Abstract
In the aftermath of spinal cord injury, glial restricted precursors (GRPs) and immature astrocytes offer the potential to modulate the inflammatory environment of the injured spinal cord and promote host axon regeneration. Nevertheless clinical application of cellular therapy for the repair of spinal cord injury requires strict quality-assured protocols for large-scale production and preservation that necessitates long-term in vitro expansion. Importantly, such processes have the potential to alter the phenotypic and functional properties and thus therapeutic potential of these cells. Furthermore, clinical use of cellular therapies may be limited by the inflammatory microenvironment of the injured spinal cord, altering the phenotypic and functional properties of grafted cells. This report simulates the process of large-scale GRP production and demonstrates the permissive properties of GRP following long-term in vitro culture. Furthermore, we defined the phenotypic and functional properties of GRP in the presence of inflammatory factors, and call attention to the importance of the microenvironment of grafted cells, underscoring the importance of modulating the environment of the injured spinal cord.
Keywords: glial restricted precursor, spinal cord injury, astrocytes, axon regeneration, inflammatory factors, long-term culture
Introduction
Astrocytes are the most abundant cell type in the central nervous system (CNS), providing crucial support for the development and maintenance of CNS function. Nevertheless, astrocytes are also involved in the injury process and in a variety of neurological disorders, in which they become activated or dysfunctional (Sloan and Barres, 2014; Ben Haim et al., 2015; Khakh and Sofroniew, 2015). Given this dichotomy, it is important not only to define their phenotypic and functional properties, but also to identify the molecular mechanisms and develop protocols for the preparation of supportive astrocyte subtypes, particularly if these cells are to be used as beneficial transplants in the CNS. These supportive astrocytes have numerous beneficial effects in the aftermath of CNS injury – promoting host axonal regeneration, limiting the inflammatory response, and reducing the inhibitory microenvironment of the injury site to create a permissive environment for axon growth and synaptic connectivity (Hill et al., 2004; Bonner et al., 2011; Jin et al., 2011; Haas and Fischer, 2014). Importantly, restoration of functional connectivity after spinal cord injury (SCI) has focused on promoting host axon regeneration and using neuronal relays with graft-derived neurons (Bonner and Steward, 2015), strategies that are facilitated by the use of supportive astrocytes (Figure 1). The objective of this paper is to summarize recent findings pertaining to the properties of glial restricted precursors (GRP) and GRP-derived astrocytes and discuss how these findings can be translated into a therapeutic strategy that promotes connectivity and recovery following SCI.
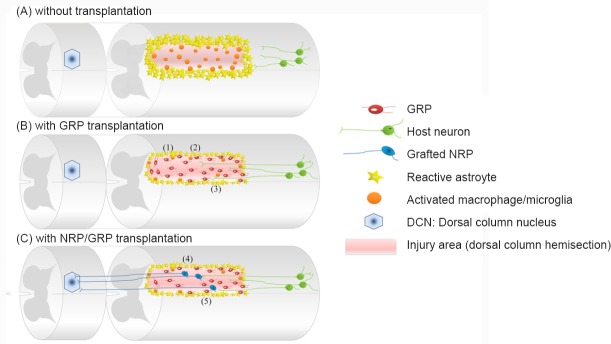
Figure 1.
Glial restricted precursor (GRP) transplants provide a supportive environment following spinal cord injury (SCI) and can be used in combinatorial strategies to restore connectivity.
(A) Diagram of the dorsal column hemisection. The lesion border contains reactive astrocytes and a scar of chondroitin sulfated proteoglycans, while the lesion core is composed of activated microglia and infiltrating macrophages. Damaged host axons retract from the site of injury and the physical, chemical, and inflammatory inhibitors of axon growth. (B) Transplantation of GRP creates a permissive environment that provides for: 1) reduction of glial scar formation, 2) reduced activation of microglia/macrophages, and 3) support for axon growth and regeneration into lesion. (C) Transplantation of GRP together with NRP allows for the formation of synaptic connections between regenerating sensory host axons and graft-derived neurons. In this case, GRP transplants also support: 4) survival and differentiation of NRP into neurons and 5) synaptic connectivity for the formation of a neuronal relay. NRP: Neuronal restricted precursors.
