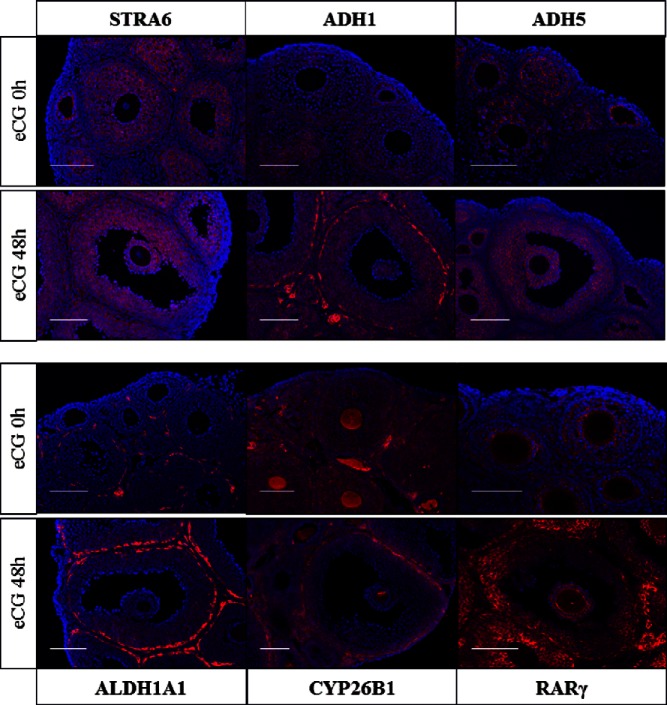
The levels of ADH1 and ALDH1A1 protein in the ovary were increased after eCG injection as were their mRNAs (Figure 1C, Supplemental Figure 1). STRA6, ADH5 and RARγ were also detected in the ovarian samples by Western blotting (Figure 1C and Supplemental Figure 1). The localization of each protein was analyzed by immunofluorescence staining using specific antibodies (Figure 2). Positive signals for STRA6 were detected in granulosa cells of ovaries with or without eCG treatment; ADH5 was predominantly localized to granulosa cells of preovulatory follicles after eCG treatment. By contrast, intense signals for ADH1 and ALDH1A1 were evident in theca cells, whereas the positive signals for both were also detected in granulosa cells after eCG injection. CYP26B1 was detected in granulosa cells before eCG injection, but its expression was decreased and was predominantly localized in theca cells after eCG injection (Figure 2). Thus, the ADH1-ALDH1A1 pathway is expressed primarily in theca cells, whereas the ADH5-ALDH1A1/7 pathway is present primarily in granulosa cells of antral follicles of the eCG-primed mouse ovary. Within the follicle, RA can either be metabolized by CYP26B1 that is induced in theca cells or can act in granulosa cells to stimulate differentiation during follicular development.
Figure 2.
The localization of factors related to RA synthesis and its functions in ovary of mice treated with or without eCG. Localizations of STRA6, ADH1, ADH5, ALDH1A1, CYP26B1, and RARγ in ovary were detected by fluorescent staining in the ovary of mice treated with or without eCG for 48 hours. Each protein was visualized with Cy3 and nuclear was stained with 4′,6′-diamino-2-phenylindole. Scale bur is 100 μm.
The de novo-synthesized RA induces gene expression in eCG-primed granulosa cells
To investigate whether RA was produced in both theca cells and granulosa cells and then acted as a ligand in these cells after eCG stimulation, we used RARE reporter mice that harbor a RA-responsive transgene (RARE-Hspa1b-LacZ), which allows the visualization of RA signaling by X-Gal staining (26). In the ovaries of 3-week-old mice, a signal was detected in granulosa cells of early secondary follicles. However, positive signals were low in the granulosa cells of large secondary follicles and antral follicles in the ovaries of untreated mice but were increased in granulosa and theca cells by eCG injections (Figure 3A). RARE-LacZ activity in whole ovary or granulosa cells during follicular development was quantitated by β-galactosidase assay system using CPRG (sensitive substrate). RARE-LacZ activity in whole ovaries and granulosa cells was significantly increased by eCG injection (Figure 3B).
Figure 3.

The detection of de novo synthesized RA in ovary using RARE reporter mice. A, Localization of LacZ-positive cells in the ovary of RARE reporter mice treated with eCG or without eCG (control). B, Activity of LacZ enzyme driven by RARE promoter in ovary (left panel) or granulosa cells (right panel) of RARE reporter mice treated with or without eCG. Values were absorbance in 595 nm after the protein samples were incubated with the substrate, CPRG, for 2 hours at 37°C. #, The RARE-LacZ activity is presented as fold induction. The control value in ovary (left panel) or granulosa cells (right panel) is set as 1. Values are mean ± SEM of three replicates. eCG treatment significantly increased the RARE-LacZ enzyme activity in ovaries (P < .01) or granulosa cells (P < .05) of mice as compared with those in mice without eCG treatment (control), respectively.
