Abstract
The choroid plexus is a complex structure which hangs inside the ventricles of the brain and consists mainly of choroid plexus epithelial (CPE) cells surrounding fenestrated capillaries. These CPE cells not only form an anatomical barrier, called the blood-cerebrospinal fluid barrier (BCSFB), but also present an active interface between blood and cerebrospinal fluid (CSF). CPE cells perform indispensable functions for the development, maintenance and functioning of the brain. Indeed, the primary role of the choroid plexus in the brain is to maintain homeostasis by secreting CSF which contains different molecules, such as nutrients, neurotrophins, and growth factors, as well as by clearing toxic and undesirable molecules from CSF. The choroid plexus also acts as a selective entry gate for leukocytes into the brain. Recent findings have revealed distinct changes in CPE cells that are associated with aging and Alzheimer's disease. In this review, we review some recent findings that highlight the importance of the CPE-CSF system in Alzheimer's disease and we summarize the recent advances in the regeneration of brain tissue through use of CPE cells as a new therapeutic strategy.
Keywords: Alzheimer's disease, choroid plexus, brain barrier, blood-CSF barrier, aging, neurodegenerative diseases
Choroid Plexus-Cerebrospinal Fluid (CSF) System
The choroid plexus is a highly vascularized structure which hangs in the brain ventricles and contains a monolayer of choroid plexus epithelial (CPE) cells. These CPE cells form the blood-cerebrospinal fluid barrier (BCSFB). In contrast to the blood-brain barrier (BBB), which is formed by endothelial cells at the level of brain capillaries, CPE cells are located on top of fenestrated capillaries. Both the CPE cells at the BCSFB and endothelial cells at the BBB are interconnected with tight junctions which restrict the entry of molecules and pathogens from blood into brain. The BCSFB apical surface has half the area of the BBB surface due to the presence of microvilli at the apical side of the CPE cells, which implies that the BCSFB also has a vital role in brain homeostasis. A major task of the CPE cells is CSF production. CSF has several established functions: it protects the brain from blood pressure changes and mechanical trauma, enables transport of various molecules through the entire central nervous system (CNS) and facilitates the removal of toxins and waste products. Consequently, the choroid plexus-CSF system plays an active role in physiological processes such as development, repair and maintenance of brain homeostasis. Additionally, this structure is important as an inflammatory sensor that detects signals originating from both the peripheral and the central nervous system (Vandenbroucke et al., 2012; Brkic et al., 2015b). Subsequently, the CPE cells can transmit the inflammatory signal, e.g., by the production of cytokines and chemokines. Additionally, the choroid plexus functions as a gateway to the brain by secreting chemoattractants that facilitate the entry of different peripheral immune cells into the brain (Demeestere et al., 2015). Although the brain was originally considered as an immune privileged site, the presence of T lymphocytes has been reported in CSF, and the choroid plexus was shown to have a niche of effector CD4(+) memory cells with a T-cell receptor repertoire specific to CNS antigens (Baruch et al., 2013). Next to their barrier function, CPE cells express on their membranes polarized and specific transport systems that enable them to control the trafficking of various brain nutrients, such as amino acids, peptide hormones, nucleosides and ions. Finally, role of CPE in the clearance of toxic molecules and drugs from the brain has been long established (de Lange, 2004).
Choroid Plexus-CSF System in Aging and Neurodegenerative Disorders
Due to the increasing number of elderly in the world's population, much attention has been recently given to the study of aging and age-related diseases, including neurodegenerative disorders. Chronic neuroinflammation has been observed in aging individuals, and in neurodegenerative diseases it leads to aggravation of the disease. Recently, it was reported that aging is associated with increased type I interferon dependent gene expression in the choroid plexus and that this expression interferes with normal brain function (Baruch et al., 2014).
