Abstract
Introduction
Comparison of rates of ventriculostomy-related infections (VRI) across institutions is difficult due to the lack of a standard definition. We sought to review published definitions of VRI and apply them to a test cohort to determine the degree of variability in VRI diagnosis.
Materials and Methods
We conducted a PubMed search for definitions of VRI using the search strings “ventriculostomy-related infection” and “ventriculostomy-associated infection.” We applied these definitions to a test cohort of 18 positive cerebrospinal fluid (CSF) cultures taken from ventriculostomies at two institutions to compare the frequency of infection using each definition.
Results
We found 16 unique definitions of VRI. When the definitions were applied to the test cohort, the frequency of infection ranged from 22–94% (median 61% with interquartile range (IQR) 56–74%). The concordance between VRI diagnosis and treatment with VRI-directed antibiotics for at least seven days ranged from 56–89% (median 72%, IQR 71–78%).
Conclusions
The myriad of definitions in the literature produce widely different frequencies of infection. In order to compare rates of VRI between institutions for the purposes of qualitative metrics and research, a consistent definition of VRI is needed.
Keywords: cerebrospinal fluid, CSF shunt, infection, ventriculitis, surgical site infection
Introduction
External ventricular drain (EVD) placement is one of the most commonly performed neurosurgical procedures1. In 2014, the International Multidisciplinary Consensus Conference on Multimodality Monitoring declared that incidence of ventriculostomy-related infections (VRIs) may be a useful indicator for intensive care unit (ICU) quality of care2.
The Centers for Disease Control (CDC), the preeminent source for infection surveillance definitions in the United States, states that a patient greater than one year of age has ventriculitis if either of the following are true: 1) organisms are cultured from the cerebrospinal fluid (CSF), or 2) the patient has at least one sign or symptom of ventriculitis including fever (>38°C), headache/stiff neck/meningeal signs/cranial nerve signs/irritability with no other recognized cause and at least one of the following: a. increased white cells, elevated protein, and decreased glucose in CSF, b. organisms seen on Gram’s stain of CSF, c. organisms cultured from blood, d. positive laboratory test of CSF, blood, or urine, e. diagnostic single antibody titer (IgM) or four-fold increase in paired sera (IgG) for the pathogen, and if the diagnosis is made antemortem, the physician institutes appropriate antimicrobial therapy3.
The frequency of VRI reported in the literature ranges from 0–45%4–8. The CDC definition is not widely employed to evaluate frequency of VRI4,9–23, largely because it does not include exclusion criteria to eliminate contaminants23 and does not contain specific timing criteria to determine when a case of ventriculitis should be attributed to a ventriculostomy. As a result, one of the hypothesized reasons for the degree of variation in frequency of VRI is the lack of consensus on the definition for VRI 9–12,14,15,19. In order to determine the effect that varying definitions of VRI have on frequency of diagnosis, we sought to review published definitions for VRI and apply them to a test cohort of positive CSF cultures taken from EVDs at two institutions then evaluate the strength of each definition by determining how accurately it identified clinically treated cases in the test cohort.
Materials and Methods
1) Collection of Definitions of VRIs
We conducted a PubMed search using the search strings “ventriculostomy-related infection” and “ventriculostomy-associated infection.” We reviewed each article to determine whether a definition of VRI was included in the manuscript. If a definition was included, we recorded the components of the definition in a database. If a previous author’s definition was cited as the source of a manuscript’s VRI definition, that definition was not repeated in the database. Manuscripts which modified a previous author’s definition with additional criteria were included as unique definitions. We reviewed each definition to see if it included information pertaining to timing, clinical signs and symptoms, laboratory results, and exclusion criteria to differentiate 1) infections secondary to another source from those that could be attributed to the EVD or 2) contaminants from true infections.
2) Acquisition of Test Cohort
We reviewed CSF culture results taken from EVDs at two institutions. Culture results for patients at New York University Langone Medical Center (NYU) who underwent EVD placement (CPT 02.21) between January 1, 2013 and June 30, 2014 were identified as part of an internal retrospective quality assessment project beginning with the hospital’s introduction of a new electronic medical record. Data for patients at Massachusetts General Hospital (MGH) was extracted from an established retrospective database of patients admitted to the MGH neurosurgery service with subarachnoid hemorrhage (SAH) between January 1, 2008 and June 30, 2012 which has been described elsewhere24. To be included in the test cohort, a CSF culture needed to grow a bacterial organism. Two positive CSF cultures from the same patient were included in the cohort separately if they grew different organisms at different time points. Cultures were excluded if they were taken from patients who were being treated for intracranial infection prior to EVD placement or if antibiotics were discontinued because care was withdrawn, as no decision about an antibiotic treatment course was made for these patients. This study was approved by the Institutional Review Board at both institutions.
Charts for patients with positive EVD-related cultures were reviewed for pertinent clinical data including: indication for EVD placement, EVD day of positive culture, presence of systemic infection, maximum temperature in the 24 hours prior to culture acquisition, presence of clinical symptoms that were not attributed to the patient’s primary neurologic disease, CSF white blood cell (WBC) count prior to and at the time of the culture and the percentage of neutrophils, CSF red blood cell (RBC) count, serum WBC count, CSF glucose, serum glucose, CSF protein, Gram stain results, organism type and quantity, number of positive cultures, growth medium, treatment, discharge condition and time to discharge.