VAD alters follicular development
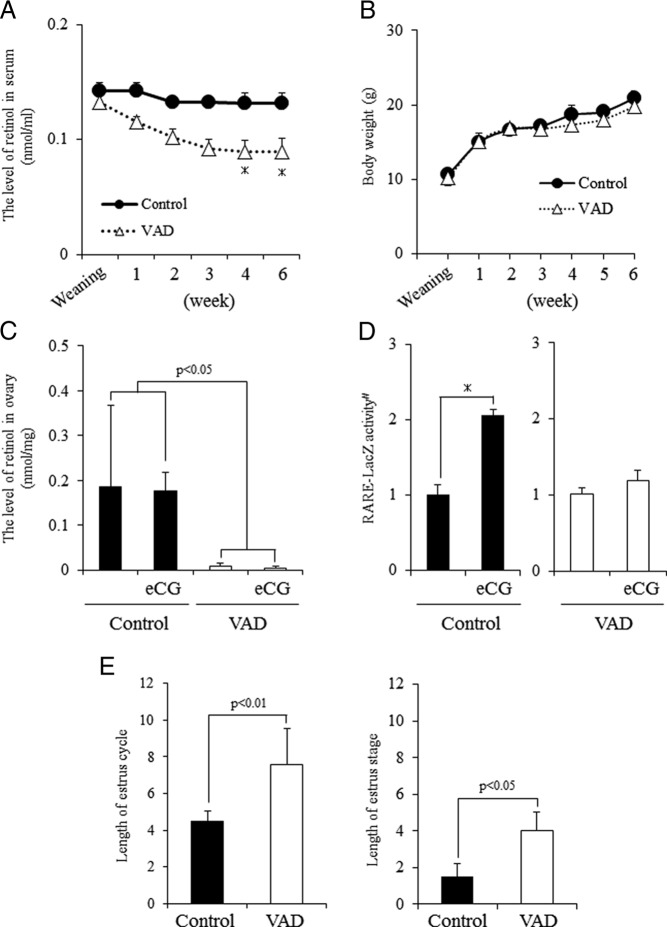
To determine the functional roles of the retinol-retinoic acid metabolic pathways in growing follicles, mice were fed a control diet or a VAD diet after weaning. The VAD diet significantly decreased the serum levels of retinol by 2 weeks after weaning with the lowest levels observed by 4 and 6 weeks (Figure 4A). The VAD diet did not affect body weight (Figure 4B). Because retinol accumulates in the liver as retinol ester (27, 28), the levels of retinol in liver tissue of control and VAD diet mice were examined. The levels of retinol in livers of the mice fed the VAD diet were significantly reduced compared with mice fed the control diet (Supplemental Figure 3B). Similarly, ovarian levels of retinol were significantly reduced in the mice fed the VAD diet compared with mice on the control diet (Figure 4C). Additionally, RARE-LacZ activity in granulosa cells was significantly increased at 48 hours after eCG injection in mice fed a control diet but was not significantly changed in mice fed the VAD diet (Figure 4D). The estrous cycle of VAD diet mice was significantly prolonged due to a long estrous stage as compared with that in control mice (Figure 4E and Supplemental Figure 3A), suggesting that abnormal follicular development were induced in VAD diet mice.
Figure 4.
Characteristics of mice fed vitamin A (retinol)-deficient diets from weaning. A, Kinetic change of retinol levels in serum in mice fed a normal diet (control) or VAD. *, Significant differences are observed at each time interval as compared with control mice (P < .05). B, The body weight of control mice or VAD mice. C, Levels of retinol in the ovary of control mice or VAD mice with or without eCG treatment. eCG, 48 hours after eCG priming. Values are mean ± SEM of three replicates. Statistical analysis was done by a two-way ANOVA. Significant differences are observed between control and VAD mice (P < .05) but not between treatment groups with or without eCG priming. D, Activity of LacZ enzyme driven by RARE promoter in granulosa cells of control or VAD mice with or without eCG priming. eCG, 48 hours after eCG priming. Values are absorbance in 595 nm after incubation for 2 hours at 37°C from the addition of LacZ enzyme substrate, CPRG. #, The RARE-LacZ activity is presented as fold induction. The eCG 0 hours value in granulosa cells is set as 1. Values are mean ± SEM of three replicates. *, Significant induction is observed by eCG treatment in control mice (P < .05). E, Length of the estrus cycle and the length of the estrous stage of control mice or VAD mice. Values are mean ± SEM of five mice.