The most prevalent neurodegenerative disorder, Alzheimer's disease (AD), is characterized by progressive loss of cognitive abilities and by brain atrophy accompanied by accumulation of amyloid β (Aβ) plaques in the brain parenchyma and neurofibrillary tangles, and aggregates of hyperphosphorylated tau protein in the neurons. While the BBB has been extensively studied in AD, the BCSFB has been largely understudied. Recent studies suggest that the choroid plexus-CSF system exhibits morphological changes and a functional decline with aging, which is believed to be exacerbated in AD (Figure 1). The most prominent changes include anomalies in CSF production and general turnover, and these have been observed in humans and in animal models of age-related diseases (Redzic et al., 2005). Changes in CPE cell metabolism such as decline in metabolic and enzymatic activity are evident. The cytoplasm of CPE cells is filled with a variety of species, such as amyloid Biondi bodies (complex filamentous ring-like structures of 8–15 nm diameter associated with lipid droplets), psammoma bodies (calcified intracellular inclusion structures), and lipofuscin (yellow or brown intracellular structures composed of lipid molecules and usually residues of lysosomal digestion process). Calcification and thickening of the basement membrane have also been noticed (Marques et al., 2013). Additionally, Aβ deposits were also observed in CPE cells in AD (Dietrich et al., 2008). In our work, using a mouse model of AD based on direct injection of Aβ1–42 oligomers into the brain ventricles, we confirmed that Aβ1–42 induces alterations in the cuboidal shape of CPE cells, and we also observed a decrease in the expression of tight junction components (Brkic et al., 2015b). Moreover, these changes were associated with increased leakiness of the BCSFB, potentially aggravating disease progression. Interestingly, it was speculated almost two decades ago that BCSFB dysfunction occurs in AD patients (Hampel et al., 1997), and this was recently confirmed by Bergen et al. (2015) who showed decreased expression of tight junction components in diseased CPE. In our mouse study, we showed that matrix metalloproteinases (MMPs), which have been implicated in several neurodegenerative diseases (Brkic et al., 2015a), are key players in the Aβ1–42 oligomer-induced barrier disruption and that barrier integrity could be preserved by applying a broad-spectrum MMP inhibitor or by abolishing MMP-3 in mice. So far, there are no clear data from humans about the role of MMPs in BCSFB breakdown in AD, although several authors reported increased presence of specific MMPs in CSF, plasma and serum of AD patients (Horstmann et al., 2010; Bjerke et al., 2011). However, the data are not consistent, and the prospect for therapeutic MMP inhibition as an AD treatment remains precarious (Brkic et al., 2015a). Indeed, MMPs play pivotal roles in many physiological conditions, including tissue repair, which could make their inhibition harmful (Vandenbroucke and Libert, 2014).
Figure 1.
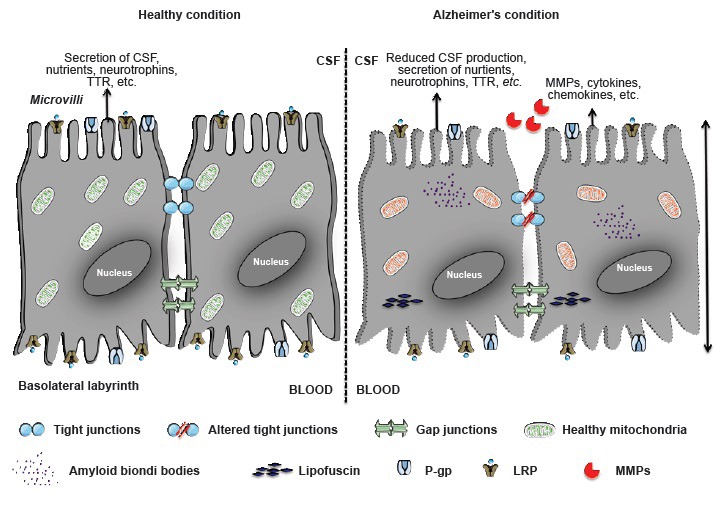
Schematic representation of choroid plexus epithelial (CPE) cells.
The left side represents healthy CPE cells, reflected by the presence of tight junctions, secretion of cerebrospinal fluid (CSF), neurotrophins, nutrients, transthyretin (TTR), etc. These cells have numerous transporters at their apical and basolateral sides. The right side represents CPE cells in Alzheimer's disease, as reflected by the alteration of altered tight junctions, reduced secretion of CSF and neurotrophins, and accumulation of toxic molecules inside the cell. Additionally, expression of several transporters is altered, e.g., lipoprotein receptor-related proteins 1 and 2 (LRP-1 and -2) and P-glycoprotein (P-gp). MMPs: Matrix metalloproteinases.