Glial Restricted Precursor (GRP) and GRP-derived Astrocytes
During CNS development, astrocytes originate from a variety of progenitor cells including GRP, which give rise to both astrocytes and oligodendrocytes (Rao and Mayer-Proschel, 1997). GRP, as well as neuronal restricted precursors (NRP), can be isolated from the rat spinal cord during embryonic development at day (E) 13.5–14 or derived in culture from multipotent neuroepithelial stem cells (Cai et al., 2002; Lepore et al., 2004). When GRP are grafted into normal adult spinal cord they show robust survival, proliferation, and migration as well as differentiation into both astrocytes and oligodendrocytes, confirming their progenitor properties (Han et al., 2004; Lepore and Fischer, 2005). Our previous studies have focused on the ability of GRP and GRP-derived astrocytes to support regeneration and connectivity following SCI (Figure 1). We and others found that GRP and GRP-derived astrocytes have remarkable supportive properties that include: 1) reduction of glial scar formation as indicated by preventing the upregulation of host glial fibrillary acid protein (GFAP) and chondroitin sulfated proteoglycan (CSPG) expression, 2) supporting axon growth and regeneration, 3) promoting the survival and neuronal differentiation of NRP, and 4) supporting structural and functional synaptic connectivity and the formation of a neuronal relay (Lepore and Fischer, 2005; Bonner et al., 2011; Jin et al., 2011; Haas and Fischer, 2014). In summary, transplantation of GRP generates supportive astrocytes, which can be used as a therapeutic platform in strategies designed for SCI repair in animal models and eventually translate into human clinical trials.
Phenotypic and Functional Characterization of GRP and GRP-derived Astrocytes
GRP are characterized by a refractive, short stellate morphology, the expression of the surface marker A2B5, and the neural precursor marker nestin (Rao and Mayer-Proschel, 1997). Traditionally, astrocytes have been defined by their multi-process stellate morphology and expression of GFAP, which is commonly used as a marker of mature astrocytes, although other markers such as S100β, ALDH1L1, and GLAST have also been used. However, there is growing evidence for a heterogeneous population of astrocytes capable of performing a diverse array of temporally, regionally, and contextually specific functions (Zhang and Barres, 2010; Khakh and Sofroniew, 2015). Our previous studies demonstrated that GRP cultured in serum-free, defined media supplemented with basic fibroblastic growth factor (bFGF) maintained their phenotypically undifferentiated state (Haas et al., 2012). Furthermore, under such conditions, these cells were capable of extensive proliferation, allowing for the expansion and cryopreservation of large, near-homogenous cell stocks. We found that in vitro differentiation of GRP into astrocytes with BMP4 or CNTF induced distinct morphological and phenotypic changes, with BMP4 generating a more mature phenotype and CNTF an intermediate state that retained progenitor markers. The use of GRP for preparation of astrocytes provides considerable advantages over isolation and culturing primary astrocytes from the CNS. These advantages are underscored by comparable protocols developed for human GRP and the availability of embryonic stem (ES) and induced pluripotent stem (iPS) cells for preparation of neural progenitors (Haas and Fischer, 2013; Roybon et al., 2013; Palm et al., 2015).
In vitro co-culture assays are useful tools to evaluate the interactions between cell populations and elucidate the mechanisms of these interactions. Such interactions can be studied by a direct co-culture model (analyzing cell-cell interactions) or an indirect model with multi-compartment slides or the use of conditioned medium (analyzing secreted factors). Studies using co-cultures of astrocytes and dorsal root ganglion (DRG) neurons have demonstrated the permissive properties of astrocytes with respect to their ability to support axon growth (Smith et al., 1990). Importantly, in these studies, only immature astrocytes, freshly isolated from early post-natal cerebral cortices, but not mature astrocytes, expanded in culture in the presence of fetal bovine serum or those isolated from the mature cortex, were able to support axon growth (Smith et al., 1990). Similar to the effects of immature astrocytes on axon growth, GRP and astrocytes pre-differentiated with either BMP4 or CNTF also supported axon growth of embryonic and adult rat DRG neurons (Haas et al., 2012). In addition, conditioned media harvested from high-density GRP cultures facilitated the growth of DRG axons on an inhibitory CSPG-enriched substrate and allowed them to cross onto a CSPG-enriched border (Ketschek et al., 2012). These data indicate that GRP and immature astrocytes, isolated from fetal tissue or derived from GRP, secrete trophic factors that support and promote axon growth even in the presence of an inhibitory environment, underscoring their potential use in SCI.