3) Evaluation of the Definitions of VRIs
The definitions were independently reviewed by two neurointensivists (A.L and A.S.L.) who determined whether they were subjective or objective. Definitions were classified as subjective if they contained vague terms that required clinical judgment and as objective if they relied only on discrete numerical laboratory and clinical data and were not open to interpretation. Both neurointensivists then independently applied the data from each subject to each of the definitions to determine if criteria for VRI were met.
4) Statistical Analysis
For each definition, we calculated the frequency of diagnosis of VRI for each neurointensivist (number of patients diagnosed with VRI/total number of positive cultures). Interrater reliability was assessed with SPSS Statistics 21 using unweighted κ statistics. For each neurointensivist, we also determined the rate of concordance between diagnosis and treatment of VRI for each definition in order to determine the accuracy of the definition. Using the decision to treat with greater than seven days of antibiotics as the “gold standard” for clinical diagnosis of VRI, we calculated the sensitivity and specificity for each definition. An accurate definition was defined as one in which all clinically treated cases in the test cohort were correctly identified.
Results
1) Definitions of Ventriculostomy-related infection
Our search yielded 17 unique definitions of VRI published from 1984–2014 4,9–23,25 (see Appendix I). One definition was based on quantification of colony forming units20 and this type of evaluation is not routinely performed at either hospital, so this definition was excluded. Of the remaining 16 definitions, seven definitions (44%) were determined to be objective and nine (56%) subjective after neurointensivist review. Criteria for each definition are in Table I.
Table I.
Criteria for definitions of ventriculostomy-related infections
| Authors | Positive CSF Culture Required | Mention of Culture Media | Timing | Clinical Signs/Symptoms | CSF WBC | CSF Glucose | CSF Protein | Exclusion Criteria | Subjective Definition |
|---|---|---|---|---|---|---|---|---|---|
| Arabi, 20051 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Bota, 20053 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Chi, 20104 | ✓ | ✓ | ✓ | ||||||
| Gozal, 20147 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Holloway, 19969 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Hopkins, 201210 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Lozier, 200215 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Lyke, 200116 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Mayhall, 198417 | ✓ | ✓ | ✓ | ✓ | |||||
| McLaughlin, 201218 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Mikhaylov, 201419 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Rath, 201421 | ✓ | ✓ | ✓ | ✓ | |||||
| Schoch, 200823 | ✓ | ✓ | ✓ | ✓ | |||||
| Tse, 201026 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Wright, 201328 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Zabramski, 200329 | ✓ | ✓ | |||||||
| Percentage | 50% | 6% | 56% | 69% | 63% | 56% | 44% | 50% | 56% |
CSF=cerebrospinal fluid, WBC=white blood cell count
a) Timing
Details about timing were noted in 9/16 (56%) of definitions. The defined time at which a positive CSF culture first qualified as a VRI varied from any time after catheter insertion9 to 2411,16 to 484 hours after placement. The timeframe at which VRI is diagnosed after EVD removal also varied from three days12 to four weeks25 after EVD removal.
b) Clinical Signs and Symptoms
Clinical signs and symptoms are included in 11/16 (69%) of the definitions. While some used vague terms such as “clinical picture”13 or “clinical signs”18,23 of infection, others used a quantitative scale18,19, or specified individual clinical signs and symptoms including cranial nerve signs, headache25, photophobia, seizures15,21, stiff neck21,25, altered mental status9,15,21, irritability9,25, inflammation at the catheter site9, and fever. In some cases fever was undefined15,18,19,21, and in others it was defined as 3825, 38.510, and 38.6 degrees Celsius12.
c) Laboratory Results
A positive CSF culture was a necessary component of the diagnosis of VRI for 8/16 (50%) of the definitions. Only one definition referred to the culture growth medium22. No definitions mandated that more than one culture be positive.
Beyond this requirement, 4/16 (25%) of the definitions did not include any further laboratory criteria for diagnosis of VRI11,16,17,22. One definition abstractly referred to “abnormal CSF parameters”23 and four definitions mentioned trends in CSF values (increased protein, decreased glucose)9,15,21,25. Of the definitions that clearly defined what CSF parameters are consistent with infection, there was inconsistency between values. Regarding glucose, the upper threshold of CSF glucose consistent with VRI was defined alternately as 15mg/dl13, 25mg/dl4, 40mg/dl or 50% of serum glucose10, and 50mg/dl or 50% of serum glucose12. Definitions of CSF WBC values consistent with diagnosis of VRI included the following: nonspecific increase in WBC15,21,25, neutrophilic pleocytosis greater than or equal to 10 cells per cubic millimiter4, increase of 100% or more in WBC18,19, WBC greater than 50 cells per cubic millimiter with greater than 50% neutrophils, and greater than 100 cells per cubic millimeter10. Only one definition included mention of the ratio of WBC to RBC14 and only one definition referred to elevated blood WBC21.
d) Criteria to Exclude Contaminants or Infections Due to Other Sources
Application of exclusion criteria was noted in 8/ 16 (50%) of the definitions such that all positive CSF cultures were not considered to be consistent with VRI. The necessity to rule out another source of CSF infection prior to attributing ventriculitis to a complication of ventriculostomy placement by screening for other foci of infection16,21, CSF leak, or penetrating injury of the central nervous system16 was included in 2/16 (13%) of definitions. Only 5/16 (31%) of definitions referred to specific criteria to diagnose CSFcontaminants as a separate entity from VRI which do not warrant treatment4,9,10,15,19.
