Drug repositioning is a strategy to identify a new application of a pre-approved drug, of which optimal dosage, administration routes, adverse effects, and contraindications are well established. Drug repositioning enables fast and cost-effective application of an identified drug in clinical settings, and is especially suitable for orphan diseases, in which the number of patients is limited (Bernard, 2014).
Motor paralyses due to damage of the peripheral nervous systems cause serious disabilities in activities of daily living (ADL). Local administration of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) induces axonal elongation of motor nerves in animal models (Boyd and Gordon, 2002). Similarly, laminins and cadherin-11 promote the axonal elongation of motor neurons. However, these molecules cannot be readily used in clinical settings, because they have to be administered locally and their half-lives are very short. Cell transplantation is one of promising modalities to treat motor nerve damage (Spejo et al., 2013), but safety concerns should be all solved before clinical application. Among chemical compounds, ibuprofen, a non-steroidal anti-inflammatory drug; valproic acid, an anti-epileptic drug; and Y27632, an inhibitor of Rho-associated kinase (ROK) are effective for motor nerve damage.
In an effort to search for a chemical compound that can be potentially used for motor nerve damage, we screened 1,186 FDA-approved drugs for enhancement of neurite elongation of a neuroblastoma and spinal motor neuron hybrid cell line, NSC34. We found that zonisamide, an anti-epileptic and anti-parkinsonian drug, promoted neurite elongation in NSC34 cells, as well as in mouse primary motor neurons, in a dose-dependent manner (Yagi et al., 2015). Zonisamide increased the neurite lengths and the number of neurite branch points, but had no effect on the ratio of neurons bearing neurites. Continuous measurement of neurite lengths of primary motor neurons cultured for 80 hours demonstrated that zonisamide did not enhance the initiation of neurite outgrowth, but enhanced the elongation of neurites. To examine the effect of zonisamide on neurite regeneration, we seeded mouse primary motor neurons in a culture dish, and allowed them to make a network of neurites. The mesh of neurites was linearly scratched off with a 200-μL pipette tip. The neurons were cultured for 48 additional hours with increasing concentrations of zonisamide, and the total length of regenerated neurites were measured. The neurite-scratch assay revealed that zonisamide enhanced neurite regeneration in a dose-dependent manner. Zonisamide induced mRNA expression of nerve growth factors (Ntf4 encoding neurotrophin-4/5, Ngf encoding nerve growth factor, and Bdnf encoding brain-derived nerve growth factor), and their receptors (Ntrk1 and Ntrk2 encoding neurotrophic tyrosine kinase receptor types 1 and 2, respectively). Ntrk1 is a receptor for NGF, and Ntrk2 is a receptor for BDNF and NT4/5. TrkB agonists promote axonal regeneration in a mouse model of peripheral nerve injury (English et al., 2013). Enhanced expression of Bdnf and its receptor Ntrk2 by zonisamide may partly account for the enhanced neurite elongation in primary motor neurons (Figure 1).
Figure 1.
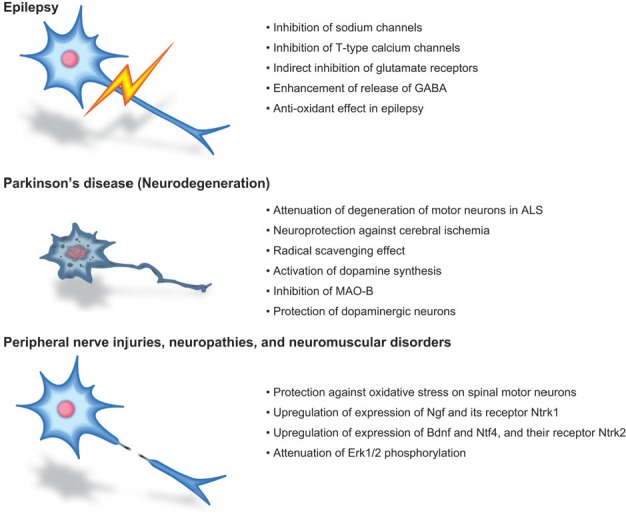
Currently available clinical application of zonisamide for epilepsy and Parkinson's disease, and its potential repositioning for nerve injuries, neuropathies, and neuromuscular disorders.
Zonisamide is an anti-epileptic agent that has been successfully repositioned for Parkinson's disease. We report that zonisamide can be potentially repositioned again for peripheral nerve injuries, neuropathies, and neuromuscular disorders. Pharmacological mechanisms of zonisamide for each disease are indicated in bullet statements. ALS: Amyotrophic lateral sclerosis; Bdnf: brain-derived neurotrophic factor; Erk1/2: extracellular signal-regulated protein kinases 1 and 2; MAO-B: monoamine oxidase B; Ngf: nerve growth factor; Ntf4: neurotrophin 4; Ntrk1: neurotrophic tyrosine kinase receptor type 1; Ntrk2: neurotrophic tyrosine kinase receptor type 2.