The large antral follicles observed in ovaries of mice fed the VAD diet 48 hours after eCG stimulation were similar to those in mice on the control diet (Supplemental Figure 4A). However, the number of oocytes ovulated by eCG/hCG treatments was significantly lower in the VAD mice than that in control mice (Table 1). Fifty percent of the oocytes ovulated in the VAD mice lacked a first polar body, and the rate of oocyte maturation in these mice was significantly reduced compared with that in control mice (Table 1). Furthermore, when the eCG-hCG primed VAD and control female mice were mated to fertile males, the fertilization rate (the number of oocytes forming pronuclear per the number of matured oocytes) was similar in the VAD mice to the controls (Table 1). However, when the putative fertilized eggs were retrieved and cultured for 3 days, more than 80% of eggs developed to the blastocyst stage in control mice compared with only about 40% in those retrieved from the VAD mice (Table 1).
Table 1.
The Effects of VAD Mice on the Fertilization and Oocyte Developmental Compartment in Vivo
| Average Number of Ovulated Oocytes | Rate of Oocyte Maturation, % | Rate of Fertilization, % | Rate of Blastocyst, % | |
|---|---|---|---|---|
| Control (n = 4) | 25.75 ± 1.75 | 87.45 ± 1.62 | 84.54 ± 3.67 | 75.68 ± 16.32 |
| VAD (n = 4) | 11.75 ± 3.15a | 45.83 ± 14.73a | 84.72 ± 11.87 | 28.98 ± 21.47a |
Rate of oocyte maturation was mature oocytes per ovulated oocytes. Fertilization rate was two cell embryos per ovulated oocytes. The rate of blastocyst stage was blastocyst stage per two cell embryos.
The significant difference was observed between the treatment groups.
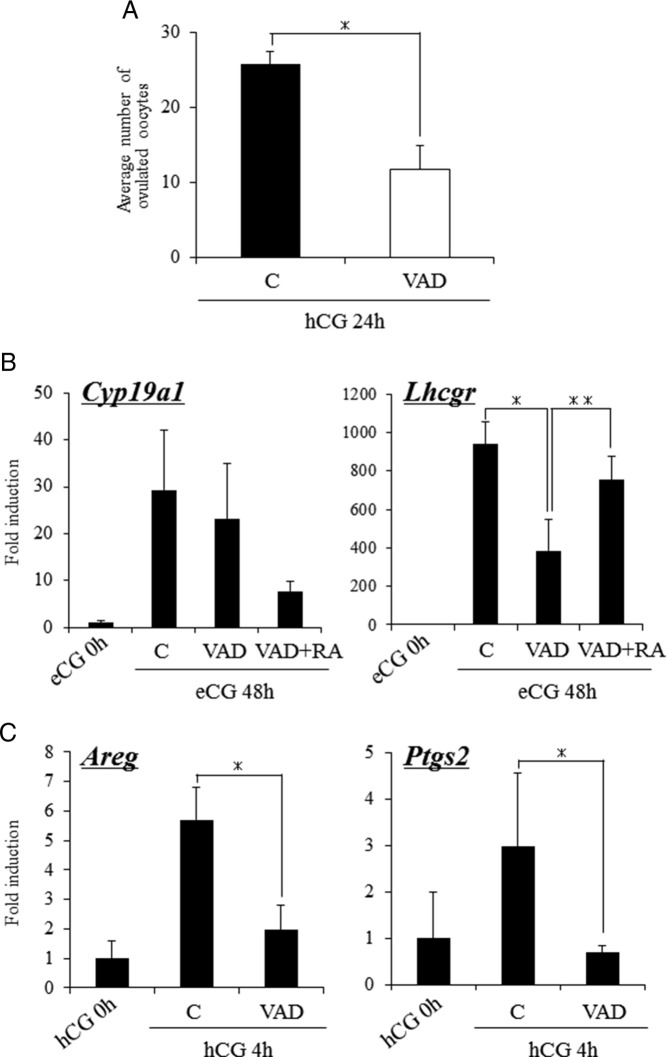
We next determined whether low levels of retinol/RA altered the expression of genes involved in ovulation. Expression of the granulosa cell marker gene Cyp19a1 was induced similarly by eCG in both control and VAD mice (Figure 5B). However, the level of Lhcgr mRNA was significantly lower in the eCG-treated VAD mice (Figure 5B) but was restored to control levels by RA administration (Figure 5B). Because the levels of Lhcgr mRNA were reduced in the VAD mice, we next determined whether genes associated with LH induction of ovulation (29) were altered in these mice. To analyze this eCG-primed control and VAD mice were injected with hCG and ovarian RNA was prepared 4 hours later. hCG significantly induced Areg and Ptgs2 mRNA in the control but not in the VAD mice (Figure 5C).