In accordance with the theory of aging as an inflammatory disease, various proinflammatory cytokines, such as TNF-α, were found in CSF of patients suffering from AD (Tarkowski et al., 2003). Likewise, we observed a significant increase in gene expression of the proinflammatory mediators Il1β, Il6, and Inos in CPE cells and increased cytokine levels in CSF; these trigger MMP activity and lead to opening of the barrier (Brkic et al., 2015b). Other researchers have identified an important role for the choroid plexus-CSF system in the clearance of toxic Aβ species from the brain into CSF via specific transporters (e.g., lipoprotein receptor-related protein 2; LRP-2) and secretion of transthyretin (TTR). Both LRP-2 and TTR can bind Aβ and facilitate its removal from the brain, thereby preventing Aβ plaque formation. Interestingly, TTR was found to be decreased in CSF in aging and in AD (Serot et al., 1997; Chen et al., 2005). In contrast however, recent findings showed no evidence of changes in CP TTR production levels in healthy or AD individuals (Bergen et al., 2015).
The CP serves as a selective entry gate for leukocytes into the CNS by surface expression of adhesion molecules that facilitate leukocyte transmigration (Demeestere et al., 2015). Recently, Baruch et al. observed that during aging and AD, nitric oxide (NO) levels were upregulated also in CP. These increased NO levels serve as a negative regulator of the CP gateway activity for immune cell infiltration into the CNS. Similarly, the 5XFAD mouse, a transgenic model of AD, is less able to reduce immune cell trafficking to the CNS. Interestingly, administration of NO scavenger molecules in these animals induced NFκB/p65 pathway activation and restored CP gateway activity (Baruch et al., 2015). Using the same mouse model, these authors also showed that depletion of Foxp3(+) regulatory T cells or inhibition of their activity by pharmaceutical compounds can alleviate plaque pathology and neuroinflammation. This suggests that targeting Treg-mediated systemic immunosuppression is a plausible strategy for treating AD (Baruch et al., 2015).
Regeneration of the Brain with CPE Cells
Because AD is a complex neurodegenerative disorder, eliminating or inhibiting the production of toxic species like Aβ alone is not sufficient to treat it, and emphasis must also be placed on the regenerative aspects of the damaged brain regions. Several studies have shown the induction of neurotrophins close to damaged sites in the brain, and injection of neurotrophins near the damaged areas was shown to improve the recovery process. Transplantation of neurotrophin releasing cells might provide sustainable continuous release of these molecules. Studies on the choroid plexus and its effect on the brain have pointed to the possibility of rejuvenating the damaged brain regions (Thanos et al., 2010). For example, Borlongan et al. (2004b) were the first to demonstrate that the choroid plexus can produce neurotrophic factors such as glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF). Conditioned medium collected from primary CPE cultures can protect serum deprived cortical neurons in a dose dependent manner. The same authors also performed intracranial transplantation of porcine CPE cells in rats, and this improved the behavioral performance and decreased the volume of infarction in the rodent stroke model (Borlongan et al., 2004b). Similarly, Bolos et al. showed that cultured CPE cells treated with oligomeric Aβ lead to increased proliferation and differentiation of neuronal progenitor cells. Moreover, transplantation of CPE cells into the APP/PS1 mouse brain induced a significant reduction in brain Aβ and p-tau levels and this revealed improved spatial and non-spatial memory in APP/PS1 mice (Bolos et al., 2014). In another study, CPE cells extracted from rat brain were encapsulated and transplanted into the brain of Aβ injected mice. The grafted CPE cells significantly reduced apoptosis, gliosis and oxidative stress and increased neurogenesis. Moreover, the mice's long-term memory improved, indicating that CPE cells might be considered for cell therapy in AD (Aliaghaei et al., 2015).