2) Characteristics of the Test Cohort
We identified 18 positive CSF bacterial cultures to be included in the test cohort. Nine cultures were sent from eight different patients with EVDs at NYU (two intracranial hemorrhages, three tumors, three SAH) and nine cultures were sent from eight different patients at MGH (all SAH).
VRI-directed antibiotics were administered to patients the treating team felt had clinical evidence of ventriculitis (based on signs/symptoms and laboratory findings) for greater than seven days to 67% (n=12) of the cohort. The treating teams did not feel that the remaining 33% (n=6) of positive cultures represented true infections and thus treated them with less than seven days of antibiotics (range 0–2 days), so we classified these cultures as contaminants. 5/6 cultures that were deemed to be contaminants grew in liquid medium only.
The clinical and laboratory findings associated with the cultures are in Table II. The most common organism was coagulase negative staphylococcus (n=5, 28%) followed by propionibacterium (n=4, 22%). Fever of at least 38.3 degrees Celsius was recorded in 56% (n=10) of the patients in the 24 hours prior to the positive culture. Clinical symptoms that were felt to not be related to the admission diagnosis were noted in 17% (n=3) of the patients on the day of the culture. In terms of CSF findings, 61% (n=11) of patients had a WBC greater than 100 cells per millimeter, 61% (n=11) had protein greater than 50 mg/dL, 39% (n=7) had glucose less than half the serum glucose, 22% (n=4) had organisms on their Gram stain, and 22% (n=4) had more than one positive culture.
Table II.
Clinical and Laboratory Findings for Test Cohort
| ID | EVD Day |
Organism | Medium | Quantity | Systemic Infection | Tmax (degrees Celsius) |
Sxms | CSF RBC (cells/ mm3) |
CSF WBC & Prior CSF WBC (cells/ mm3) |
CSF PMN (%) |
CSF Protein (mg/dL) |
CSF Glc (mg/dL) |
Serum Glc (mg/dL) |
Serum WBC (K/ul) |
Gram Stain |
# | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 5 | S.epi | Liquid | -- | No | 36.9 | No | 2,320 | 2 (2) | 88 | 13 | 96 | 131 | 15.3 | No | 1 | IV x 2 days |
| N2 | 2 | P.acnes | Liquid | -- | No | 36.8 | No | 878 | 18 (N/A) | 6 | <10 | 89 | 89 | 11 | No | 1 | No |
| N2* | 2 | MSSA | Liquid | -- | No | 36.6 | No | 132,000 | 2200 (N/A) | 92 | 361 | 77 | 149 | 12.2 | No | 1 | IV x 2 days |
| N3 | 5 | Bacillus | Solid | Innum | No | 36.8 | HA/N | 150 | 0 (1) | N/A | <10 | 84 | 117 | 13.9 | GVR | 4 | IV x 14 days, IT x 5 days |
| N4 | 1 | P.acnes | Liquid | -- | No | 37.4 | No | 3,103 | 145 (2860) | 77 | 45 | 76 | 90 | 12.1 | No | 1 | No |
| N5 | 14 | S.epi | Solid | Many | No | 35.7 | No | 73 | 3 (3) | N/A | 33 | 65 | 115 | 10 | No | 1 | IV x 10 days |
| N6 | 6 | Klebsiella | Solid | Mod | Klebsiella UTI | 37.6 | AMS | 737,000 | 1730 (305) | 79 | 733 | 111 | 174 | 14.9 | No | 3 | IV x 22 days |
| N7 | 9 | S.epi | Liquid | -- | Enterobacter PNA | 38.8 | No | 390 | 1 (1) | N/A | 31 | 89 | 130 | 9.7 | No | 1 | No |
| N8 | 5 | Serratia | Solid | Mod | No | 38.6 | No | 17,000 | 107 (11) | 83 | 159 | 48 | 105 | 11.8 | No | 19 | IV x 37 days |
| M1 | 16 | Providencia Rettgeri | Solid | Mod | E.coli PNA | 39.4 | No | 533 | 411 (603) | 83 | 67 | 92 | 205 | 12.7 | Few GNR | 1 | IV x 10 days |
| M2 | 4 | CN Staph | Solid | Rare | PNA | 38.3 | No | 196,000 | 940 (N/A) | 85 | 176 | 53 | 139 | 10.8 | Mod PMN | 2 | IV x 23 days |
| M3 | 8 | P.acnes | Solid | -- | E.coli UTI | 39.4 | No | 9,125 | 295 (13) | 61 | 72 | 86 | 164 | 15.2 | No | 1 | No |
| M4 | 6 | Acinetobacter | Solid | Mod | PNA (No organism) | 39.1 | No | N/A | 256 (44) | 77 | 60 | 78 | 120 | 24.7 | Mod GNR | 1 | IV x 22 days |