Figure 5.
Ovarian function of mice fed a VAD diet. A, The effects of the VAD diet on the ovulation of hormone-treated mice. Mice fed normal diet (control) and VAD diet were injected with eCG. After 48 hours, these mice were further treated with hCG. For 24 hours after hCG injection, the ovulated oocytes were collected from oviducts and then the number of them was counted. Values are mean ± SEM of 4 mice. *, Significant differences are observed as compared with those in control mice (P < .05). B, The expression of genes known to be markers of follicular development, Cyp19a1 and Lhcgr, in granulosa cells of control mice and VAD mice injected with eCG and/or RA. For reference, the eCG 0 hours values are set as 1, and the data are presented as fold induction. *, Significant differences are observed between control (C) and VAD groups (P < .05); **, the addition of RA to VAD mice (RA+VAD) significantly increased the expression of Lhcgr in VAD mice (P < .05). Values are mean ± SEM of three replicates. RA, coinjection with eCG and RA (2.5 mg/kg) for 48 hours. C, The expression of genes known to be the target of the LH-LH receptor (LHR)-dependent pathway in granulosa cells of control mice and VAD mice injected with hCG for 4 hours. For reference, the hCG 0 hour values are set as 1, and the data are presented as fold induction. *, Significant differences are observed as compared with those in ovaries of mice fed normal diet between control and VAD mice. Values are mean ± SEM of three replicates.
Effects of an ADH inhibitor on ovarian responses to gonadotropins
To determine the roles of the retinol-retinoic acid metabolic pathway in more detail, we injected the ADH inhibitor, 4MP, to immature hormonally primed mice. More than 30 oocytes were collected from the oviduct of eCG-hCG treated mice (Figure 6A). The number of ovulated oocytes was significantly lower in mice that were treated with eCG+4MP prior to hCG (Figure 6A). On the other hand, when 4MP was injected with hCG to eCG-primed mice, the number of ovulated COCs was similar to that in control mice without any 4MP (Figure 6A). These results indicate that the de novo synthesis of RA in antral follicles is required during follicular development to enhance responsiveness to the ovulatory LH surge.
Figure 6.
The effects of ADH inhibitor on follicular development and/or ovulation process. A, The number of ovulated oocytes when the mice were coadministered with eCG and ADH inhibitor (4MP) followed by hCG injection or hCG and 4MP after eCG stimulation. Day 23 immature female mice were coinjected with eCG and 4MP (8 mg/kg). After 48 hours, these mice were treated with hCG. In other group, day 23 immature female mice treated with eCG for 48 hours were coinjected with hCG and 4MP. For 16 hours after hCG injection, the ovulated oocytes were collected from oviducts and counted. Values are mean ± SEM of three mice. *, Significant differences were observed as compared with that in control (P < .05). Control, immature female mice were treated with eCG followed by hCG stimulation. eCG+4MP; the mice were coinjected with eCG and 4MP (8 mg/kg) for 48 hours, and then hCG was injected into the mice. hCG+4MP, the mice were coinjected with hCG and 4MP (8 mg/kg) 48 hours after eCG injection. B, The expression of Lhcgr and Cyp19a1in granulosa cells of mice treated with eCG and 4MP for 48 hours or the expression of LH-LHR target genes, Areg and Ptgs2, in granulosa cells of mice injected with hCG for 4 hours after eCG and 4MP treatment. For reference, the values of eCG 0 hour are set as 1, and the data are presented as fold induction. *, 4MP treatment significantly suppressed the gene expressions as compared with those in control mice treated with eCG alone (P < .05). Values are mean ± SEM of three replicates. C, Activity of LacZ enzyme driven by RARE promoter in granulosa cells of eCG-stimulated mice with or without 4MP treatment for 48 hours. Values are absorbance in 595 nm after the protein samples are incubated for 2 hours at 37°C with CPRG, LacZ substrate. #, The RARE-LacZ activity is presented as fold induction. The eCG 0 hour value in granulosa cells is set as 1. Values are mean ± SEM of three replicates. *, 4MP treatment significantly decreased the level of LacZ enzyme activity after eCG stimulation (P < .05). D, Circular levels of estrogen in mice treated with eCG and/or 4MP, RA, or 17β-estradiol. *, The estrogen level was significantly increased by eCG injection (P < .05); however, there was no significant difference among the treatment group of eCG-primed mice (P < .05). Values are mean ± SEM of three replicates. 4MP, Mice were coinjected with eCG and 4MP (8 mg/kg) for 48 hours. E2, Mice were coinjected with eCG+4MP and 17β-estradiol (10 mg/kg) for 48 hours. RA; mice were coinjected with eCG+4MP and RA (2.5 mg/kg) for 48 hours. E, The addition of RA treatment overcomes 4MP-induced negative effect of Lhcgr expression in granulosa cells. #, The expression level of Lhcgr is presented as fold induction. The eCG 0 hour value is set as 1. *, 4MP treatment significantly decreased the expression of Lhcgr in granulosa cells of eCG-stimulated mice (P < .05); **, the addition of RA but not 17β-estradiol (E2) significantly increased the level of Lhcgr expression as compared with that in granulosa cells of mice cotreated with eCG and 4MP. Values are mean ± SEM of three replicates.