CPE cells are also involved in Aβ clearance. Aβ is taken up by CPE cells for degradation, but since CPE lysosomal function is compromised in AD, Aβ accumulates rather than being cleared. Like the BBB, the BCSFB possesses transporters that can transport Aβ from CSF to blood, i.e., LRP-1, LRP-2/megalin, receptors for advanced glycation end (RAGE) and P-glycoprotein (P-gp). However, several of these transporters are compromised in AD, which contributes to Aβ accumulation in the brain (Mesquita et al., 2012).
The above-mentioned findings shed light on the neuroprotective capacity of CPE cells and on their potential use in cell therapy to treat neurodegenerative diseases. Moreover, CPE cell transplantation studies conducted in other neurodegenerative diseases, such as Huntington's disease, were also successful (Emerich and Borlongan, 2009). Furthermore, choroid plexus cell transplantation also alleviated other pathological brain conditions, such as cerebral ischemia in adult rats (Borlongan et al., 2004a).
The presence of stem cells in the brain near the subventricular zone (SVZ) was reported recently, but the mechanisms regulating their differentiation and migration into the CNS is not clear. However, the proximity of the pluripotent stem cells to the choroid plexus-CSF system suggests that CPE-derived growth factors are required for the maintenance and differentiation of the stem cell lineage. Interestingly, Ide et al. (2001) reported that choroid plexus ependymal cells (CPEC) isolated from the fourth ventricle can induce axonal growth when transplanted into the dorsal funiculus of rat spinal cord. Results from the same group demonstrated that one week after grafting green fluorescent protein (GFP)-positive CPEC cells at the injured spinal cord site, some of the GFP-CPEC cells became also positive for the glial fibrillary acidic protein (GFAP), a marker for astrocytes. In support of those results, CPE cells were shown to have the capacity to differentiate into neural precursor cells after focal cerebral ischemia (Li et al., 2002). Although these studies are preliminary, they point to the possibility of rejuvenating the damaged brain via the choroid plexus. However, the biologically active factors that are secreted by the CPE cells at the grafted site have first to be identified.
Conclusion
The choroid plexus plays a pivotal role in brain homeostasis. Indeed, the structure, function and location of the choroid plexus-CSF system provides it with a significant influence on physiological and pathological CNS functioning. CPE cells are tightly connected, which prevents uncontrolled leakage from blood into the CSF and enables the choroid plexus to control inflammatory cell influx into the brain. CPE cells contain numerous transporters and can actively secrete molecules that spread the inflammatory response into the brain. Moreover, CPE cells also play a significant role in the clearance of toxic molecules such as Aβ. In aging and AD, CPE cell functionality is heavily affected, reflected by loss of structural integrity and changes in secretory activity, and this might play a central role in disease initiation or exacerbation of disease progression. This indicates the possibility of rejuvenating CPE cells to restore their functions and thereby treat neurodegenerative diseases such as AD by targeting the choroid plexus‒CSF system.
Footnotes
Funding: This research was supported by the Research Foundation - Flanders (FWO), the Concerted Research Actions (GOA) of Ghent University, the Belgian Science Policy (Interuniversity Attraction Pools - IAP7/07), the Belgain Foundation of Alzheimer's Researoh (SAO), the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant ON173056) and COST Action BM1402.
Conflicts of interest: The authors declare no competing financial interests.