| M5 | 14 | Propionibact | Liquid | -- | No | 38.4 | No | 1,397 | 6 (1,190) | 32 | 14 | 72 | 144 | 12.1 | No | 1 | IV x 21 days |
| M6 | 13 | Enterobacter Aerogenes | Solid | Abund | No | 38.9 | HA/Nuch | 32,500 | 1,550 (N/A) | 92 | 233 | 64 | 172 | 8.1 | Abund PMN | 1 | IV x 16 days |
| M7 | 18+1 | Klebsiella | Solid | -- | No | 39.3 | No | 744 | 13,700 (640) | 90 | 820 | < 2 | 106 | 9.6 | Abund PMN | 1 | IV x 14 days |
| M8 | 9 | CN Staph | Solid | -- | No | 39.2 | No | 3,400 | 71 (26) | 82 | 117 | 40 | 177 | 10.7 | No | 1 | IV x 30 days |
| M8* | 17 | GNR | Solid | Abund | CN Staph Ventriculitis | N/A | No | 328 | 117 (1975) | 76 | 126 | < 2 | 149 | 7.6 | Abund GNR | 1 | IV x 30 days |
ID=identification (values that begin with N are New York University patients and those that begin with M are Massachusetts General Hospital patients), *=patients with positive cultures that grew two distinct organisms (N2 had positive cultures on two separate admissions and M8 grew two different organisms at different times on the same admission), ICH=intracranial hemorrhage, SAH=subarachnoid hemorrhage, IVH=intraventricular hemorrhage, EVD=external ventricular drain, EVD Day= number of days EVD was in place (placement day = day 1; patients who were diagnosed with infection after EVD removal are denoted at EVD day of removal + number of days after removal), S.epi= Staphylococcus epidermidis, P.acnes= Propionibacterium acnes, MSSA=Methicillin Sensitive Staphylococcus aureus, CN staph=coagulase negative Staphylococcus, E.coli=Escherichia coli, Propionibact=Propionibacterium species, Quantity= not available for organisms that grew in liquid only (and in some cases no data on quantity was available for organisms that grew on solid media), Innum=innumerable bacteria, Mod=moderate, Abund=abundant, Systemic Infection=presence of systemic infection on day of positive culture, UTI=urinary tract infection, PNA=pneumonia, Tmax=maximum temperature in the 24 hours prior to the culture, Sxms=symptoms not attributed or possibly worse than would be attributed to primary pathology, HA=headache, N=nausea, AMS=altered mental status, Nuch=nuchal rigidity, CSF=cerebrospinal fluid, WBC=white blood cells, PMN=polymorphic neutrophils, N/A=not applicable (no results available), Glc=glucose, GVR=gram variable rods, GNR=gram negative rods, #=number of positive cultures with the same organism, IV=intravenous antibiotics, IT= intrathecal antibiotics
All patients in the test cohort were discharged alive. Patients whose cultures were treated as true infections were discharged at a median of 16.5 days (IQR 10–21) after their positive culture. Patients whose cultures were considered contaminants were discharged at a median of five days (IQR 4.25–8) after their positive culture.
3) Application of VRI Definitions to the Test Cohort
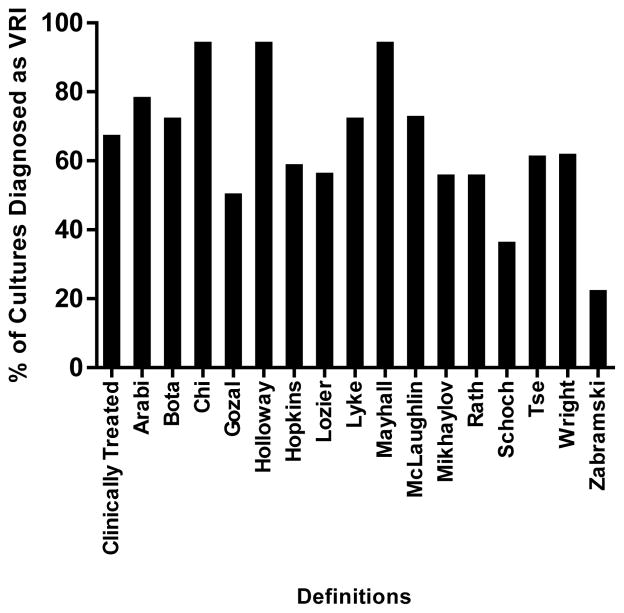
After application of the sixteen different definitions, the percentage of positive cultures diagnosed as VRI ranged from 22–94% (median of 61% with IQR 56–74%), as shown in Figure I. The objective definitions yielded a range of 22–94% of VRI diagnoses (median of 61% and IQR 61–74%) while the subjective definitions produced a range of 33–78% of VRI diagnoses (median of 59% and IQR 56–62%). No definition was 100% accurate at identifying cases that were clinically treated as VRI. Concordance rates between diagnosis and treatment ranged from 56–89% (median of 72% IQR of 71–78%). Three definitions demonstrated 100% sensitivity, but all of them had very low specificities (17% each).11,13,16 See Table III.
Figure I.
Diagnosis of Ventriculostomy-Related Infection (VRI)
This bar graph demonstrates the variation in percentage of cultures in the test cohort diagnosed as ventriculostomy-related infections.
Table III.