Cotreatment of mice with 4MP and eCG significantly decreased the induction of Lhcgr similar to that observed in the eCG-treated VAD mice with the reduction of RARE-LacZ activity in granulosa cells (Figure 6, B and C). However, large antral follicles were observed in ovaries of mice injected with eCG and 4MP (Supplemental Figure 4B). It has been reported that the expression of Lhcgr in granulosa cells is associated with the production of estrogen in preovulatory follicles (30). Therefore, serum levels of estrogen in mice injected with eCG or eCG+4MP were analyzed; no differences among treatments were detected (Figure 6D). Moreover, administration of RA to mice injected with eCG+4MP overcame the low level expression of Lhcgr, whereas coinjection of estrogen had no effect (Figure 6E). The injection of RA also reversed the negative effects of 4MP not only on ovulation after hCG injection but also the developmental competence of ovulated oocytes (Table 2).
Table 2.
Ovulation and Oocyte Developmental Competence eCG/hCG-Primed Mice Further Treated With 4MP and/or RA
| Number of Ovulated Oocytes | Rate of Fertilization, % | Rate of Blastocyst, % | |
|---|---|---|---|
| Control (n = 3) | 55.30 ± 2.91 | 71.98 ± 6.91 | 45.02 ± 4.50 |
| 4MP (n = 4) | 44.00 ± 2.04a | 14.79 ± 5.13a | 0.00 ± 0.00a |
| 4MP+RA (n = 4) | 53.75 ± 3.97b | 78.60 ± 3.98b | 50.23 ± 2.63b |
Fertilization rate was two cell embryos per ovulated oocytes. The rate of blastocyst stage was blastocyst stage per ovulated oocytes.
The significant difference was observed between the control group and the 4MP treatment group.
The significant difference was observed between the 4MP treatment group and the 4MP+RA treatment group.
Thus, the de novo-synthesized RA impacts the expression of Lhcgr in granulosa cells to acquire the responsible ability to LH, which induces successful ovulation process.
Effects of retinol on Lhcgr expression in granulosa cells in vitro
The addition of FSH+T did not induce the Lhcgr expression as compared with that in control (without any hormone), whereas the addition of retinol to the FSH+T containing medium increased Lhcgr mRNA levels in a dose-dependent manner with the maximum effect observed at 10 nM (Figure 7A). The retinol-induced Lhcgr expression in ovary cultured with FSH+T was significantly suppressed by 4MP in a dose-dependent manner (Figure 7B). Moreover, the expression of Lhcgr, when the ovary was cultured with FSH+T in the presence of serum containing physiological levels of retinol, was also significantly suppressed by 4MP in a dose-dependent manner (Figure 7C). These results suggest that the uptake of retinol in granulosa cells from serum and its conversion to retinoic acid enhance the induction of granulosa cell differentiation in response to FSH.
Discussion
RA plays a key role in many developmental processes (31) including gonadal specification (32) in which the expression of the RA-metabolizing enzyme CYP26B1 is critical for early testis development (33). In the ovary, regulated expression of Cyp26b1 by activin has been documented and is inversely related to granulosa cell proliferation and antral follicle development from secondary follicles (10). In other words, ovarian produced RA may impact granulosa cell proliferation by facilitating FSH-mediated functions. However, there have been no reports determining how RA is produced within the ovary or antral follicles to induce the differentiation of granulosa cells. Herein we document that the expression of genes involved in the cellular uptake of retinol from serum and the biosynthesis of RA from retinol are highly and differentially regulated in theca cells and granulosa cells during antral and preovulatory follicular development. Specifically, we show that the enzymes of the ADH family that are involved in the conversion of retinol to retinal and those of the ALDH family that convert retinal to RA (14) are preferentially expressed in either granulosa cells (Adh5-Aldh1a7) or theca cells (Adh1-Aldh1a1). Moreover, we show that CYP26B1 is also differentially localized in the ovary before and after eCG injection. Before eCG injection, CYP26B1 is expressed in both granulosa cells and theca cells. However, the expression was down-regulated in granulosa cells but was induced in theca cells in preovulatory follicles. These results were correlated with RA activity as detected in granulosa cells of LacZ-RA-RAR reporter mice. Therefore, FSH enhances the biosynthesis of RA from retinol, and the de novo synthesized RA in granulosa cells, especially, appears to be required for further progression of follicular development from the antral to preovulatory stage.