We next made a mouse model of sciatic nerve autograft (Yagi et al., 2015). The sciatic nerve of 8-week-old C57BL/6J male mouse was completely transected at two sites and sutured again. Mice took 30 mg/kg/day zonisamide once a day from a day after surgery. The amount of zonisamide used for the mouse model (30 mg/kg/day) was three times more than the maximum dose used for human (10 mg/kg/day), which is below the dose translation factor between mouse and human of 12 (Reagan-Shaw et al., 2008). Administration of zonisamide for 1 week increased the size of axons distal to the transected site 3.9-fold, suggesting enhancement of nerve regeneration by zonisamide. Zonisamide also improved the sciatic function index, a marker for motor function of a hindlimb after sciatic nerve injury, from 6 weeks after surgery. Muscle pathology at 8 weeks after surgery revealed that zonisamide was protective against muscle degeneration. Similarly, gene expression levels of Chrne encoding the acetylcholine receptor ε subunit, Colq encoding collagen Q, and Rapsn encoding rapsyn were increased in the tibialis anterior muscle at 8 weeks after surgery. Chrne, Colq and Rapsn are specifically expressed at the neuromuscular junction. Structural proteins for neurites (Map2 encoding microtubule-associated protein 2 enriched in dendrites, Mapt encoding microtubule-associated protein tau enriched in axon, and Gap43 encoding growth-associated protein 43 enriched in the growth cone) were also upregulated in the autografted sciatic nerve of the mouse model. Similar to primary spinal motor neurons, zonisamide induced mRNA expression of nerve growth factors (Ntf4 and Ngf, but not Bdnf), and their receptors (Ntrk1 and Ntrk2) in the model mice (Figure 1).
The antiepileptic effects of zonisamide are accounted for by inhibition of sodium channels, inhibition of T-type calcium channels, indirect inhibition of glutamate receptors, and enhancement of release of the inhibitory neurotransmitter GABA (Figure 1). In addition, zonisamide shows attenuation of degeneration of motor neurons and loss of astrocytes in a mouse model of amyotrophic lateral sclerosis (ALS), neuroprotection against cerebral ischemia, anti-oxidant effect in epilepsy, and radical scavenging effect (Figure 1). As zonisamide has anti-oxidant effects (Kawajiri et al., 2010), and as this effect has not been reported in spinal motor neurons, we examined the effect of zonisamide on survival of primary spinal motor neurons exposed to hydrogen peroxide (H2O2) (Yagi et al., 2015). H2O2 reduced the number of surviving cells to 74.4 ± 5.8% of the control and 10 μM zonisamide rescued the ratio to 89.3 ± 5.0%. Although zonisamide increased the number of surviving cells only by 14.9%, the effect was statistically significant.
In neurite outgrowth, Erk1/2 and JNK1/2/3 are activated, but we observed that zonisamide rather attenuated Erk1/2 phosphorylation and had no effect on JNK1/2/3 phosphorylation. As Erk is also activated in apoptosis of neuroblastoma cells, and as inhibition of Erk activation by a MEK inhibitor is protective against oxidative stress in a mouse model, the neuroprotective effect of zonisamide against H2O2-exposed primary spinal motor neurons might have been partly conferred by attenuation of Erk phosphorylation.
Zonisamide is an anti-epileptic agent widely used for adjunctive treatment for partial seizures in human. Murata and colleagues noticed in a patient with Parkinson's disease and epilepsy that administration of zonisamide for epilepsy improved symptoms of Parkinson's disease (Murata, 2004). Sumitomo Dainippon Pharma Co., Ltd. conducted a clinical trial in collaboration with Murata and colleagues, and obtained an approval in Japan as a repositioned drug for Parkinson's disease. Zonisamide activates dopamine synthesis and inhibits monoamine oxidase B (MAO-B), which catalyzes dopamine (Murata, 2004) (Figure 1). Zonisamide also has a protective effect for dopaminergic neurons (Asanuma et al., 2010) (Figure 1). An enigma still remains to be solved whether the anti-parkinsonian effect and the neurite-elongation effect of zonisamide share the same pharmacological mechanisms or not. Zonisamide has been used for more than 30 years for epilepsy without major adverse effects. We hope that zonisamide is a clinically applicable therapeutic agent used for peripheral nerve injuries, neuropathies, and neuromuscular disorders, in which terminal sprouting of an axon of a motor neuron compensate for defective neuromuscular signal transmission (Ohno et al., 2014).
Zonisamide was kindly provided by Sumitomo Dainippon Pharma Co., Ltd., Japan. Studies conducted in the authors’ laboratory were supported by Grants-in-Aid from the MEXT, MHLW, and AMED of Japan.
References
- Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, Miyoshi K, Murata M. Neuroprotective effects of zonisamide target astrocyte. Ann Neurol. 2010;67:239–249. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- Bernard NJ. Osteoarthritis: Repositioning verapamil-for Wnt of an OA treatment. Nat Rev Rheumatol. 2014;10:260. doi: 10.1038/nrrheum.2014.60. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- English AW, Liu K, Nicolini JM, Mulligan AM, Ye K. Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves. Proc Natl Acad Sci U S A. 2013;110:16217–16222. doi: 10.1073/pnas.1303646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri S, Machida Y, Saiki S, Sato S, Hattori N. Zonisamide reduces cell death in SH-SY5Y cells via an anti-apoptotic effect and by upregulating MnSOD. Neurosci Lett. 2010;481:88–91. doi: 10.1016/j.neulet.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Murata M. Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson's disease. Curr Pharm Des. 2004;10:687–693. doi: 10.2174/1381612043453180. [DOI] [PubMed] [Google Scholar]
- Ohno K, Ohkawara B, Ito M, Engel AG. eLS. John Wiley & Sons, Inc; 2014. Molecular Genetics of Congenital Myasthenic Syndromes. doi: 10.1002/9780470015902.a9780470024314. [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Spejo AB, Carvalho JL, Goes AM, Oliveira AL. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience. 2013;250:715–732. doi: 10.1016/j.neuroscience.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Yagi H, Ohkawara B, Nakashima H, Ito K, Tsushima M, Ishii H, Noto K, Ohta K, Masuda M, Imagama S, Ishiguro N, Ohno K. Zonisamide enhances neurite elongation of primary motor neurons and facilitates peripheral nerve regeneration in vitro and in a mouse model. PLoS One. 2015;10:e0142786. doi: 10.1371/journal.pone.0142786. [DOI] [PMC free article] [PubMed] [Google Scholar]