To test the data obtained from in vitro experiments in animal models, we examined the properties of GRP using a C4 dorsal column hemisection of SCI and found that ascending sensory axons, labeled with cholera toxin B, regenerated into the injury/transplant site as shown in Figure 1 (Hill et al., 2004; Haas et al., 2012; Haas and Fischer, 2013). Importantly, both GRP and GRP-derived astrocytes prepared with BMP4 or CNTF showed comparable effects, creating a permissive environment for host axon growth into but not out of the injury site. The finding that GRP transplants were capable of inducing only a modest regenerative response, which did not extent beyond the injury site, may be related to the limited regenerative response of axons in the CNS and the permissive nature of the GRP for axon growth in absence of directional guidance molecules outside the transplant. It is important to note that the regenerative capacity of the motor system, particularly that of the corticospinal tract (CST), is poor even in the presence of GRP or astrocytes (Hill et al., 2004, and unpublished data). In this setting, GRP were only able to prevent the significant CST retraction that occurs in the aftermath of SCI. Taken together, GRP and GRP-derived astrocytes create an improved supportive environment for axon regeneration in both in vitro and in vivo systems.
Evaluation of Late Passage GRP
The clinical use of cellular products for transplantation requires large-scale preparation, cryopreservation, and a strict process of quality assurance to ensure the standardized properties of cellular therapeutics with minimal lot-to-lot variability. To guarantee a supply of supportive astrocytes it will be necessary to expand GRP cultures while ensuring that the therapeutic properties of these cells have not changed. There have been several reports examining the phenotypic and functional properties of neural cells cultured for extensive periods of time with respect to their supportive properties. For example, multipotent neural stem cells isolated from embryonic human spinal cord and grown as neurospheres can be expanded for up to 25 passages (over 350 days) in vitro and maintain their multipotent capacity in vivo when grafted into an SCI model (Akesson et al., 2007). Similarly, oligodendrocyte precursor cells (OPC) can be maintained as oligospheres for several months and retain their ability to differentiate into functional oligodendrocytes capable of myelinating axons of primary cortical neurons in a co-culture assay (Zhu et al., 2014). In contrast, olfactory ensheathing cells (OEC), specialized glial cells with Schwann cell and astrocyte-like properties, can lose their ability to remyelinate the injured cord after 6 weeks in culture (Radtke et al., 2010). These examples emphasize the importance of testing and identifying the appropriate temporal parameters and culture conditions for expansion of cells to be used in transplantation studies.
In a recent study, we examined the long-term properties of GRP following expansion of adherent cultures in serum-free, defined media supplemented with the bFGF mitogen (Hayakawa et al., 2015). Specifically, we compared the morphological, phenotypic, and functional properties of early (10–20 days in culture) and late (120–140 days in culture) passage GRP. Our results demonstrated that late passage GRP were maintained in an undifferentiated state, and more importantly, showed comparable axon growth-promoting effects on adult DRG axon growth in both direct co-culture and indirect conditioned medium experiments (Figure 2A) (Hayakawa et al., 2015). Taken together, we established an in vitro culture protocol that enables GRP to maintain their progenitor characteristics and supportive properties relative to axon growth even after long-term culture. It is now important to confirm that late passage GRP maintain their permissiveness in vivo and support axon growth when transplanted into animal models of SCI.
Figure 2.
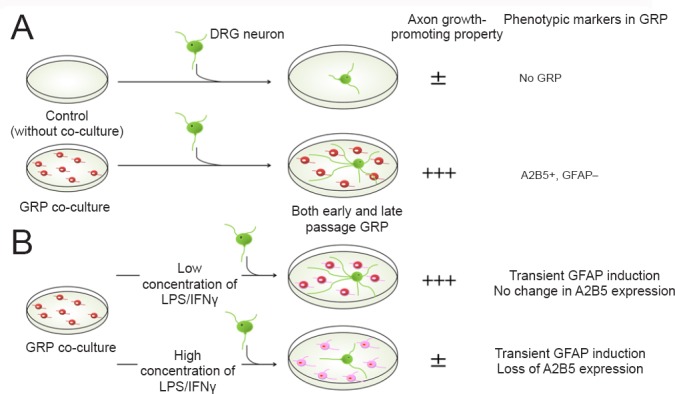
Axon growth-promoting properties of glial restricted precursors (GRP) in vitro.