To further define the role of retinol and RA in ovarian follicular development and fertility in vivo, female mice were fed a retinol-free diet (VAD) after weaning for 6 weeks to reduce to basal levels of serum retinol. The estrous cycle in the VAD-treated mice was disrupted and constant estrous was maintained for up to 4 days, suggesting that ovulation and release of the LH surge was disrupted in the mice (34–36). To avoid the possibility of defective pituitary functions by low levels of retinol, mice were treated with a superovulatory regimen of with eCG and hCG (37). Although large antral follicles developed in response to eCG in the VAD mice (as in control mice), ovulation in response to hCG was reduced and was associated with low expression of LH receptor mRNA, Lhcgr, in the VAD mice. Additionally, when the ADH inhibitor, 4MP, was coinjected with either eCG or hCG to immature mice, 4MP specifically suppressed follicular development from the antral to preovulatory stage and impaired oocyte developmental competence but did not acutely block hCG induction of ovulation. Injections of exogenous RA to eCG+4MP treated mice completely reversed the inhibitory effects of 4MP on preovulatory follicle development and oocyte developmental competence. These results indicate further that de novo-synthesized RA in FSH-stimulated granulosa cells impacts granulosa cell differentiation, the acquisition of responsiveness to LH/hCG, and oocyte development.
ADH and ALDH family members are known to be involved in alcohol metabolism in the liver (15, 24, 38, 39). Specifically, ALDH family members degrade the metabolic byproduct acetaldehyde, eliminate its toxic activity, and thereby protect normal cell functions and viability (18). In the ovary, acetaldehyde is produced as a byproduct of steroidogenesis from cholesterol and is increased in ovary of eCG-primed mice (17). We previously showed that when the activity of ALDH was suppressed by the injection of a specific inhibitor, the expression of genes involved in FSH-induced follicular development was significantly impaired and granulosa cells exhibited increased apoptosis (17). However, whether the physiological roles of the ALDH family were the reduction of toxic activity of acetaldehyde, production of RA, or both was not resolved (17). In the present study, we have used two different approaches to define the functions of the ADH-ALDH pathways in the ovary in vivo: one is the use of an ADH inhibitor that does not suppress ALDH-dependent acetaldehyde degradation, and the other reduced the levels of the precursor substrate retinol. Both treatments impaired preovulatory follicular development, expression of Lhcgr, ovulation, and expression of ovulation dependent genes but did not increase granulosa cell apoptosis. These results indicated that both reduced acetaldehyde degradation and de novo RA synthesis by ALDH family contribute to normal follicular development and the release the oocytes with the fertilization competence.
The induction and expression of Lhcgr in granulosa cells is the hallmark of preovulatory follicles in all mammalian species studies (40). Androgens (41), estrogens (42), and RA (43) have been shown to enhance FSH induction of Lhcgr expression in cultured granulosa cells of rat, mouse, and other species. Estrogen is presumed to mediate its effects by estrogen receptor beta (NR3A2) (44); however, a putative consensus estrogen response element binding site on the promoter region of Lhcgr has not been reported. Herein we show that depleting RA by a retinol-free diet or by injecting an ADH inhibitor in vivo the expression of Lhcgr but not Cyp19a1 is reduced. Moreover, the serum levels of 17β-estradiol were similar in the ADH inhibitor-treated and control mice and injections of 17β-estradiol did not restore the expression level of Lhcgr in the ADH inhibitor-exposed granulosa cells. Conversely, injections of RA not only restored RA levels in vivo but also blocked the inhibitor-induced reduction of Lhcgr expression. Furthermore, we show herein that when antral follicles from immature mice were cultured in serum-free medium, FSH did not induce Lhcgr expression unless a low concentration of retinol was also added to the culture medium. However, high doses of retinol suppressed the expression of Lhcgr, indicating that the response to retinol/RA is dose dependent. These results extend those of Bagavandoss and Midgley (21), who reported that when rat granulosa cells were cultured in serum-free media, cotreatment with FSH and RA increased the expression of Lhcgr as compared with that by FSH alone and other groups who have reported that RA suppresses FSH-induced Lhcgr expression in granulosa cells (43, 45). These previous discrepancies may be due to the presence or absence of serum that contains high levels of retinol or to the dose of RA used. Collectively it appears that intrafollicular levels of RA, mediated by biosynthesis (herein) and accumulation (10), is required for FSH-induced Lhcgr expression in granulosa cells during follicular development process.