Evaluation of Test Cohort Using Varying Definitions of Ventriculostomy-Related Infections
| Authors | Frequency of VRI (%) | Concordance Rate Between Intensivists (kappa) | Concordance with Treatment (%) | Sensitivity* | Specificity* | ||
|---|---|---|---|---|---|---|---|
| MD1 | MD2 | MD1 | MD2 | ||||
| Arabi, 20051 | 78% | 78% | 1 | 78% | 78% | 92% | 50% |
| Bota, 20053 | 72% | 72% | 1 | 61% | 61% | 75% | 33% |
| Chi, 20104 | 94% | 94% | 1 | 72% | 72% | 100% | 17% |
| Gozal, 20147 | 50% | 50% | 1 | 72% | 72% | 67% | 83% |
| Holloway, 19969 | 94% | 94% | 1 | 72% | 72% | 100% | 17% |
| Hopkins, 201210 | 67% | 50% | 0.667 | 89% | 83% | 75–92% | 83% |
| Lozier, 200215 | 56% | 56% | 1 | 67% | 67% | 67% | 67% |
| Lyke, 200116 | 72% | 72% | 1 | 72% | 72% | 83% | 50% |
| Mayhall, 198417 | 94% | 94% | 1 | 72% | 72% | 100% | 17% |
| McLaughlin, 201218 | 67% | 78% | 0.727 | 78% | 78% | 83–92% | 50–67% |
| Mikhaylov, 201419 | 61% | 50% | 0.556 | 83% | 83% | 75–83% | 83–100% |
| Rath, 201421 | 67% | 44% | 0.571 | 78% | 67% | 58–83% | 100% |
| Schoch, 200823 | 39% | 33% | 0.880 | 72% | 67% | 50–58% | 100% |
| Tse, 201026 | 61% | 61% | 0.532 | 83% | 72% | 75–83% | 67–83% |
| Wright, 201328 | 56% | 67% | 0.769 | 78% | 89% | 75–92% | 83% |
| Zabramski, 200329 | 22% | 22% | 1 | 56% | 56% | 33% | 100% |
VRI=ventriculostomy-related infection, MD1=neurointensivist one, MD2=neurointensivist two,*=data is listed as a range if the sensitivity/specificity were not the same for MD1 and MD2, Grey rows: subjective definitions, white rows: objective definitions
Discussion
Despite the high risk of infection associated with EVDs, the CDC does not have a device-related infection definition for EVDs as it does for ventilators, Foley catheters, and central venous catheters3. Therefore, a wide variety of definitions are employed and there is no universal definition for VRI4,9–23,25. In fact, of the seventeen different definitions of VRI we found in the literature4,9–23,25, only McLaughlin et al.’s25 is grossly similar to the CDC’s definition (it differs only in that it stipulates an infection is consistent with VRI up to four weeks after EVD removal). We found that application of sixteen different definitions taken from the literature to eighteen positive CSF cultures yielded a wide range of VRI frequency (22–94%) and a wide degree of variation in concordance rates between diagnosis and treatment (56–89%) which likely contributes to the large variation in reported VRI rates in the literature4–8. The necessity for a uniform definition is clear, as this would allow for comparison of infection rates between institutions as a metric of ICU quality of care2 and for research.
It would seem that the ideal definition for VRI would be as objective as possible such that there would be no confusion as to whether a patient meets criteria. However, of the sixteen definitions we evaluated, seven (44%) were objective. In comparison to the percentage of patients treated for VRI, the objective definitions were only 56–72% consistent. In some cases, use of the strict objective criteria led to underdiagnosis of VRI in comparison to the number of patients who were treated22, and in other cases, it led to overdiagnosis11,13,16, and application of some definitions led to a combination of patients who were not treated who met criteria and patients who were treated who did not meet criteria4,10,12. No definition was 100% accurate. This degree of variation is related to the fact that patients who are treated for VRI are not a homogeneous population in terms of laboratory or clinical findings. While patients without EVDs routinely present with CSF pleocytosis, elevated protein, and decreased glucose in the setting of meningitis/ventriculitis, the composition of the CSF in patients with EVDs varies depending on the underlying pathology, so reliance on CSF findings to diagnose VRI is difficult12,15,26.
Schade et al. compared CSF findings in patients with EVDs who had positive CSF cultures and clinical signs of ventriculitis (fever, headache, nuchal rigidity, altered mental status) and control patients with EVDs with negative cultures and no signs of ventriculitis and found no significant difference in CSF leukocyte count, protein, IL-6, or CSF to blood glucose ratio26. Although the clinical symptoms and signs typically associated with ventriculitis include headache, nuchal rigidity, altered mental status, and fever, these symptoms are often present in the neurosurgical population so it can be difficult to attribute them to VRI, and the presence of systemic infections or the need for intubation and sedation may further complicate recognition of clinical changes27,28. Thus, we do not believe the ideal definition for VRI should include explicit requirements regarding clinical and laboratory data. However, in the setting of a positive CSF culture, worsening fever trends, increasing WBC in CSF or blood, or other worsening markers of inflammation such as c-reactive protein or erythrocyte sedimentation rate, may aid the clinician in arriving to a diagnosis of infection. The growth of multiple positive cultures may be associated with increased likelihood that a patient has an infection.