References
- Aliaghaei A, Digaleh H, Khodagholi F, Ahmadiani A. Encapsulated choroid plexus epithelial cells actively protect against intrahippocampal Abeta-induced long-term memory dysfunction; upregulation of effective neurogenesis with the abrogated apoptosis and neuroinflammation. J Mol Neurosci. 2015;56:708–721. doi: 10.1007/s12031-015-0492-y. [DOI] [PubMed] [Google Scholar]
- Baruch K, Kertser A, Porat Z, Schwartz M. Cerebral nitric oxide represses choroid plexus NFkappaB-dependent gateway activity for leukocyte trafficking. EMBO J. 2015;34:1816–1828. doi: 10.15252/embj.201591468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Kaing S, Ten Brink JB, Netherlands Brain B, Gorgels TG, Janssen SF. Gene expression and functional annotation of human choroid plexus epithelium failure in Alzheimer's disease. BMC Genomics. 2015;16:956. doi: 10.1186/s12864-015-2159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke M, Zetterberg H, Edman A, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer's disease. J Alzheimers Dis. 2011;27:665–676. doi: 10.3233/JAD-2011-110566. [DOI] [PubMed] [Google Scholar]
- Bolos M, Antequera D, Aldudo J, Kristen H, Bullido MJ, Carro E. Choroid plexus implants rescue Alzheimer's disease-like pathologies by modulating amyloid-beta degradation. Cell Mol Life Sci. 2014;71:2947–2955. doi: 10.1007/s00018-013-1529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport. 2004a;15:1543–1547. doi: 10.1097/01.wnr.0000133298.84901.cf. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004b;35:2206–2210. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- Brkic M, Balusu S, Libert C, Vandenbroucke RE. Friends or foes: matrix metalloproteinases and their multifaceted roles in neurodegenerative diseases. Mediators Inflamm 2015. 2015a doi: 10.1155/2015/620581. 620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkic M, Balusu S, Van Wonterghem E, Gorle N, Benilova I, Kremer A, Van Hove I, Moons L, De Strooper B, Kanazir S, Libert C, Vandenbroucke RE. Amyloid beta oligomers disrupt blood-CSF barrier integrity by activating matrix metalloproteinases. J Neurosci. 2015b;35:12766–12778. doi: 10.1523/JNEUROSCI.0006-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RL, Athauda SB, Kassem NA, Zhang Y, Segal MB, Preston JE. Decrease of transthyretin synthesis at the blood-cerebrospinal fluid barrier of old sheep. J Gerontol A Biol Sci Med Sci. 2005;60:852–858. doi: 10.1093/gerona/60.7.852. [DOI] [PubMed] [Google Scholar]
- de Lange EC. Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1793–1809. doi: 10.1016/j.addr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Demeestere D, Libert C, Vandenbroucke RE. Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov Today. 2015;20:928–941. doi: 10.1016/j.drudis.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Borlongan CV. Potential of choroid plexus epithelial cell grafts for neuroprotection in Huntington's disease: what remains before considering clinical trials. Neurotox Res. 2009;15:205–211. doi: 10.1007/s12640-009-9021-5. [DOI] [PubMed] [Google Scholar]
- Hampel H, Kotter HU, Moller HJ. Blood-cerebrospinal fluid barrier dysfunction for high molecular weight proteins in Alzheimer disease and major depression: indication for disease subsets. Alzheimer Dis Assoc Disord. 1997;11:78–87. doi: 10.1097/00002093-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Budig L, Gardner H, Koziol J, Deuschle M, Schilling C, Wagner S. Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer's disease. Int Psychogeriatr. 2010;22:966–972. doi: 10.1017/S1041610210000827. [DOI] [PubMed] [Google Scholar]
- Ide C, Kitada M, Chakrabortty S, Taketomi M, Matsumoto N, Kikukawa S, Mizoguchi A, Kawaguchi S, Endoh K, Suzuki Y. Grafting of choroid plexus ependymal cells promotes the growth of regenerating axons in the dorsal funiculus of rat spinal cord: a preliminary report. Exp Neurol. 2001;167:242–251. doi: 10.1006/exnr.2000.7566. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J Neurol Sci. 2002;193:137–146. doi: 10.1016/s0022-510x(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer's disease. Mol Neurodegener. 2013;8:38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, Palha JA, Marques F. Modulation of iron metabolism in aging and in Alzheimer's disease: relevance of the choroid plexus. Front Cell Neurosci. 2012;6:25. doi: 10.3389/fncel.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: aging and late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63:506–508. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–260. doi: 10.1016/s0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Thanos CG, Bintz B, Emerich DF. Microencapsulated choroid plexus epithelial cell transplants for repair of the brain. Adv Exp Med Biol. 2010;670:80–91. doi: 10.1007/978-1-4419-5786-3_8. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke RE, Dejonckheere E, Van Lint P, Demeestere D, Van Wonterghem E, Vanlaere I, Puimege L, Van Hauwermeiren F, De Rycke R, Mc Guire C, Campestre C, Lopez-Otin C, Matthys P, Leclercq G, Libert C. Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J Neurosci. 2012;32:9805–9816. doi: 10.1523/JNEUROSCI.0967-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