The mechanisms by which RA enhances FSH induction of Lhcgr remain to be determined but based on computer analyses of −5000 bp of the Lhcgr promoter using the JASPAR database (http://jaspar.binf.ku.dk/), a putative RARE is not observed. Thus, RAR, like estrogen receptor-β (NR3A2), may enhance Lhcgr expression via other mechanisms including methylation of the guanine-cytosine (GC) rich region in the Lhcgr promoter (46) and possibly interactions with the transcription factor specificity protein-1 (47) that occurs with other steroid receptors (48).
In conclusion, specific genes of the Adh/Aldh families that are involved in RA biosynthesis are regulated by eCG/FSH and selectively expressed in either theca cells or granulosa cells during follicular development. RA is essential for facilitating FSH induction of the LH receptor. Moreover, RA is also essential for oocyte maturation and fertility because female mice fed a retinol-deficient diet for 3 weeks ovulated fewer COCs, and the oocytes within the ovulated complexes exhibited impaired developmental competence. Thus, increased RA biosynthesis is required for not only preovulatory follicular development but also for oocyte maturation during ovulation.
Acknowledgments
Ovine FSH was kindly provided by Dr A. F. Parlow (the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, Torrance California). We are grateful to Dr H. Kuniyoshi (Hiroshima University) for kindly helping in the detection of retinol and Dr Y. Hoshino (Hiroshima University) for insightful comments and suggestions concerning the manuscript.
This work was supported in part by Grants-in-Aid for Scientific Research 24688028 and 25132708 (to M.S.) and 13J02153 (to T.K.) from the Japan Society for the Promotion of Science and National Institutes of Health Grant HD-076980 (to J.S.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADH
- alcohol dehydrogenases
- ALDH
- acetaldehyde dehydrogenase
- COC
- cumulus-oocyte complex
- CPRG
- chlorophenolred-β-D-galactopyranoside
- eCG
- equine chorionic gonadotropin
- hCG
- human chorionic gonadotropin
- 4MP
- 4-methylpyrazole
- RA
- retinoic acid
- RAR
- RA receptor
- RARE
- RA-responsible element
- VAD
- vitamin A deficient
- X-Gal
- X-galactosidase.
References
- 1. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. [DOI] [PubMed] [Google Scholar]
- 2. Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-β superfamily members in female fertility. Mol Cell Endocrinol. 2000;159:1–5. [DOI] [PubMed] [Google Scholar]
- 3. Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. [DOI] [PubMed] [Google Scholar]
- 4. Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod. 2006;12:537–555. [DOI] [PubMed] [Google Scholar]
- 5. Shimasaki S, Zachow RJ, Li D, et al. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci USA. 1999;96:7282–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) α and ER β. Biol Reprod. 2006;655:1534–1541. [DOI] [PubMed] [Google Scholar]
- 7. Woodruff TK, Lyon RJ, Hansen SE, Rice GC, Mather JP. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990;127:3196–3205. [DOI] [PubMed] [Google Scholar]
- 8. Yamoto M, Shima K, Nakano R. Gonadotropin receptors in human ovarian follicles and corpora lutea throughout the menstrual cycle. Horm Res. 1992;1:5–11. [DOI] [PubMed] [Google Scholar]
- 9. Matzuk MM. Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol. 2000;163:61–66. [DOI] [PubMed] [Google Scholar]
- 10. Kipp JL, Golebiowski A, Rodriguez G, Demczuk M, Kilen SM, Mayo KE. Gene expression profiling reveals Cyp26b1 to be an activin regulated gene involved in ovarian granulosa cell proliferation. Endocrinology. 2011;152:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J Biol Chem. 1994;269:32821–32827. [PubMed] [Google Scholar]
- 12. Vernet N, Dennefeld C, Rochette-Egly C, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. [DOI] [PubMed] [Google Scholar]
- 13. Deuster G. Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. Am Soc Nutr Sci. 1998;98:459–462. [DOI] [PubMed] [Google Scholar]
- 14. Lieber CS. Hepatic and metabolic effects of ethanol: pathogenesis and prevention. Ann Med. 1994;26:325–330. [DOI] [PubMed] [Google Scholar]
- 15. Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 16. Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–4456. [DOI] [PubMed] [Google Scholar]
- 17. Kawai T, Mihara T, Kawashima I, et al. Endogenous acetaldehyde toxicity during antral follicular development in the mouse ovary. Reprod Toxicol. 2012;33:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao Q, Weiner H, Crabb DW. The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion. J Clin Invest. 1996;98:2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 20. Zhuang YH, Ylikomi T, Lindfors M, Piippo S, Tuohimaa P. Immunolocalization of retinoic acid receptors in rat, mouse and human ovary and uterus. J Steroid Biochem Mol Biol. 1994;48:61–68. [DOI] [PubMed] [Google Scholar]