Differences in the phenotypic and the functional properties of GRP using in vitro co-culture experiments with dorsal root ganglion (DRG) neurons. (A) Comparison of early and late passage GRP. Both early and late passage GRP were characterized by high expression of A2B5 and low expression of glial fibrillary acid protein (GFAP) typical of an immature state. Early and late passage GRP promoted comparable axon growth relative to control cultures consisting of DRG neurons alone. (B) GRP treated with inflammatory factors and their ability to support axon growth. GRP were exposed to various concentrations of Lipopolysaccharide (LPS)/Interferon gamma (IFNγ) for 24 hours and tested in co-culture experiments. GRP treated with low concentrations of LPS/IFNγ retained their axon growth supportive properties. In contrast, GRP treated with high concentrations of LPS/IFNγ showed a transient GFAP expression that was followed by a loss of A2B5 expression, and displayed an attenuation of their axon growth supporting properties.
GRP and the Inflammatory Microenvironment
Traumatic injury to the spinal cord initiates an inflammatory cascade mediated by a multitude of signaling molecules in a stage-specific manner (Alexander and Popovich, 2009; Bowes and Yip, 2014). Astrocytes respond to injury by undergoing a spectrum of morphological, biochemical, and functional alterations in a process known as reactive astrocytosis (Fitch and Silver, 2008; Sofroniew, 2009). During this process, astrocytes become hypertrophic, upregulate GFAP expression, and form a glial scar. The consequences of reactive astrocytosis depend on the location, severity, and temporal relationship to the injury, ranging from reversible alterations with preservation of cellular domains and tissue structure to, in severe cases, irreversible changes resulting in scar formation with permanent rearrangement of tissue structure. Accordingly, there is growing evidence for the presence of different types of reactive astrocytes with diverse morphological, molecular, and functional properties (Bowes and Yip, 2014; Khakh and Sofroniew, 2015).
Given the dramatic effects of inflammatory factors on the properties of mature astrocytes, we examined how the inflammatory environment may directly affect the morphological, phenotypic, and functional properties of GRP using the DRG co-culture system (Figure 2). We used a combination of Lipopolysaccharide (LPS) and Interferon gamma (IFNγ), maximizing the pro-inflammatory effects by signaling through two divergent pathways (Schroder et al., 2006). We found that GRP exposed to either low or high concentrations of LPS/IFNγ for 24 hours resulted in transient GFAP expression, which returned to normal levels by 72 hours after treatment cessation (Hayakawa et al., 2015). Although expression of GFAP was transient and normalized following withdrawal of the inflammatory factors, the expression of A2B5, a marker of GRP, was significantly reduced in GRP treated with high concentrations of LPS/IFNγ. These results suggest that the exposure of GRP to a severe pro-inflammatory microenvironment induces progressive changes in the phenotypic characteristics of these cells in a dose dependent manner. Importantly, these phenotypic alterations were mirrored by the reduced ability of GRP to support axon growth after exposure to high concentrations of LPS/IFNγ as evaluated by direct co-culture assays (Figure 2B). These observations are consistent with reports demonstrating alterations in transcriptional profiles of astrocytes following LPS exposure, consistent with reactive astrogliosis (Zamanian et al., 2012). They also highlight the importance of the microenvironment on the properties of transplanted cells and suggest that modulation of the injury environment may be important in creating more favorable conditions for cell transplantation.
As we relate these results to our previous studies, which demonstrated that GRP create a permissive environment when grafted in an acute SCI model (Bonner et al., 2011; Jin et al., 2011; Haas et al., 2012), we need to consider: 1) the dynamic nature of the injury environment characterized by a complex cascade of pro-inflammatory and anti-inflammatory signaling molecules, 2) the diversity of cells present and/or recruited in the injury site (e.g., resident microglia and recruited macrophages) and their plasticity in response to inflammation that provides both beneficial and detrimental effects, and 3) the complex bi-directional interactions between grafted cells and the injured environment. While this complex environment is difficult to recreate in culture conditions, in vitro experiments serve as a model to define not only the direct interactions between cells, but also with signaling molecules, contributing to an understanding of the mechanism of the inflammatory process.