The definition of VRI cannot be so basic, though, that a positive CSF culture is reflexively diagnosed as a VRI, because cultures can be contaminated with skin flora, and the definition of VRI should only encompass positive cultures that are clinically relevant4,9,10,15,19. It is interesting to note that only one definition mentioned culture media, but amplification of an organism in liquid media is more likely to be a contaminant than that on solid media22,29. Skin flora are notably the most common source of VRI10,15,26,28, so the criteria applied to identify whether an organism is a contaminant or a true infection should not be so stringent that patients who are treated with antibiotics due to the possibility of infection are not diagnosed as infected. A liberal definition of VRI that allows for interpretation by the physician under individual clinical circumstances needs to be applied in order for a definition to achieve 100% accuracy at identifying clinically treated cases of VRI27. In fact, the definition that showed the greatest degree of concordance with treatment (83%) and the highest sensitivity (75–83%) and specificity (83–100%) stated that in the setting of a positive CSF culture, the diagnosis of VRI is made if there is a clinical picture of infection14.
This definition is actually quite simplistic and other than a reference to the clinical picture (which encompasses clinical signs, symptoms, and laboratory findings) it does not include mention of timing of the culture or exclusion criteria. In terms of timing, we believe it is reasonable to allow passage of a full 24 hours after EVD insertion before a positive culture from the EVD is attributed to the EVD itself11,13,16, similar to the manner in which the CDC requires a Foley catheter to be in place for 48 hours before calling a urinary tract infection a catheter associated urinary tract infections (CAUTI)3. The definitions we found had cutoffs for diagnosis of VRI after EVD removal ranging from three days to four weeks4,9,12,17,25. It is interesting to note that the CDC criteria for ventriculoperitoneal shunt related infection are applicable for ninety days after shunt placement3. The appropriate length of time after EVD removal to consider ventriculitis to be EVD-related is unclear, but it is necessary to exclude clearly identified cases of community-acquired meningitis when defining VRI.
In terms of exclusion criteria, we believe it is important that the definition of VRI exclude: 1) patients who are diagnosed with intracranial infection prior to or at the time of EVD placement, as these infections cannot be attributed to the EVD, and 2) patients who have other sources of intracranial infection or systemic infection with the same organism found in the CSF11,13,16.
Due to the great degree of variability we found in VRI diagnosis using the available definitions in the literature, it is important for there to be discussion amongst infectious disease specialists, neurointensivists, and neurosurgeons regarding the ideal definition for VRI. Such discussion should include mention of timing from EVD placement and removal, clinical scenario, and exclusion of patients with prior or systemic infections.
Limitations
Our search for VRI definitions was not meant to be all-encompassing, rather to provide a sample of the variety of interpretations of the term in the literature. We are certain that if we used other search strings (such as the term “external ventricular drainage”) or search engines, we would find additional definitions.
Although the CDC does not necessitate a positive CSF culture to make the diagnosis of ventriculitis3, we chose to limit our cohort to patients who had positive CSF cultures. We recognize that there are times that clinicians may feel that a patient warrants treatment for ventriculitis based on clinical and laboratory features despite having negative cultures9,13–15,18,19,23,25, but our specific goal was to evaluate the variant interpretations of a positive CSF culture using different definitions of VRI. Of note, the CDC’s definitions for CAUTIs and central line associated bloodstream infections (CLABSIs) both require a positive culture3.
Our test cohort contained samples from two different time periods at two institutions with multiple treating physicians and varying underlying pathology, but this provided variability in CSF flora and mimicked the subjectivity regarding decision to treat that is sometimes associated with the art of medicine, and we do not feel it adversely affected our findings. Unfortunately, neither institution’s microbiology laboratory routinely quantifies culture results, so we were not able to evaluate the utility of colony counts for distinguishing contaminants from true infections.20
When clinical signs or symptoms and laboratory data are employed to define ventriculitis by CDC criteria, the definition also requires a physician institute “appropriate antimicrobial therapy.” We chose to make this criterion more specific by indicating the need for treatment with antibiotics for greater than seven days, and applied this stipulation to patients with positive CSF cultures. We used the seven day rule as our “gold standard” for diagnosis of VRI by the treatment team, but of course, this is in some ways a self-fulfilling prophecy. Due to the frequency of contaminants found in CSF taken from EVDs4,9,10,15,19,23, we felt that application of the seven day rule distinguished between patients the clinical team believed had positive cultures due to true ventriculitis from those who merely had contaminated CSF. Inclusion of this criterion in a formal definition of VRI would of course render the definition applicable only to surveillance reporting, not to clinical diagnosis and treatment, given that it relies in part on knowing whether the treatment team elected to administer antibiotics. We acknowledge that there may be variability between physicians as to what clinical circumstances warrant treatment with a full course of antibiotics, as has been noted with respect to discordance in physician preference regarding administration of antimicrobials in patients with pneumonia27,30. However, we believe that treatment teams regularly weigh the risks of antibiotics (development of Clostridium difficile 31, adverse drug reactions including anaphylaxis or systemic toxicity 32,33, and development of infections due to resistant pathogens 32,34–36) against the degree of clinical concern for infection when assessing a positive culture. As such, if a treatment team feels a patient warrants administration of at least seven days of antibiotics, provided that there is robust clinical data to support this, the patient should be categorized as having a VRI, and if a treatment team feels a positive culture should be treated as a contaminant, the patient should not be classified as having a VRI. Whether or not a positive CSF culture or a specified antibiotic course should be mandated as part of a universal definition of VRI needs to be debated by a committee of specialists.