- 21. Bagavandoss P, Midgley AR., Jr Biphasic action of retinoids on gonadotropin receptor induction in rat granulosa cells in vitro. Life Sci. 1988;43:1607–1614. [DOI] [PubMed] [Google Scholar]
- 22. Figueiredo JR, Hulshof SC, Van den Hurk R, et al. Preservation of oocyte and granulosa cell morphology in bovine preantral follicles cultured in vitro. Theriogenology. 1994;41:1333–1346. [DOI] [PubMed] [Google Scholar]
- 23. Zheng WL, Bucco RA, Sierra-Rievera E, Osteen KG, Melner MH, Ong DE. Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-II. Biol Reprod. 1999;60:110–114. [DOI] [PubMed] [Google Scholar]
- 24. Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. [DOI] [PubMed] [Google Scholar]
- 25. Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. J Biochem. 2005;388:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacDonald PN, Bok D, Ong DE. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat and human. Proc Natl Acad Sci USA. 1990;87:4265–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Randolph RK, Ross AC. Vitamin A status regulates hepatic lecithin: retinol acyltransferase activity in rats. J Biol Chem. 1991;266:16453–16457. [PubMed] [Google Scholar]
- 28. Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez KF, Couse JF, Jayes FL, et al. Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-β. Endocrinology. 2010;151:2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White JC, Highland M, Clagett-Dame M. Abnormal development of the sinuatrial venous valve and posterior hindbrain may contribute to late fetal resorption of vitamin A-deficient rat embryos. Teratology. 2000;62:374–384. [DOI] [PubMed] [Google Scholar]
- 32. Yokobayashi S, Liang CY, Kohler H, et al. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature. 2013;495:236–240. [DOI] [PubMed] [Google Scholar]
- 33. MacLean G, Abu-Abed S, Dollé P, Tahayato A, Chambon P, Petkovich M. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev. 2001;107:195–201. [DOI] [PubMed] [Google Scholar]
- 34. Hisaw FL. Development of the graafian follicle and ovulation. Physiol Rev. 1947;27:95–119. [DOI] [PubMed] [Google Scholar]
- 35. Ying SY, Fang VS, Greep RO. Changes in concentration of serum LH and FSH associated with estrogen-advanced ovulation in 4-day cyclic rats. Proc Soc Exp Biol Med. 1972;139:738–740. [DOI] [PubMed] [Google Scholar]
- 36. Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. New York: Raven Press; 1994:725–780. [Google Scholar]
- 37. Fowler RE, Edwards RG. Induction of superovulation and pregnancy in mature mice by gonadotropin. J Endocrinol. 1957;15:374–384. [DOI] [PubMed] [Google Scholar]
- 38. Lieber CS. Metabolic consequences of ethanol. Endocrinologist. 1994;4:127–139. [Google Scholar]
- 39. Rivera-Meza M, Quintanilla ME, Tampier L, Mura CV, Sapag A, Israel Y. Mechanism of protection against alcoholism by an alcohol dehydrogenase polymorphism: development of an animal model. FASEB J. 2010;24:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. [DOI] [PubMed] [Google Scholar]
- 41. Farookhi R, Desjardins J. Luteinizing hormone receptor induction in dispersed granulosa cells requires estrogen. Mol Cell Endocrinol. 1986;47:13–24. [DOI] [PubMed] [Google Scholar]
- 42. Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–1865. [DOI] [PubMed] [Google Scholar]
- 43. Hattori M, Takesue K, Nishida N, Kato Y, Fujihara N. Inhibitory effect of retinoic acid on the development of immature porcine granulosa cells to mature cells. J Mol Endocrinol. 2000;25:53–61. [DOI] [PubMed] [Google Scholar]
- 44. Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. [DOI] [PubMed] [Google Scholar]
- 45. Minegishi T, Hirakawa T, Kishi H, et al. The mechanisms of retinoic acid-induced regulation on the follicle-stimulating hormone receptor in rat granulosa cells. Biochim Biophys Acta. 2000;1495:203–211. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Fatima N, Dufau ML. Coordinated changes in DNA methylation and histone modifications regulate silencing/derepression of luteinizing hormone receptor gene transcription. Mol Cell Biol. 2005;25:7929–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Dufau ML. Repression of the luteinizing hormone receptor gene promoter by crosstalk among EAR3/COUP-TFI, Sp1/Sp3, and TFIIB. Mol Cell Biol. 2003;23:6958–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6:44–55. [DOI] [PubMed] [Google Scholar]