Conclusions and Future Perspectives
The complexity of SCI is underscored by changes that occur in distinct spatial and temporal patterns, variations in injury models and experimental design, and the need for combination therapy targeting multiple aspects of the injury. It is therefore important to continue research using not only in vitro systems to identify potential therapeutic candidates and evaluate their mechanism of action, but also in vivo models of SCI to validate promising strategies with respect to functional recovery. Our work with GRP has emphasized the value of GRP as a therapeutic platform in the repair of SCI. The in vitro co-culture studies identified the axon growth-supporting properties of GRP, the means to expand GRP cultures to prepare sufficient stocks for transplantation experiments, and the direct impact of pro-inflammatory factors on the phenotypic and functional properties of these cells. Subsequently, GRP have been evaluated as a therapeutic strategy in animal models of SCI, which confirmed the therapeutic potential of these cells and their derived astrocytes with respect to modulation of the injured environment, host axon growth, and synaptic connectivity. It is likely that GRP transplants will be used as a therapeutic platform in combination with additional strategies to reconnect the injured nervous system, such as: 1) NRP, for relay formation, 2) Trophic molecules, to enhance GRP migration or guide axon regeneration, 3) Neuronal modification, to enhance the intrinsic regenerative capacity of injured neurons, 4) Anti-inflammatory agents, to limit the inflammatory microenvironment, 5) Anti-scar agents, to reduce glial scarring in order to achieve more efficient cell survival and axonal regeneration, and 6) Biomaterials, to create a niche capable of providing a more permissive environment for transplantation.
Finally, it is also important to note that the lessons and experience gained from SCI studies could also be applied to the treatment of various other neurological diseases, such as Amyotrophic lateral sclerosis (ALS), where transplantation of GRP and immature astrocytes could be used to provide neuroprotection and slow progression of the neurodegenerative process (Lepore et al., 2008).
Footnotes
Funding: This work was supported by NIH PO1 NS055976, Craig H. Neilsen Foundation.
References
- Akesson E, Piao JH, Samuelsson EB, Holmberg L, Kjaeldgaard A, Falci S, Sundstrom E, Seiger A. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 2007;92:60–66. doi: 10.1016/j.physbeh.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: From fetal grafts to neural stem cells. Brain Res. 2015;1619:115–123. doi: 10.1016/j.brainres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes AL, Yip PK. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma. 2014;31:1753–1766. doi: 10.1089/neu.2014.3429. [DOI] [PubMed] [Google Scholar]
- Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Transplanting neural progenitors to built a neuronal relay across the injured spinal cord. Neural Regen Res. 2014;9:1173–1176. doi: 10.4103/1673-5374.135321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Haas C, Jin Y, Bouyer J, Otsuka T, Fischer I. Glial restricted precursors maintain their permissive properties after long-term expansion but not following exposure to pro-inflammatory factors. Brain Res. 2015;1629:113–125. doi: 10.1016/j.brainres.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Han SS, Tyler-Polsz CJ, Cai J, Rao MS, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–126. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm T, Bolognin S, Meiser J, Nickels S, Trager C, Meilenbrock RL, Brockhaus J, Schreitmuller M, Missler M, Schwamborn JC. Rapid and robust generation of long-term self-renewing human neural stem cells with the ability to generate mature astroglia. Sci Rep. 2015;5:16321. doi: 10.1038/srep16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke C, Lankford KL, Wewetzer K, Imaizumi T, Fodor WL, Kocsis JD. Impaired spinal cord remyelination by long-term cultured adult porcine olfactory ensheathing cells correlates with altered in vitro phenotypic properties. Xenotransplantation. 2010;17:71–80. doi: 10.1111/j.1399-3089.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia-Diaz A, Yang EJ, Sattler R, Jackson-Lewis V, Kim YA, Kachel CA, Rothstein JD, Przedborski S, Wichterle H, Henderson CE. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zhu B, Zhao C, Young FI, Franklin RJ, Song B. Isolation and long-term expansion of functional, myelinating oligodendrocyte progenitor cells from neonatal rat brain. Curr Protoc Stem Cell Biol. 2014;31:20.17.1–15. doi: 10.1002/9780470151808.sc02d17s31. [DOI] [PubMed] [Google Scholar]