Conclusion
The CDC does not have a distinct definition for VRI apart from its general definition of hospital-acquired meningitis, so there are a myriad of varied definitions in the literature. In order to facilitate congruous evaluation of infection rates for both quality metrics and research, a universal definition of VRI would be of great value.
Supplementary Material
Abbreviations
- CAUTI
catheter associated urinary tract infection
- CDC
Centers for Disease Control
- CLABSI
central line associated bloodstream infection
- CSF
cerebrospinal fluid
- EVD
external ventricular drain
- ICU
intensive care unit
- IQR
interquartile range
- MGH
Massachusetts General Hospital
- NYU
New York University
- RBC
red blood cells
- SAH
subarachnoid hemorrhage
- VRI
ventriculostomy-related infection
- WBC
white blood cells
Footnotes
Contributions
Ariane Lewis: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, final appraisal for publication Sarah Wahlster: acquisition of data, critical revision of manuscript, final appraisal for publication Sarah Karinja: study conception and design, critical revision of manuscript, final appraisal for publication Barry Czeisler: critical revision of manuscript, final appraisal for publication W. Taylor Kimberly: critical revision of manuscript, final appraisal for publication Aaron Lord: study conception and design, analysis and interpretation of data, critical revision of manuscript, final appraisal for publication The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Declaration of Interest
Ariane Lewis, Sarah Wahlster, Sarah Karinja, and Barry Czeisler do not have any financial conflicts of interest. Aaron Lord received support from the NYU-HHC Clinical and Translational Science Institute via grant UL1 TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health. W. Taylor Kimberly receives support from the Andrew David Heitman Neurovascular Research Foundation. The Foundation had no role in study design, collection, analysis, interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
References
- 1.Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage. J Neurosurg. 2014;120:228–36. doi: 10.3171/2013.6.JNS121577. [DOI] [PubMed] [Google Scholar]
- 2.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care. Neurocrit Care. 2014 doi: 10.1007/s12028-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Definitions S. CDC / NHSN Surveillance Definitions for Specific Types of Infections. 2014. [Google Scholar]
- 4.Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33:2028–33. doi: 10.1086/324492. [DOI] [PubMed] [Google Scholar]
- 5.Williams T, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. J Neurosurg. 2011;115:1040–6. doi: 10.3171/2011.6.JNS11167. [DOI] [PubMed] [Google Scholar]
- 6.Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: improved technique can reduce infection rates. Br J Neurosurg. 2011;25:632–5. doi: 10.3109/02688697.2011.578770. [DOI] [PubMed] [Google Scholar]
- 7.Bader MK, Littlejohns L, Palmer S. Ventriculostomy and intracranial pressure monitoring: In search of a 0% infection rate. Hear Lung J Acute Crit Care. 1995;24:166–72. doi: 10.1016/s0147-9563(05)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Desai NS, Ricci J, Stieg PE, Rosengart AJ, Hrtl R, et al. Factors contributing to ventriculostomy infection. World Neurosurg. 2012;77:135–40. doi: 10.1016/j.wneu.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33:137–43. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent J-L. Ventriculostomy-related infections in critically ill patients: a 6-year experience. J Neurosurg. 2005;103:468–72. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 11.Chi H, Chang K-Y, Chang H-C, Chiu N-C, Huang F-Y. Infections associated with indwelling ventriculostomy catheters in a teaching hospital. Int J Infect Dis. 2010;14:e216–9. doi: 10.1016/j.ijid.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Gozal YM, Farley CW, Hanseman DJ, Harwell D, Magner M, Andaluz N, et al. Ventriculostomy-associated infection: a new, standardized reporting definition and institutional experience. Neurocrit Care. 2014;21:147–51. doi: 10.1007/s12028-013-9936-9. [DOI] [PubMed] [Google Scholar]
- 13.Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24. doi: 10.3171/jns.1996.85.3.0419. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins SJ, McMahon CJ, Singh N, Galea J, Hoadley M, Scarth S, et al. Cerebrospinal fluid and plasma cytokines after subarachnoid haemorrhage: CSF interleukin-6 may be an early marker of infection. J Neuroinflammation. 2012;9:255. doi: 10.1186/1742-2094-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170–81. doi: 10.1097/00006123-200207000-00024. discussion 181–2. [DOI] [PubMed] [Google Scholar]
- 16.Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310:553–9. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 17.Mikhaylov Y, Wilson TJ, Rajajee V, Thompson BG, Maher CO, Sullivan SE, et al. Efficacy of antibiotic-impregnated external ventricular drains in reducing ventriculostomy-associated infections. J Clin Neurosci. 2014;21:765–8. doi: 10.1016/j.jocn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Rath P-M, Schoch B, Adamzik M, Steinmann E, Buer J, Steinmann J. Value of multiplex PCR using cerebrospinal fluid for the diagnosis of ventriculostomy-related meningitis in neurosurgery patients. Infection. 2014;42:621–7. doi: 10.1007/s15010-014-0590-8. [DOI] [PubMed] [Google Scholar]
- 19.Schoch B, Regel JP, Nierhaus a, Wichert M, Mueller OM, Sandalcioglu IE, et al. Predictive value of intrathecal interleukin-6 for ventriculostomy-related Infection. Zentralbl Neurochir. 2008;69:80–6. doi: 10.1055/s-2007-1022559. [DOI] [PubMed] [Google Scholar]
- 20.Stenager E, Gerner-Smidt P, Kock-Jensen C. Ventriculostomy-related infections--an epidemiological study. Acta Neurochir (Wien) 1986;83:20–3. doi: 10.1007/BF01420503. [DOI] [PubMed] [Google Scholar]
- 21.Tse T, Cheng K, Wong K, Pang K, Wong C. Ventriculostomy and Infection: A 4-year-review in a local hospital. Surg Neurol Int. 2010;1:47. doi: 10.4103/2152-7806.69033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725–30. doi: 10.3171/jns.2003.98.4.0725. [DOI] [PubMed] [Google Scholar]
- 23.Wright K, Young P, Brickman C, Sam T, Badjatia N, Pereira M, et al. Rates and determinants of ventriculostomy-related infections during a hospital transition to use of antibiotic-coated external ventricular drains. Neurosurg Focus. 2013;34:E12. doi: 10.3171/2013.2.FOCUS12271. [DOI] [PubMed] [Google Scholar]
- 24.Lewis A, Irvine H, Ogilvy C, Kimberly WT. Predictors for delayed ventriculoperitoneal shunt placement after external ventricular drain removal in patients with subarachnoid hemorrhage. Br J Neurosurg. 2014:1–6. doi: 10.3109/02688697.2014.967753. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin N, St-Antoine P, Bojanowski MW. Impact of antibiotic-impregnated catheters on the timing of cerebrospinal fluid infections in non-traumatic subarachnoid hemorrhage. Acta Neurochir (Wien) 2012;154:761–6. doi: 10.1007/s00701-012-1276-6. discussion 767. [DOI] [PubMed] [Google Scholar]
- 26.Schade RP, Schinkel J, Roelandse FWC, Geskus RB, Visser LG, Van Dijk MC, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104:101–8. doi: 10.3171/jns.2006.104.1.101. [DOI] [PubMed] [Google Scholar]
- 27.Freeman WD, Ziai WC, Hanley D. Ventriculostomy-Associated Infection (VAI): In Search of a Definition. Neurocrit Care. 2014 doi: 10.1007/s12028-014-0055-z. [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez-González R, Boto GR, Pérez-Zamarrón Á. Cerebrospinal fluid diversion devices and infection. A comprehensive review. Eur J Clin Microbiol Infect Dis. 2012;31:889–97. doi: 10.1007/s10096-011-1420-x. [DOI] [PubMed] [Google Scholar]
- 29.Bhadange Y, Sharma S, Das S, Sahu SK. Role of liquid culture media in the laboratory diagnosis of microbial keratitis. Am J Ophthalmol. 2013;156:745–51. doi: 10.1016/j.ajo.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Nussenblatt V, Avdic E, Berenholtz S, Daugherty E, Hadhazy E, Lipsett PA, et al. Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol. 2014;35:278–84. doi: 10.1086/675279. [DOI] [PubMed] [Google Scholar]
- 31.Dellit TH, Chan JD, Fulton C, Pergamit RF, McNamara EA, Kim LJ, et al. Reduction in Clostridium difficile infections among neurosurgical patients associated with discontinuation of antimicrobial prophylaxis for the duration of external ventricular drain placement. Infect Control Hosp Epidemiol. 2014;35:589–90. doi: 10.1086/675828. [DOI] [PubMed] [Google Scholar]
- 32.Flibotte JJ, Lee KE, Koroshetz WJ, Rosand J, McDonald CT. Continuous antibiotic prophylaxis and cerebral spinal fluid infection in patients with intracranial pressure monitors. Neurocrit Care. 2004;1:61–8. doi: 10.1385/NCC:1:1:61. [DOI] [PubMed] [Google Scholar]
- 33.Gelabert-González M, Ginesta-Galan V, Sernamito-García R, Allut AG, Bandin-Diéguez J, Rumbo RM. The Camino intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochir (Wien) 2006;148:435–41. doi: 10.1007/s00701-005-0683-3. [DOI] [PubMed] [Google Scholar]
- 34.Alleyne CH, Hassan M, Zabramski JM. The Efficacy and Cost of Prophylactic and Periprocedural Antibiotics in Patients with External Ventricular Drains. Neurosurgery. 2000;47:1124–9. doi: 10.1097/00006123-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Dimick JB, Lipsett PA, Kostuik JP. Spine update: antimicrobial prophylaxis in spine surgery: basic principles and recent advances. Spine (Phila Pa 1976) 2000;25:2544–8. doi: 10.1097/00007632-200010010-00020. [DOI] [PubMed] [Google Scholar]
- 36.May AK, Fleming SB, Carpenter RO, Diaz JJ, Guillamondegui OD, Deppen SA, et al. Influence of broad-spectrum antibiotic prophylaxis on intracranial pressure monitor infections and subsequent infectious complications in head-injured patients. Surg Infect (Larchmt) 2006;7:409–17. doi: 10.1089/sur.2006.7.409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.